Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture
Abstract
1. Introduction
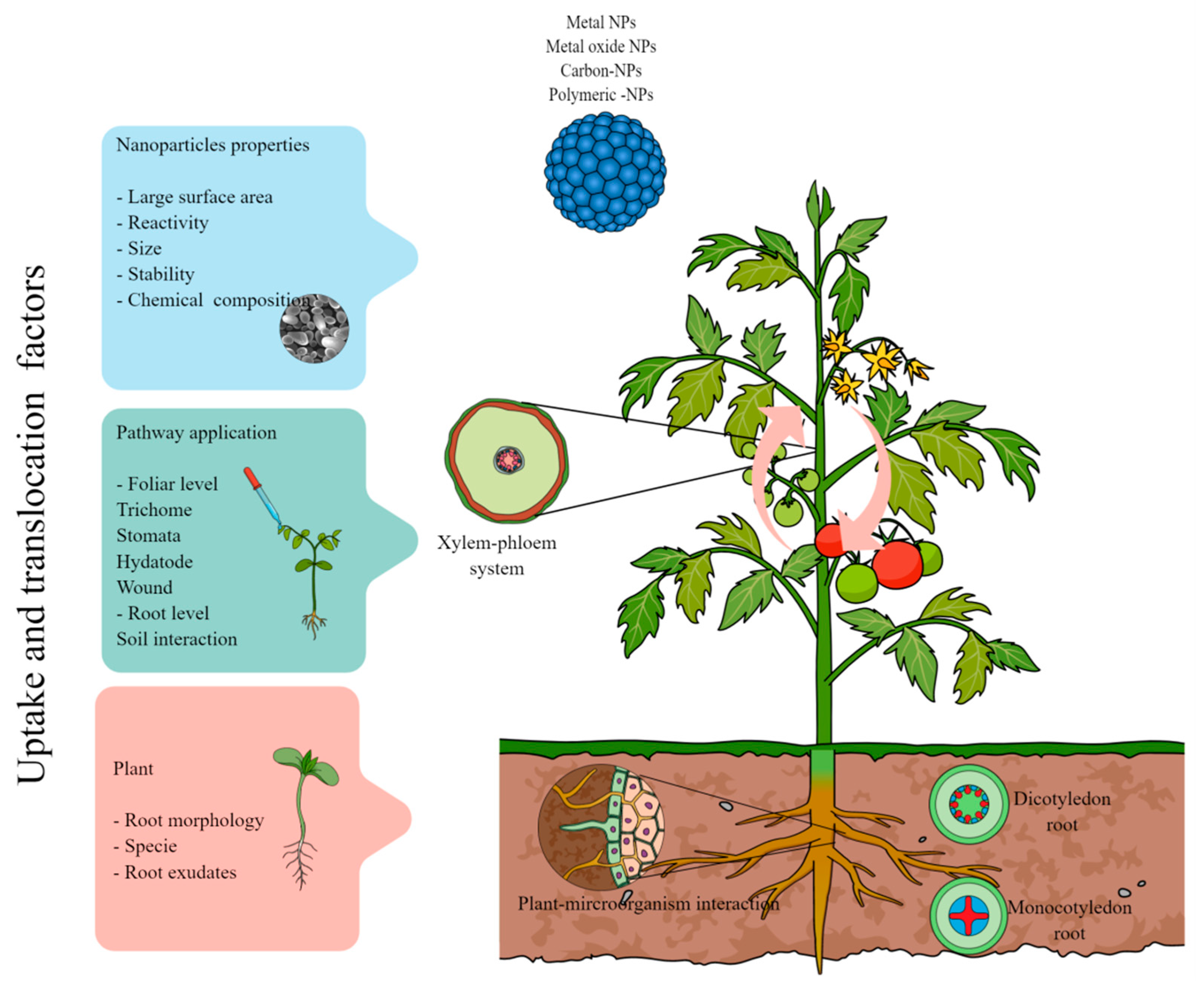
2. Uptake and Translocation of Nanoparticles in Plants
3. Potential Adverse Effects of Nanoparticles on Plants
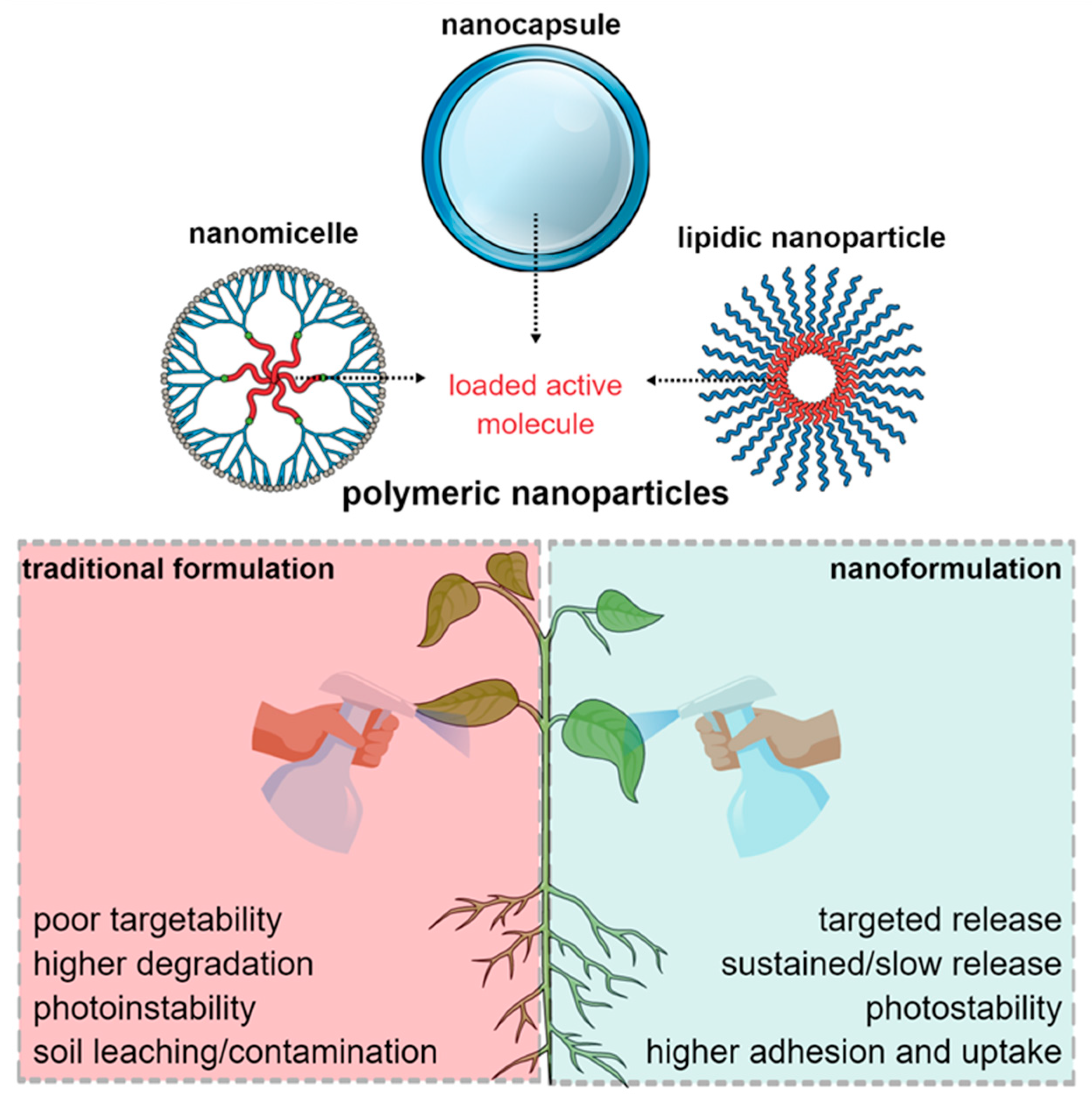
4. Potential Use of Polymeric Nanoparticles
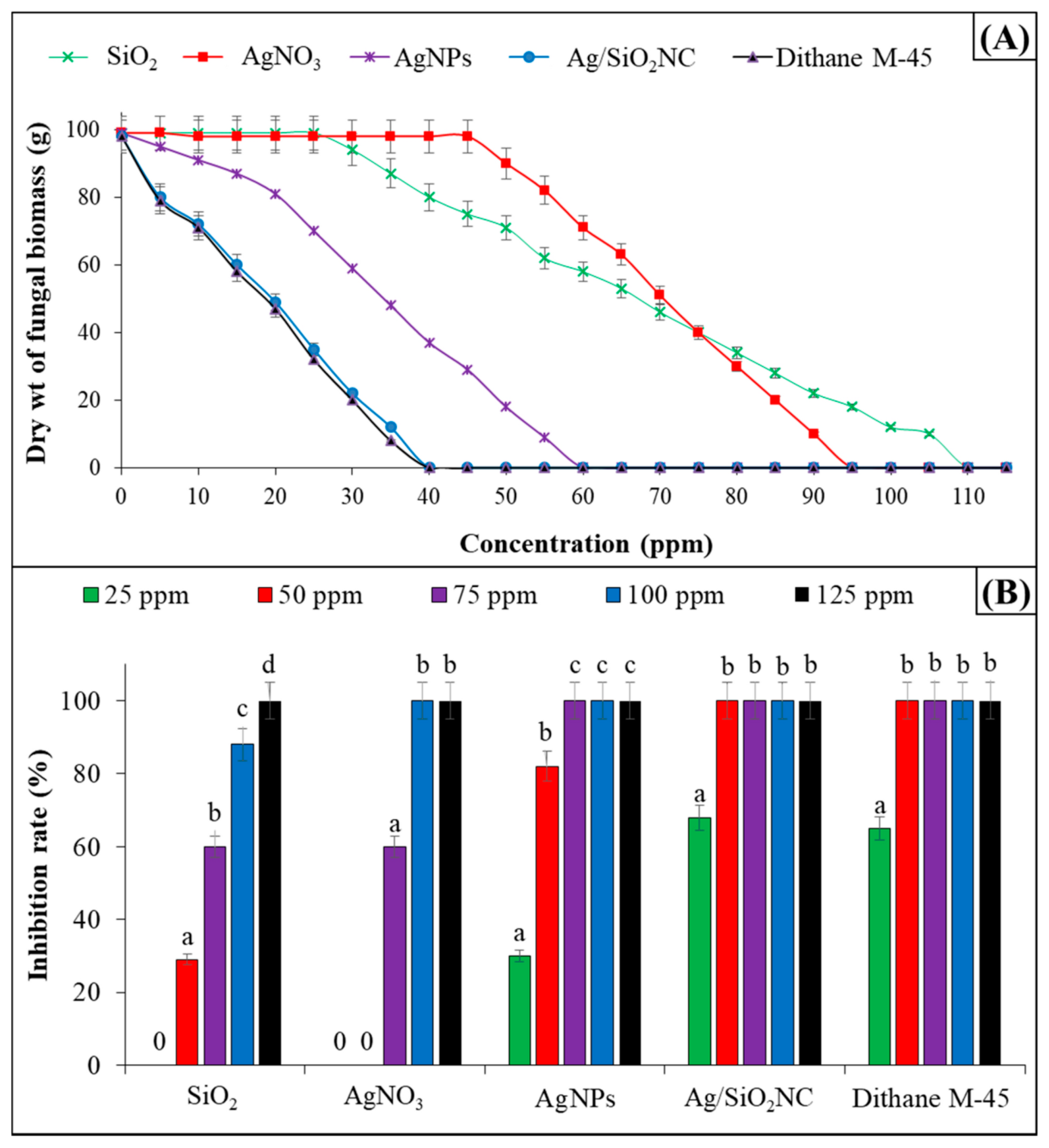
5. Potential Uses of Metal and Metal Oxide Nanoparticles
6. Other Nanoparticles
7. Conclusions, Challenges and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Varah, A.; Ahodo, K.; Coutts, S.R.; Hicks, H.L.; Comont, D.; Crook, L.; Hull, R.; Neve, P.; Childs, D.Z.; Freckleton, R.P.; et al. The costs of human-induced evolution in an agricultural system. Nat. Sustain. 2020, 3, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cai, X. Climate change impacts on global agricultural land availability. Environ. Res. Lett. 2011, 6, 014014. [Google Scholar] [CrossRef]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry Under a Changing Climate. Front. Plant Sci. 2020, 11, 601009. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, H.; Hwang, H.-M. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Guzmán, M.G.; Cellini, F.; Fotopoulos, V.; Balestrini, R.; Arbona, V. New approaches to improve crop tolerance to biotic and abiotic stresses. Physiol. Plant. 2022, 174, e13547. [Google Scholar] [CrossRef]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; Von Der Kammer, F.; Praetorius, A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef]
- Wohlleben, W.; Mielke, J.; Bianchin, A.; Ghanem, A.; Freiberger, H.; Rauscher, H.; Gemeinert, M.; Hodoroaba, V.-D. Reliable nanomaterial classification of powders using the volume-specific surface area method. J. Nanopart. Res. 2017, 19, 61. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and Toxicity: The Magnitude of the Impact of Nanomaterials in Microorganisms and Plants. J. Adv. Res. 2021, 31, 113–126. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- El-Kady, M.M.; Ansari, I.; Arora, C.; Rai, N.; Soni, S.; Verma, D.K.; Singh, P.; Mahmoud, A.E.D. Nanomaterials: A Comprehensive Review of Applications, Toxicity, Impact, and Fate to Environment. J. Mol. Liq. 2023, 370, 121046. [Google Scholar] [CrossRef]
