Evaluation of Antiproliferative, Antimicrobial, Antioxidant, Antidiabetic and Phytochemical Analysis of Anogeissus dhofarica A. J. Scott
Abstract
1. Introduction
2. Material and Methods
2.1. Apparatus and Reagents
2.2. Collection and Identification of Plant Samples
2.3. Extraction and Fractionation
2.4. Phytochemical Analysis
2.4.1. Qualitative Assessment
Phenols
Flavonoids
Carbohydrates
Alkaloids
Saponins
Glycosides
Tannins
2.5. Phenolic and Flavonoid Content Quantification
2.5.1. Quantification of Total Phenolic Content (TPC)
Preparation of Standard and Its Dilutions
Preparation of Samples for the Estimation of Total Phenolic Contents
2.5.2. Quantification of Total Flavonoid Content (TFC)
Preparation of Standard Quercetin for Calibration Curve
Preparation of Samples for Estimation of Total Flavonoid Contents
2.6. HR-ESI-MS Analysis
2.7. Biological Activities
2.7.1. Antimicrobial Activity
Bacterial and Fungal Culture
Preparation of Bacterial Inoculum
Preparation of Fungal Inoculum
Antibacterial Assessment
Antifungal Assessment
Minimum Inhibitory Concentration Determination
2.7.2. Antioxidant Activity
2.7.3. α-Glucosidase Assay
2.7.4. Cytotoxicity Assay
2.7.5. Cytotoxic Assay (Brine Shrimp Lethality Assay)
Shrimp Larvae Hatching
Brine Shrimp Lethality Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Qualitative Phytochemical Analysis
3.2. Total Phenolic and Flavonoid Contents
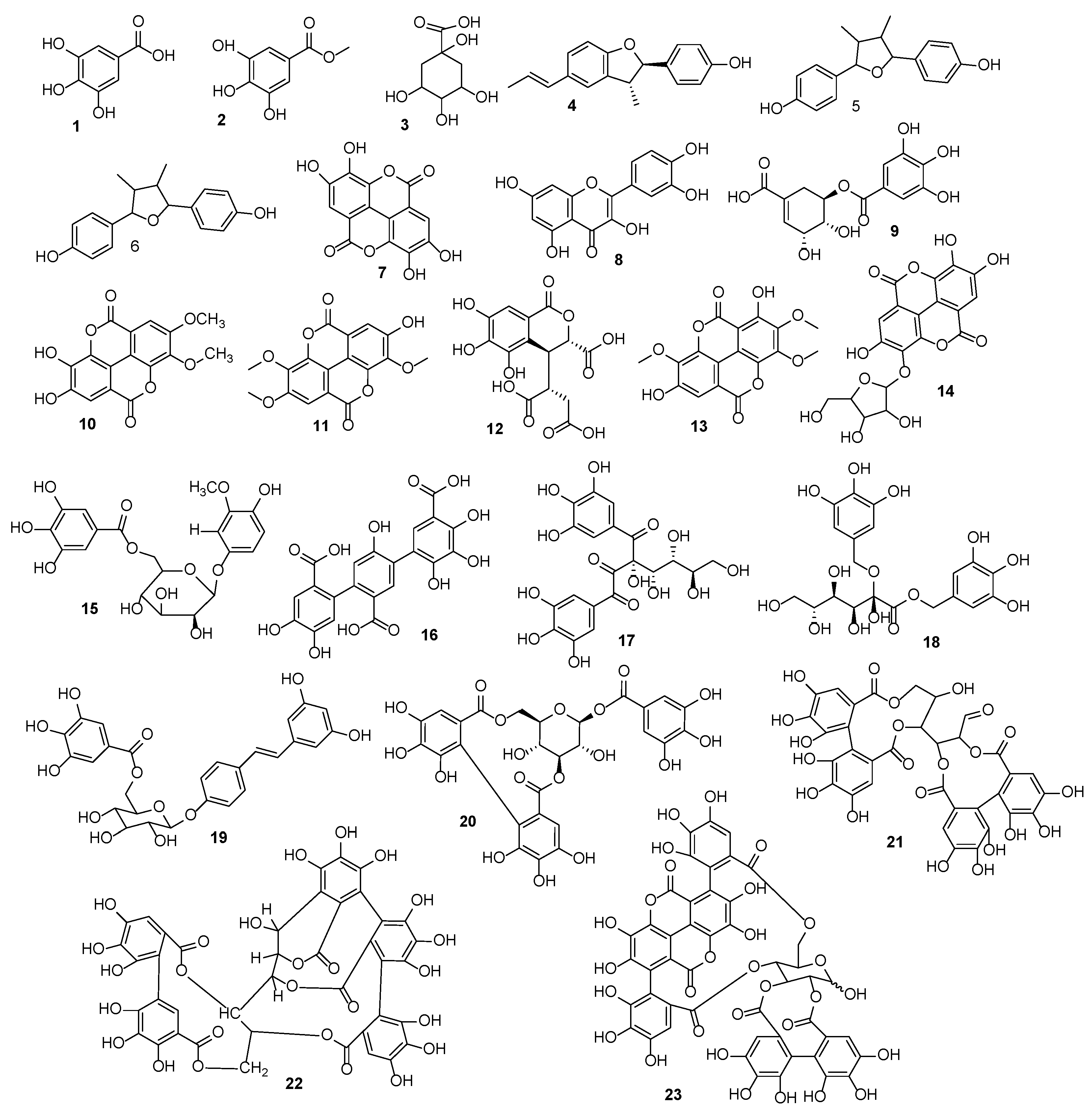
3.3. HR-ESI-MS Assessment
3.4. Antimicrobial Potential
3.4.1. Antibacterial and Antifungal Assessment
3.4.2. Antimicrobial MIC Evaluation
3.5. Antioxidant Assessment
3.6. In-Vitro Antidiabetic Assay
3.7. Cytotoxic Activity
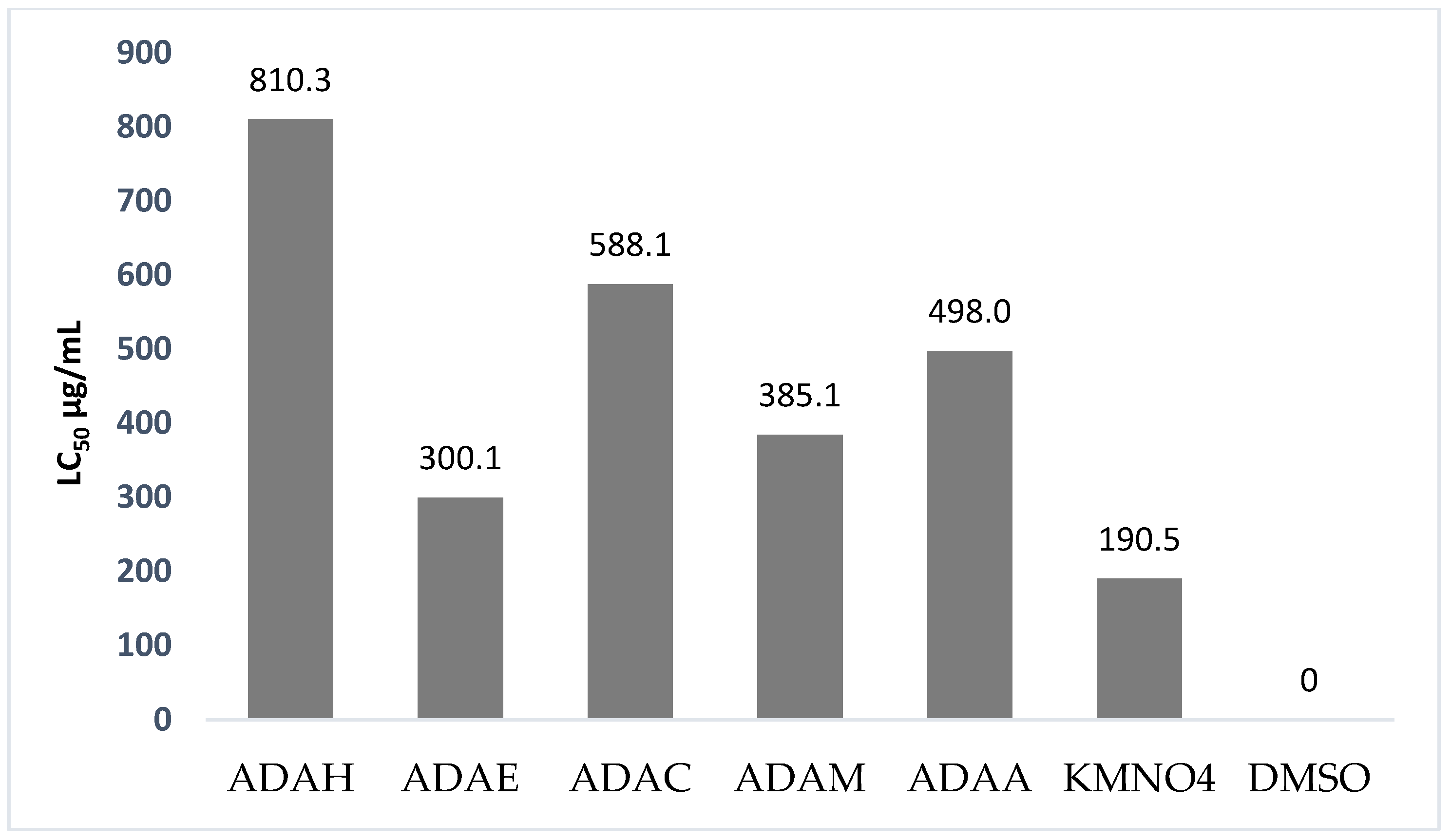
3.8. Brine Shrimp Lethality Assay (Cytotoxic activity)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raina, H.; Soni, G.; Jauhari, N.; Sharma, N.; Bharadvaja, N. Phytochemical importance of medicinal plants as potential sources of anticancer agents. Turk. J. Bot. 2014, 38, 1027–1035. [Google Scholar] [CrossRef]
- Mohammed, A.H. Importance of medicinal plants. Res. Pharm. Health Sci. 2019, 5, 124–125. [Google Scholar] [CrossRef]
- Muhammad, Z.; Nasir, R.; Asim, M.; Fozia, A.; Munawar, I.; Muhammad, M.; Muhammad, S. Antioxidant, antibacterial, antifungal activities and phytochemical analysis of dagger (Yucca aloifolia) leaves extracts. J. Med. Plants Res. 2013, 7, 243–249. [Google Scholar]
- Ur Rehman, F.; Kalsoom, M.; Adnan, M.; Fazeli-Nasab, B.; Naz, N.; Ilahi, H.; Ali, M.F.; Ilyas, M.; Yousaf, G.; Toor, M.D. Importance of medicinal plants in human and plant pathology: A review. Int. J. Pharm. Biomed. Res. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.; Luximon-Ramma, A.; Aruoma, O.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Rane, R.A.; Jain, K.; Shaikh, M.; Hampannavar, G.; Karpoormath, R. A recent perspective on discovery and development of diverse therapeutic agents inspired from isatin alkaloids. Curr. Top. Med. Chem. 2016, 16, 1262–1289. [Google Scholar] [CrossRef]
- Olugbami, J.; Gbadegesin, M.; Odunola, O. In vitro evaluation of the antioxidant potential, phenolic and flavonoid contents of the stem bark ethanol extract of Anogeissus leiocarpus. Afr. J. Med. Med. Sci. 2014, 43, 101. [Google Scholar]