- Pokropivny, V.; Skorokhod, V. Classification of nanostructures by dimensionality and concept of surface forms engineering in nanomaterial science. Mater. Sci. Eng. C 2007, 27, 990–993. [Google Scholar] [CrossRef]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of Silica Nanoparticles in Abiotic and Biotic Stress Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef]
- Kohatsu, M.Y.; Lange, C.N.; Pelegrino, M.T.; Pieretti, J.C.; Tortella, G.; Rubilar, O.; Batista, B.L.; Seabra, A.B.; de Jesus, T.A. Foliar spraying of biogenic CuO nanoparticles protects the defence system and photosynthetic pigments of lettuce (Lactuca sativa). J. Clean. Prod. 2021, 324, 129264. [Google Scholar] [CrossRef]
- Liu, J.; Qi, W.-Y.; Chen, H.; Song, C.; Li, Q.; Wang, S.-G. Selenium Nanoparticles as an Innovative Selenium Fertilizer Exert Less Disturbance to Soil Microorganisms. Front. Microbiol. 2021, 12, 746046. [Google Scholar] [CrossRef]
- Ghani, M.I.; Saleem, S.; Rather, S.A.; Rehmani, M.S.; Alamri, S.; Rajput, V.D.; Kalaji, H.M.; Saleem, N.; Sial, T.A.; Liu, M. Foliar application of zinc oxide nanoparticles: An effective strategy to mitigate drought stress in cucumber seedling by modulating antioxidant defense system and osmolytes accumulation. Chemosphere 2022, 289, 133202. [Google Scholar] [CrossRef]
- Tripathi, D.; Singh, M.; Pandey-Rai, S. Crosstalk of nanoparticles and phytohormones regulate plant growth and metabolism under abiotic and biotic stress. Plant Stress 2022, 6, 100107. [Google Scholar] [CrossRef]
- García-Ovando, A.E.; Piña, J.E.R.; Naranjo, E.U.E.; Chávez, J.A.C.; Esquivel, K. Biosynthesized nanoparticles and implications by their use in crops: Effects over physiology, action mechanisms, plant stress responses and toxicity. Plant Stress 2022, 6, 100109. [Google Scholar] [CrossRef]
- Oliveira, H.C.; Gomes, B.C.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef]
- Gomes, D.G.; Debiasi, T.V.; Pelegrino, M.T.; Pereira, R.M.; Ondrasek, G.; Batista, B.L.; Seabra, A.B.; Oliveira, H.C. Soil Treatment with Nitric Oxide-Releasing Chitosan Nanoparticles Protects the Root System and Promotes the Growth of Soybean Plants under Copper Stress. Plants 2022, 11, 3245. [Google Scholar] [CrossRef]
- Longano, D.; Ditaranto, N.; Sabbatini, L.; Torsi, L.; Cioffi, N. Synthesis and Antimicrobial Activity of Copper Nanomaterials. Nano Antimicrob. 2011, 26, 85–117. [Google Scholar] [CrossRef]
- Bruna, T.; Maldonado-Bravo, F.; Jara, P.; Caro, N. Silver Nanoparticles and Their Antibacterial Applications. Int. J. Mol. Sci. 2021, 22, 7202. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. Funct. Chitosan 2020, 6, 457–489. [Google Scholar]
- Singh, A.; Kumar, H.; Kumar, S.; Dutta, P. Chapter 15—Role of chitosan and chitosan-based nanoparticles in antioxidant regulation of plants. In Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences; Kumar, S., Madihally, S.V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 321–341. ISBN 9780323853910. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Soroush, F.; Varma, R.S. Nano/microencapsulation of plant biocontrol agents by chitosan, alginate, and other important biopolymers as a novel strategy for alleviating plant biotic stresses. Int. J. Biol. Macromol. 2022, 222, 1589–1604. [Google Scholar] [CrossRef]
- Sobral, M.C.; Martins, I.M.; Sobral, A.J. Chapter 13—Role of chitosan and chitosan-based nanoparticles against heavy metal stress in plants. In Role of Chitosan and Chitosan-Based Nanomaterials in Plant Sciences; Kumar, S., Madihally, S.V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 273–296. ISBN 9780323853910. [Google Scholar] [CrossRef]
- Kumari, M.; Pandey, S.; Mishra, S.K.; Giri, V.P.; Agarwal, L.; Dwivedi, S.; Pandey, A.K.; Nautiyal, C.S.; Mishra, A. Omics-Based Mechanistic Insight into the Role of Bioengineered Nanoparticles for Biotic Stress Amelioration by Modulating Plant Metabolic Pathways. Front. Bioeng. Biotechnol. 2020, 8, 242. [Google Scholar] [CrossRef]
- Rahman Khan, M.; Adam, V.; Fatima Rizvi, T.; Zhang, B.; Ahamad, F.; Jośko, I.; Zhu, Y.; Yang, M.; Mao, C. Nanoparticle-plant interactions: A two-way traffic. Small 2019, 15, e1901794. [Google Scholar] [CrossRef]