- Farooq, U.; Waseem, B.; Muzaffar, R.; Tripathi, J.; Tharani, M.; Sharma, M. A comparative study of phytochemical investigation of Xanthium strumarium medicinal plant. Int. J. Res. Pharm. Chem. 2014, 4, 96–100. [Google Scholar]
- Osuagwu, G.; Okwulehie, I.; Emenike, J. Phytochemical and Mineral content of the leaves of four Nigerian Pterocarpus species. Int. J. Mol. Med. Adv. Sci 2007, 3, 6–11. [Google Scholar]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Falcone, M.; Paterson, D. Spotlight on ceftazidime/avibactam: A new option for MDR Gram-negative infections. J. Antimicrob. Chemother. 2016, 71, 2713–2722. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.J.; Tor, Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Med. Chem. 2014, 6, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, P.; Purkayastha, S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J. Pharm. Sci. 2012, 74, 443. [Google Scholar]
- Ur Rehman, N.; Rafiq, K.; Khan, A.; Ahsan Halim, S.; Ali, L.; Al-Saady, N.; Hilal Al-Balushi, A.; Al-Busaidi, H.K.; Al-Harrasi, A. α-Glucosidase inhibition and molecular docking studies of natural brominated metabolites from marine macro brown alga Dictyopteris Hoytii. Mar. Drugs 2019, 17, 666. [Google Scholar] [CrossRef] [PubMed]
- Boerma, T.; Mathers, C.D. The World Health Organization and global health estimates: Improving collaboration and capacity. BMC Med. 2015, 13, 50. [Google Scholar] [CrossRef]
- Ur Rehman, N.; Halim, S.A.; Khan, M.; Hussain, H.; Yar Khan, H.; Khan, A.; Abbas, G.; Rafiq, K.; Al-Harrasi, A. Antiproliferative and carbonic anhydrase II inhibitory potential of chemical constituents from Lycium shawii and Aloe vera: Evidence from in silico target fishing and in vitro testing. Pharmaceuticals 2020, 13, 94. [Google Scholar] [CrossRef]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.G.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M.A. Status and challenges of plant-anticancer compounds in cancer treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef]
- Bagayatkar, M.A.; Garge, V.N. Evaluation of cytotoxic activity of hydro alcoholic extract of Anogeissus latifolia with brine shrimp lethality assay. Lat. Am. Appl. Res. 2018, 7, 1182–1189. [Google Scholar]
- Zahoor, M.; Bari, W.U.; Zeb, A.; Khan, I. Toxicological, anticholinesterase, antilipidemic, antidiabetic and antioxidant potentials of Grewia optiva Drummond ex Burret extracts. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190220. [Google Scholar] [CrossRef]
- Sabu, M.; Kuttan, R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J. Ethnopharmacol. 2002, 81, 155–160. [Google Scholar] [CrossRef]
- Singh, D.; Baghel, U.S.; Pannu, M.S.; Yadav, R. Ethnopharmacological based evaluation of Anogeissus pendula Edgew extracts for antioxidant and hepatoprotective potential. Anc. Sci. Life 2017, 36, 136. [Google Scholar] [PubMed]
- Singh, D.; Baghel, U.S.; Gautam, A.; Baghel, D.S.; Yadav, D.; Malik, J.; Yadav, R. The genus Anogeissus: A review on ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 194, 30–56. [Google Scholar] [CrossRef]
- Mann, A.; Yusuf, A.; Daniyan, S. TLC analysis and bioactivity screening of the stem bark extract of Anogeissus leiocarpus against multi-resistant Staphylococcus aureus and quantification of its phytoconstituents. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 192–194. [Google Scholar]