- Lv, J.; Christie, P.; Zhang, S. Uptake, translocation, and transformation of metal-based nanoparticles in plants: Recent advances and methodological challenges. Environ. Sci. Nano 2019, 6, 41–59. [Google Scholar] [CrossRef]
- Azim, Z.; Singh, N.B.; Singh, A.; Amist, N.; Niharika; Khare, S.; Yadav, R.K.; Bano, C.; Yadav, V. A review summarizing uptake, translocation and accumulation of nanoparticles within the plants: Current status and future prospectus. J. Plant Biochem. Biotechnol. 2022. [Google Scholar] [CrossRef]
- Fincheira, P.; Tortella, G.; Duran, N.; Seabra, A.B.; Rubilar, O. Current applications of nanotechnology to develop plant growth inducer agents as an innovation strategy. Crit. Rev. Biotechnol. 2020, 40, 15–30. [Google Scholar] [CrossRef]
- Ali, S.; Mehmood, A.; Khan, N. Uptake, Translocation, and Consequences of Nanomaterials on Plant Growth and Stress Adaptation. J. Nanomater. 2021, 2021, 6677616. [Google Scholar] [CrossRef]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, S.; Jiang, C.; Wu, C.; Gao, M.; Wang, Q. A review of root exudates and rhizosphere microbiome for crop production. Environ. Sci. Pollut. Res. 2021, 28, 54497–54510. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Huang, X.; Keller, A.A. Dissolution and Aggregation of Metal Oxide Nanoparticles in Root Exudates and Soil Leachate: Implications for Nanoagrochemical Application. Environ. Sci. Technol. 2021, 55, 13443–13451. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Khan, I.; Afzal Awan, S.; Rizwan, M.; Hassan, Z.U.; Adnan Akram, M.; Tariq, R.; Brestic, M.; Xie, W. Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: A review. Environ. Sci. Pollut. Res. 2022, 29, 89823–89833. [Google Scholar] [CrossRef]
- Pérez-De-Luque, A. Interaction of Nanomaterials with Plants: What Do We Need for Real Applications in Agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Domínguez, E.; A Heredia-Guerrero, J.; Heredia, A. The plant cuticle: Olds challenges, new perspectives. J. Exp. Bot. 2017, 68, 5251–5255. [Google Scholar] [CrossRef]
- Pillitteri, L.J.; Torii, K.U. Mechanisms of Stomatal Development. Annu. Rev. Plant Biol. 2012, 63, 591–614. [Google Scholar] [CrossRef]