- Oberprieler, C.; Meister, J.; Schneider, C.; Kilian, N. Genetic structure of Anogeissus dhofarica (Combretaceae) populations endemic to the monsoonal fog oases of the southern Arabian Peninsula. Biol. J. Linn. Soc. 2009, 97, 40–51. [Google Scholar] [CrossRef]
- Miller, A.; Morris, M. Plants of Dhofar, the Southern Region of Oman: Traditional, Economic and Medicinal Uses; Diwan of Royal Court: Muscat, Oman, 1988; p. 102. [Google Scholar]
- Kürschner, H.; Hein, P.; Kilian, N.; Hubaishan, M.A. The Hybantho durae-Anogeissetum dhofaricae ass. nova-phytosociology, structure and ecology of an endemic South Arabian forest community. Phytocoenologia 2004, 34, 569–612. [Google Scholar] [CrossRef]
- Gul, R.; Jan, S.U.; Faridullah, S.; Sherani, S.; Jahan, N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. Sci. World J. 2017, 2017, 5873648. [Google Scholar] [CrossRef]
- Auwal, M.S.; Saka, S.; Mairiga, I.A.; Sanda, K.A.; Shuaibu, A.; Ibrahim, A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet. Res. Forum Int. Q. J. 2014, 5, 95. [Google Scholar]
- Gacem, M.A.; Telli, A.; Gacem, H.; Ould-El-Hadj-Khelil, A. Phytochemical screening, antifungal and antioxidant activities of three medicinal plants from Algerian steppe and Sahara (preliminary screening studies). SN Appl. Sci. 2019, 1, 1721. [Google Scholar] [CrossRef]
- Shah, M.; Murad, W.; Ur Rehman, N.; Halim, S.A.; Ahmed, M.; Rehman, H.; Zahoor, M.; Mubin, S.; Khan, A.; Nassan, M.A. Biomedical applications of Scutellaria edelbergii Rech. f.: In vitro and in vivo approach. Molecules 2021, 26, 3740. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Kaur, S.; Mondal, P. Study of total phenolic and flavonoid content, antioxidant activity and antimicrobial properties of medicinal plants. J. Microbiol. Exp. 2014, 1, 00005. [Google Scholar] [CrossRef]
- Adeleye, I.; Ogunniyi, A.; Omonigbehin, E. Antimicrobial activity of some local herbs on common skin pathogens. Biosci. Res. J. 2003, 15, 231–236. [Google Scholar]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Rahman, H.; Khan, A.; Bibi, S.; Ullah, O.; Ullah, S.; Ur Rehman, N.; Murad, W.; Al-Harrasi, A. Identification of α-Glucosidase Inhibitors from Scutellaria edelbergii: ESI-LC-MS and Computational Approach. Molecules 2022, 27, 1322. [Google Scholar] [CrossRef] [PubMed]
- Kamuhabwa, A.; Nshimo, C.; de Witte, P. Cytotoxicity of some medicinal plant extracts used in Tanzanian traditional medicine. J. Ethnopharmacol. 2000, 70, 143–149. [Google Scholar] [CrossRef]
- Sakhi, M.; Khan, A.; Iqbal, Z.; Khan, I.; Raza, A.; Ullah, A.; Nasir, F.; Khan, S.A. Design and Characterization of Paclitaxel-Loaded Polymeric Nanoparticles Decorated with Trastuzumab for the Effective Treatment of Breast Cancer. Front. Pharmacol. 2022, 13, 855294. [Google Scholar] [CrossRef] [PubMed]
- Al-matani, S.K.; Al-Wahaibi, R.N.S.; Hossain, M.A. In vitro evaluation of the total phenolic and flavonoid contents and the antimicrobial and cytotoxicity activities of crude fruit extracts with different polarities from Ficus sycomorus. Pac. Sci. Rev. A Nat. Sci. Eng. 2015, 17, 103–108. [Google Scholar] [CrossRef]