- Essa, H.L.; Abdelfattah, M.S.; Marzouk, A.S.; Shedeed, Z.; Guirguis, H.A.; El-Sayed, M.M.H. Biogenic copper nanoparticles from Avicennia marina leaves: Impact on seed germination, detoxification enzymes, chlorophyll content and uptake by wheat seedlings. PLoS ONE 2021, 16, e0249764. [Google Scholar] [CrossRef]
- Lopez-Lima, D.; Mtz-Enriquez, A.I.; Carrión, G.; Basurto-Cereceda, S.; Pariona, N. The bifunctional role of copper nanoparticles in tomato: Effective treatment for Fusarium wilt and plant growth promoter. Sci. Hortic. 2021, 277, 109810. [Google Scholar] [CrossRef]
- Ranjan, A.; Rajput, V.D.; Minkina, T.; Bauer, T.; Chauhan, A.; Jindal, T. Nanoparticles induced stress and toxicity in plants. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100457. [Google Scholar] [CrossRef]
- Da Costa, M.V.J.; Sharma, P.K. Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 2016, 54, 110–119. [Google Scholar] [CrossRef]
- Margenot, A.J.; Rippner, D.A.; Dumlao, M.R.; Nezami, S.; Green, P.G.; Parikh, S.J.; McElrone, A.J. Copper oxide nanoparticle effects on root growth and hydraulic conductivity of two vegetable crops. Plant Soil 2018, 431, 333–345. [Google Scholar] [CrossRef]
- Balážová, Ľ.; Babula, P.; Baláž, M.; Bačkorová, M.; Bujňáková, Z.; Briančin, J.; Kurmanbayeva, A.; Sagi, M. Zinc oxide nanoparticles phytotoxicity on halophyte from genus Salicornia. Plant Physiol. Biochem. 2018, 130, 30–42. [Google Scholar] [CrossRef]
- Pavani, K.V.; Poojitha, G.S.; Beulah, M. Phytotoxicity of Zinc-Oxide Nanoparticles on Seedling Growth and Antioxidant Activity of Red Gram (Cajanus cajan L.) Seed. Jordan J. Biol. Sci. 2020, 13, 153–158. [Google Scholar]
- García-Gómez, C.; Obrador, A.; González, D.; Babín, M.; Fernández, M.D. Comparative study of the phytotoxicity of ZnO nanoparticles and Zn accumulation in nine crops grown in a calcareous soil and an acidic soil. Sci. Total Environ. 2018, 644, 770–780. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Biba, R.; Peharec Štefanić, P.; Cvjetko, P.; Tkalec, M.; Balen, B. Silver nanoparticles phytotoxicity mechanisms. In Silver Nanomaterials for Agri-Food Applications; Kamel, A.A.-E., Ed.; Elsevier: Amsterdam, Netherlands, 2021; pp. 317–356. ISBN 9780128235287. [Google Scholar]