- Weli, A.M.; Al-Harrasi, A.; Al Baiti, N.H.; Philip, A.; Hossain, A.; Gilani, S.A.; Banioraba, N. Biological and toxicological evaluation of aerial parts extracts of locally grown Cleome austroarabica. J. King Saud Univ.-Sci. 2020, 32, 753–757. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Mann, A.; Amupitan, J.; Oyewale, A.; Okogun, J.; Ibrahim, K. Chemistry of secondary metabolites and their antimicrobial activity in the drug development process: A review of the genus Anogeissus. Med. Plants—Int. J. Phytomed. Relat. Ind. 2009, 1, 55–77. [Google Scholar] [CrossRef]
- Konaté, K.; Sanou, D.O.; Santara, B.; Dicko, M.H. Qualitative analysis of phenolic compounds and antibacterial activity of trunk bark extracts of Anogeissus leiocarpus (dc.) guill. and perrot (combretaceae) traditionally used for the management of infectious diseases in village poultry. World J. Pharm. Res. 2022. [Google Scholar]
- Elufioye, T.; Olaifa, O. A comparison of the antioxidant activity with the Total Phenolic and Total Flavonoid Contents of the leaves and stem-bark of Anogeissus leiocarpa (DC.) Guill& Pirr.(Combretaceae). Niger. J. Pharm. Res. 2016, 12, 75–85. [Google Scholar]
- Danai, P.; Pandey, V.; Agrawal, T. In vitro antioxidant potential and antimicrobial activity of leaves and stem extracts of Anogeissus pendula Edgew. Plant Sci. Today 2021, 8, 873–881. [Google Scholar] [CrossRef]
- Annegowda, H.; Nee, C.W.; Mordi, M.; Ramanathan, S.; Mansor, S. Evaluation of phenolic content and antioxidant property of hydrolysed extracts of Terminalia catappa L. leaf. Asian J. Plant Sci. 2010, 9, 479. [Google Scholar] [CrossRef]
- Holasova, M.; Fiedlerova, V.; Smrcinova, H.; Orsak, M.; Lachman, J.; Vavreinova, S. Buckwheat—The source of antioxidant activity in functional foods. Food Res. Int. 2002, 35, 207–211. [Google Scholar] [CrossRef]
- Reddy, K.; Srimannarayana, G.; Rao, N.S. Isolation of 3, 3′, 4-tri-o-methyl-flavellagic acid from bark of Anogeissus latifolia. Curr. Sci. 1974, 43, 544–545. [Google Scholar]
- Orlando, G.; Ferrante, C.; Zengin, G.; Sinan, K.I.; Bene, K.; Diuzheva, A.; Jekő, J.; Cziáky, Z.; Di Simone, S.; Recinella, L. Qualitative chemical characterization and multidirectional biological investigation of leaves and bark extracts of Anogeissus leiocarpus (DC.) Guill. & Perr. (Combretaceae). Antioxidants 2019, 8, 343. [Google Scholar]
- Rimando, A.M.; Pezzuto, J.M.; Farnsworth, N.R.; Santisuk, T.; Reutrakul, V.; Kawanishi, K. New lignans from Anogeissus acuminata with HIV-1 reverse transcriptase inhibitory activity. J. Nat. Prod. 1994, 57, 896–904. [Google Scholar] [CrossRef]
- Nduji, A.; Okwute, S. Co-occurrence of 3,3′,’-tri-O-methylflavellagic acid and 3,3′-di-O-methylellagic acid in the bark of Anogeissus schimperii. Phytochemistry 1988, 27, 1548–1550. [Google Scholar] [CrossRef]
- Formato, M.; Vastolo, A.; Piccolella, S.; Calabrò, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Antioxidants in Animal Nutrition: UHPLC-ESI-QqTOF Analysis and Effects on In Vitro Rumen Fermentation of Oak Leaf Extracts. Antioxidants 2022, 11, 2366. [Google Scholar] [CrossRef]
- Attioua, B.; Lagnikab, L.; Yeoc, D.; Antheaumed, C.; Kaisere, M.; Wenigerf, B.; Lobsteinf, A.; Vonthron-Sénécheauf, C. In vitro antiplasmodial and antileishmanial activities of flavonoids from Anogeissus leiocarpus (Combretaceae). Int. J. Pharm. Rev. Res. 2011, 11, 1–6. [Google Scholar]
- Fyhrquist, P.; Salih, E.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R. Ellagitannins, ellagic acid derivatives and ampelopsin in antimicrobial root and stem bark extracts of some selected African species of Terminalia and Anogeissus leiocarpus. Planta Med. 2014, 80, P1L10. [Google Scholar] [CrossRef]
- Gupta, R. Pharmacognostic studies on “Dhava” (Anogeissus latifolia Bedd.)—I. Stem-bark and root. Proc. Plant Sci. 1985, 94, 589–606. [Google Scholar] [CrossRef]
- Garbi, M.; Kabbashi, A.; El-Badri Elamin, O.; Koko, W.; Dahab, M.; Elshikh, A. In Vitro gardicidal and amoebicidal activity of Anogeissus leiocarpus leaves extracts. Hortic. Int. J. 2018, 2, 232–235. [Google Scholar] [CrossRef]
- Akande, T.; Khatib, M.; Salawu, S.O.; Akindahunsi, A.A.; Mannelli, L.D.C.; Ghelardini, C.; Balli, D.; Cecchi, L.; Mulinacci, N. 1H NMR and HPLC-DAD-MS for the characterization of ellagitannins and triterpenoids of less investigated Anogeissus leiocarpus DC (Combretaceae) stem bark. Food Chem. 2022, 375, 131813. [Google Scholar] [CrossRef]
- Salih, E.Y.A.; Kanninen, M.; Sipi, M.; Luukkanen, O.; Hiltunen, R.; Vuorela, H.; Julkunen-Tiitto, R.; Fyhrquist, P. Tannins, flavonoids and stilbenes in extracts of African savanna woodland trees Terminalia brownii, Terminalia laxiflora and Anogeissus leiocarpus showing promising antibacterial potential. S. Afr. J. Bot. 2017, 108, 370–386. [Google Scholar] [CrossRef]
- Chaabi, M.; Benayache, S.; Benayache, F.; N’Gom, S.; Koné, M.; Anton, R.; Weniger, B.; Lobstein, A. Triterpenes and polyphenols from Anogeissus leiocarpus (Combretaceae). Biochem. Syst. Ecol. 2008, 36, 59–62. [Google Scholar] [CrossRef]
- Lin, T.C.; Tanaka, T.; Nonaka, G.I.; Nishioka, I.; Young, T.J. Tannins and related compounds. CVIII. Isolation and characterization of novel complex tannins (flavono-ellagitannins), anogeissinin and anogeissusins A and B, from Anogeissus acuminata (ROXB ex DC.) GUILL. et PERR. var. lanceolata WALL. ex CLARKE. Chem. Pharm. Bull. 1991, 39, 1144–1147. [Google Scholar] [CrossRef]
- Sani, H.; Aliyu, B. In-vitro Antibacterial Activity of Anogeissus leiocarpus Dc (Stem Bark) Extracts against Escherichia coli and Staphylococcus aureus. Bayero J. Pure Appl. Sci. 2011, 4, 56–59. [Google Scholar] [CrossRef]
- Govindarajan, R.; Vijayakumar, M.; VEnkateshwara Rao, C.; Shirwaikar, A.; Mehrotra, S.; Pushpangadan, P. Healing potential of Anogeissus latifolia for dermal wounds in rats. Acta Pharm. 2004, 54, 331–338. [Google Scholar]
- Shi, G.-X.; Shao, J.; Wang, T.-M.; Wang, C.-Z. New advance in studies on antimicrobal activity of Scutellaria baicalensis and its effective ingredients. China J. Chin. Mater. Med. 2014, 24, 3713–3718. [Google Scholar]
- Batawila, K.; Kokou, K.; Koumaglo, K.; Gbéassor, M.; De Foucault, B.; Bouchet, P.; Akpagana, K. Antifungal activities of five Combretaceae used in Togolese traditional medicine. Fitoterapia 2005, 76, 264–268. [Google Scholar] [CrossRef]
- Issah, A.; Azeez, I.; Boyejo, A.; Owolabi, S.; Buhari, O.; Ikeola, M. Antibacterial Activities of Some Commonly Used Medicinal Plants against Bacteria Isolates. Am. J. Med. Biol. Res. 2020, 8, 1–11. [Google Scholar]
- Baba-Moussa, F.; Akpagana, K.; Bouchet, P. Antifungal activities of seven West African Combretaceae used in traditional medicine. J. Ethnopharmacol. 1999, 66, 335–338. [Google Scholar] [CrossRef]
- Tona, L.; Kambu, K.; Ngimbi, N.; Cimanga, K.; Vlietinck, A. Antiamoebic and phytochemical screening of some Congolese medicinal plants. J. Ethnopharmacol. 1998, 61, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Motto, A.E.; Lawson-Evi, P.; Eklu-Gadegbeku, K. Antidiabetic and antioxidant potential of total extract and supernatant fraction of the roots of Anogeissus leiocarpus in HFD-fed and Streptozocin-induced diabetic rats. Biomed. Pharmacother. 2022, 154, 113578. [Google Scholar] [CrossRef] [PubMed]
- Kodama, D.H.; Gonçalves, A.E.D.S.S.; Lajolo, F.M.; Genovese, M.I. Flavonoids, total phenolics and antioxidant capacity: Comparison between commercial green tea preparations. Food Sci. Technol. 2010, 30, 1077–1082. [Google Scholar] [CrossRef]
- Zadra, M.; Piana, M.; de Brum, T.F.; Boligon, A.A.; de Freitas, R.B.; Machado, M.M.; Stefanello, S.T.; Soares, F.A.A.; Athayde, M.L. Antioxidant activity and phytochemical composition of the leaves of Solanum guaraniticum A. St.-Hil. Molecules 2012, 17, 12560–12574. [Google Scholar] [CrossRef]
- Govindarajan, R.; Vijayakumar, M.; Rao, C.V.; Shirwaikar, A.; Rawat, A.K.S.; Mehrotra, S.; Pushpangadan, P. Antioxidant potential of Anogeissus latifolia. Biol. Pharm. Bull. 2004, 27, 1266–1269. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oboh, G.; Omojokun, O.S.; Jimoh, T.O.; Oyeleye, S.I. In vitro antioxidant activities of African birch (Anogeissus leiocarpus) leaf and its effect on the α-amylase and α-glucosidase inhibitory properties of acarbose. J. Taibah Univ. Med. Sci. 2016, 11, 236–242. [Google Scholar] [CrossRef]
- Hassana, L.E.A.; Al-Suadea, F.; Fadul, S.M.; Majida, A. Evaluation of antioxidant, antiangiogenic and antitumor properties of Anogeissus leiocarpus against colon cancer. Angiotherapy 2018, 1, 56–66. [Google Scholar] [CrossRef]
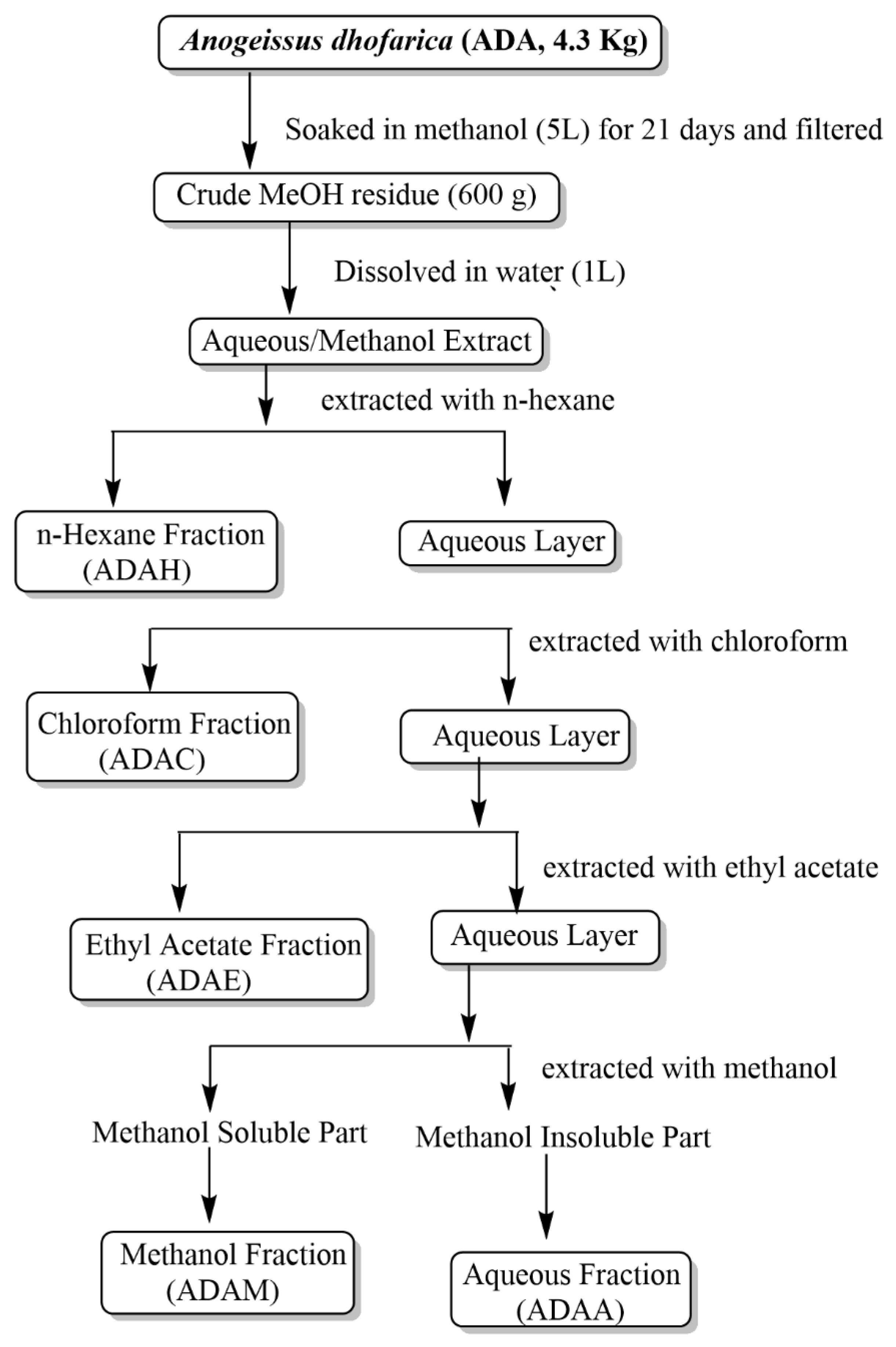
| Fractions | Obtained Mass (g) | Yield (%) |
|---|---|---|
| ADAH | 31.34 | 6.26 |
| ADAC | 24.95 | 4.99 |
| ADAE | 29.42 | 5.88 |
| ADAA | 65.32 | 13.06 |
| Fractions | Phenols | Flavonoids | Carbohydrates | Tannins | Alkaloids | Saponins | Glycosides |
|---|---|---|---|---|---|---|---|
| ADAC | + | + | + | + | – | + | – |
| ADAH | + | – | – | – | – | – | – |
| ADAE | + | + | + | + | – | + | + |
| ADAM | + | + | + | + | – | + | – |
| ADAA | – | – | + | + | – | – | + |
| Extracts | Total Flavonoid Contents (mg QE/g) of Dry Fractions Mean ± SEM | Total Phenolic Contents (mg GAE/g) of Dry Fractions Mean ± SEM |
|---|---|---|
| ADAH | 34.4 ± 0.4 | 83.14 ± 0.5 |
| ADAE | 80.2 ± 0.1 | 420.4 ± 1.9 |
| ADAC | 56.2 ± 0.3 | 148.2 ± 0.5 |
| ADAM | 94.1 ± 0.3 | 299.9 ± 0.5 |
| ADAA | 69.6 ± 0.2 | 210.8 ± 1.2 |
| S. No | Name | Molecular Formula | M-H Calculated | M-H Observed | Reference Species | Classification |
|---|---|---|---|---|---|---|
| 1 | Gallic acid | C7H12O6 | 169.0137 | 169.0115 | A. latifolia [48] | Phenolic acid |
| 2 | Methyl gallate | C8H8O5 | 183.0262 | 183.0293 | A. latifolia [48] | Phenolic acid |
| 3 | Quinic acid | C7H12O6 | 191.0555 | 191.0510 | A. leiocarpus [49] | Aliphatic carboxylic acid |
| 4 | Conocarpan | C18H8O2 | 265.1225 | 265.1387 | A. acuminate [50] | Lignan |
| 5 | Anolignan B | C18H8O2 | 265.1225 | 265.1387 | A. acuminate [50] | Lignan |
| 6 | 4,4’-(3,4-dimethyltetrahydrofuran-2,5-diyl) diphenol | C18H20O3 | 283.1334 | 283.0493 | A. Acuminate [50] | Lignan |
| 7 | Ellagic acid | C14H6O8 | 300.9984 | 300.9893 | A. letifolia [48] | Flavanone |
| 8 | Quercetin | 301.0348 | 301.0241 | A. letifolia [48] | Flavonol | |
| 9 | 5-O-Galloylshikimic acid | C14H14O9 | 325.0559 | 325.1695 | A. letifolia [55] | Phenolic acid |
| 10 | 2,3-Di-O-methylellagic acid | C16H10O8 | 329.0297 | 329.0193 | A. letifolia [48] | Flavanone |
| 11 | 3,4,3′-Tri-O-methylellagic acid | C17H12O8 | 343.0453 | 343.1137 | A. schimperi [51] | Flavanone |
| 12 | Chebulic acid | C14H12O11 | 355.0301 | 355.3078 | A. leiocarpus [56] | Phenolic acid |
| 13 | 3,4,3′-Tri-O-methylflavellagic acid | C17H12O9 | 359.0403 | 359.0235 | A. Letifolia [48] | Flavanone |
| 14 | Ellagic acid pentoside | C19H14O12 | 433.0407 | 433.2132 | A. leiocarpus [57] | Flavanone |
| 15 | 3-Methoxy-4-hydroxyphenol-1-O-𝛽-D-(6′-O-galloyl)-glucoside | C20H22O12 | 453.1039 | 453.0877 | A. leiocarpus [58] | Gallotannin |
| 16 | (+)-gallocatechin-3-O-gallate | C22H18O11 | 457.0776 | 457.3076 | A. pendula [52] | Catechin allates |
| 17 | Digalloylglucose | C20H20O14 | 483.0774 | 483.0627 | A. leiocarpus [58] | Gallotannin |
| 18 | Digalloxyglucose | C20H24O14 | 487.1087 | 487.3117 | A. leiocarpus [58] | Gallotannin |
| 19 | Resveratrol-4’-O-(6″-galloyl) glucoside | C27H25O12 | 541.1346 | 541.0059 | A. latifolia [59] | Gallotannin |
| 20 | Corillagin | C27H22O18 | 633.0727 | 633.0506 | A. latifolia [59] | Ellagitannin |
| 21 | Bis-HHDP glucose | C34H23O22 | 783.0681 | 783.3675 | A. pendula [52] | Ellagitannin |
| 22 | Castalagin | C41H26O26 | 933.0634 | 933.0250 | A. acuminate [60] | Ellagitannin glycoside |
| 23 | Punicalagin | C48H28O30 | 1083.0590 | 1083.0131 | A. leiocarpus [58] | Ellagitannin |
| Extracts | ZOI (mm ± SEM). | ||
|---|---|---|---|
| Sample Codes | Bacteria | Fungi | |
| S. aureus | C. albicans | C. kruzei | |
| ADAH | 17 ± 0.3 | NA | NA |
| ADAE | 28 ± 0.4 | 14 ± 0.5 | 26 ± 0.1 |
| ADAC | 16 ± 0.1 | NA | NA |
| ADAM | 26 ± 0.2 | 16 ± 0.2 | 30 ± 0.2 |
| ADAA | 26 ± 0.3 | 18 ± 0.2 | 34 ± 0.1 |
| DMSO (Blank) | NA | NA | NA |
| Standards | 31.6 ± 0.3 | 16.1 ± 0.1 | 20.5 ± 0.4 |
| Extracts | MIC (mg/mL) | ||
|---|---|---|---|
| Sample Codes | Bacteria | Fungi | |
| S. aureus | C. albicans | C. kruzei | |
| ADAH | 3.12 ± 0.5 | - | - |
| ADAE | 0.78 ± 0.3 | 50 ± 0.5 | 3.12 ± 0.3 |
| ADAC | 3.12 ± 0.2 | - | - |
| ADAM | 1.56 ± 0.1 | 25 ± 0.1 | 3.12 ± 0.5 |
| ADAA | 1.56 ± 0.4 | 25 ± 0.1 | 0.78 ± 0.2 |
| Extracts | Conc. (μg/mL) | % Inhibition | IC50 Values (μg/mL) ± SEM |
|---|---|---|---|
| ADAH | 1000 | 92.72 | |
| 500 | 87.15 | ||
| 250 | 76.32 | 37.8 ± 0.2 | |
| 125 | 66.17 | ||
| 62.5 | 55.15 | ||
| ADAC | 1000 | 88.92 | |
| 500 | 83.75 | ||
| 250 | 69.811 | 104.9 ± 0.3 | |
| 125 | 54.17 | ||
| 62.5 | 42.97 | ||
| ADAE | 1000 | 96.2 | |
| 500 | 93.95 | ||
| 250 | 87.75 | 9.8 ± 1.2 | |
| 125 | 79.24 | ||
| 62.5 | 70.60 | ||
| ADAA | 1000 | 95.12 | |
| 500 | 91.825 | ||
| 250 | 85.75 | 25.8 ± 0.1 | |
| 125 | 74.454 | ||
| 62.5 | 65.80 | ||
| ADAM | 1000 | 97.54 | |
| 500 | 93.32 | ||
| 250 | 86.45 | 17.4 ± 0.4 | |
| 125 | 76.60 | ||
| 62.5 | 67.60 | ||
| 1000 | 96.12 | ||
| 500 | 94.02 | ||
| Ascorbic Acid | 250 | 89.61 | 13.8 ± 0.4 |
| (Standard) | 125 | 80.14 | |
| 62.5 | 68.60 |
| Extracts | % Inhibition | IC50 ± μg/mL (SEM) |
|---|---|---|
| ADAH | 94.30 | 10.1 ± 0.31 |
| ADAE | 94.86 | 6.4 ± 0.12 |
| ADAC | 91.21 | 20.1 ± 0.42 |
| ADAM | 93.60 | 2.1 ± 0.05 |
| ADAA | 93.37 | 2.4 ± 0.10 |
| Acarbose | 64.23 | 377.0 ± 1.06 |
| Extracts | Conc. (mg/mL) | % Viability | % Inhibition | IC50 Values (mg/mL) |
|---|---|---|---|---|
| ADAM | 1.25 | 98.57 | 1.42 | 8.2 ± 0.4 |
| 2.5 | 92.94 | 7.05 | ||
| 5 | 67.69 | 32.30 | ||
| 10 | 39.53 | 60.46 | ||
| ADAA | 1.25 | 103.99 | −3.99 | NA |
| 2.5 | 110.7 | −10.77 | ||
| 5 | 107.85 | −7.85 | ||
| 10 | 89.91 | 10.08 | ||
| ADAE | 1.25 | 96.17 | 3.82 | 6.2 ± 0.3 |
| 2.5 | 83.54 | 16.44 | ||
| 5 | 59.24 | 40.75 | ||
| 10 | 30.87 | 69.12 | ||
| ADAH | 1.25 | 97.53 | 2.46 | 8.8 ± 0.6 |
| 2.5 | 87.72 | 12.27 | ||
| 5 | 66.13 | 33.86 | ||
| 10 | 45.27 | 54.72 | ||
| ADAC | 1.25 | 96.90 | 3.09 | 7.2 ± 0.4 |
| 2.5 | 86.99 | 13.00 | ||
| 5 | 79.69 | 20.30 | ||
| 10 | 37.55 | 62.44 |
| Extracts | Conc. (mg/mL) | % Viability | % Inhibition |
|---|---|---|---|
| ADAM | 1.25 | 96.15 | 3.84 |
| 2.5 | 93.66 | 6.33 | |
| 5 | 82.46 | 17.53 | |
| 10 | 80.57 | 19.42 | |
| ADAA | 1.25 | 93.18 | 6.81 |
| 2.5 | 94.64 | 5.35 | |
| 5 | 89.50 | 10.49 | |
| 10 | 80.79 | 19.20 | |
| ADAE | 1.25 | 97.67 | 2.32 |
| 2.5 | 94.20 | 5.79 | |
| 5 | 85.55 | 14.44 | |
| 10 | 78.46 | 21.53 | |
| ADAH | 1.25 | 94.64 | 5.35 |
| 2.5 | 95.29 | 4.70 | |
| 5 | 89.12 | 10.87 | |
| 10 | 84.95 | 15.04 | |
| ADAC | 1.25 | 95.50 | 4.49 |
| 2.5 | 91.88 | 8.11 | |
| 5 | 86.79 | 13.20 | |
| 10 | 79.38 | 20.61 |
| Mean % Mortality of Brine Shrimp Larvae (nauplii) | ||||
|---|---|---|---|---|
| Concentrations | 10 | 100 | 250 | 500 |
| ADAH | 0 | 10 ± 0.09 | 20 ± 0.3 | 30 ± 0.3 |
| ADAE | 10 ± 0.1 | 30 ± 0.1 | 50 ± 0.5 | 70 ± 0.2 |
| ADAC | 0 | 20 ± 0.1 | 30 ± 0.2 | 40 ± 0.5 |
| ADAM | 10 ± 0.2 | 20 ± 0.2 | 40 ± 0.5 | 60 ± 0.1 |
| ADAA | 10 ± 0.07 | 20 ± 0.4 | 30 ± 0.3 | 50 ± 0.6 |
| KMNO4 (Positive control) | 30 ± 0.4 | 40 ± 0.3 | 60 ± 0.7 | 80 ± 0.5 |
| DMSO (Negative control) | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsood, R.; Khan, F.; Ullah, S.; Khan, A.; Al-Jahdhami, H.; Hussain, J.; M. Weli, A.; Maqsood, D.; Rahman, S.M.; Hussain, A.; et al. Evaluation of Antiproliferative, Antimicrobial, Antioxidant, Antidiabetic and Phytochemical Analysis of Anogeissus dhofarica A. J. Scott. Antibiotics 2023, 12, 354. https://doi.org/10.3390/antibiotics12020354
Maqsood R, Khan F, Ullah S, Khan A, Al-Jahdhami H, Hussain J, M. Weli A, Maqsood D, Rahman SM, Hussain A, et al. Evaluation of Antiproliferative, Antimicrobial, Antioxidant, Antidiabetic and Phytochemical Analysis of Anogeissus dhofarica A. J. Scott. Antibiotics. 2023; 12(2):354. https://doi.org/10.3390/antibiotics12020354
Chicago/Turabian StyleMaqsood, Rabia, Faizullah Khan, Saeed Ullah, Ajmal Khan, Habib Al-Jahdhami, Javid Hussain, Afaf M. Weli, Danial Maqsood, Shaikh Mizanoor Rahman, Amjad Hussain, and et al. 2023. "Evaluation of Antiproliferative, Antimicrobial, Antioxidant, Antidiabetic and Phytochemical Analysis of Anogeissus dhofarica A. J. Scott" Antibiotics 12, no. 2: 354. https://doi.org/10.3390/antibiotics12020354
APA StyleMaqsood, R., Khan, F., Ullah, S., Khan, A., Al-Jahdhami, H., Hussain, J., M. Weli, A., Maqsood, D., Rahman, S. M., Hussain, A., Rehman, N. U., & Al-Harrasi, A. (2023). Evaluation of Antiproliferative, Antimicrobial, Antioxidant, Antidiabetic and Phytochemical Analysis of Anogeissus dhofarica A. J. Scott. Antibiotics, 12(2), 354. https://doi.org/10.3390/antibiotics12020354