- Hasanzadeh, A.; Gholipour, B.; Rostamnia, S.; Eftekhari, A.; Tanomand, A.; Valizadeh, K.A.; Khaksar, S.; Khalilov, R. Biosynthesis of AgNPs onto the urea-based periodic mesoporous organosilica (AgxNPs/Ur-PMO) for antibacterial and cell viability assay. J. Colloid Interface Sci. 2020, 585, 676–683. [Google Scholar] [CrossRef]
- Baran, A.; Fırat Baran, M.; Keskin, C.; Hatipoğlu, A.; Yavuz, Ö.; İrtegün Kandemir, S.; Adican, M.T.; Khalilov, R.; Mammadova, A.; Ahmadian, E.; et al. Investigation of Antimicrobial and Cytotoxic Properties and Specification of Silver Nanoparticles (AgNPs) Derived from Cicer arietinum L. Green Leaf Extract. Front. Bioeng. Biotechnol. 2022, 10, 855136. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Shweta; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.; Tripathi, D.K.; Sharma, S. Differential Phytotoxic Impact of Plant Mediated Silver Nanoparticles (AgNPs) and Silver Nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Matras, E.; Gorczyca, A.; Pociecha, E.; Przemieniecki, S.W.; Oćwieja, M. Phytotoxicity of Silver Nanoparticles with Different Surface Properties on Monocots and Dicots Model Plants. J. Soil Sci. Plant Nutr. 2022, 22, 1647–1664. [Google Scholar] [CrossRef]
- Asli, S.; Neumann, P.M. Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant Cell Environ. 2009, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wu, X.; Wu, F.; Chen, W.; White, J.C.; Yang, Y.; Wang, B.; Xing, B.; Tao, S.; Wang, X. Potential application of titanium dioxide nanoparticles to improve the nutritional quality of coriander (Coriandrum sativum L.). J. Hazard. Mater. 2020, 389, 121837. [Google Scholar] [CrossRef]
- Chahardoli, A.; Sharifan, H.; Karimi, N.; Kakavand, S.N. Uptake, translocation, phytotoxicity, and hormetic effects of titanium dioxide nanoparticles (TiO2NPs) in Nigella arvensis L. Sci. Total Environ. 2021, 806, 151222. [Google Scholar] [CrossRef]
- Pascoli, M.; Lopes-Oliveira, P.J.; Fraceto, L.F.; Seabra, A.B.; Oliveira, H.C. State of the art of polymeric nanoparticles as carrier systems with agricultural applications: A minireview. Energy Ecol. Environ. 2018, 3, 137–148. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Bogdanchikova, N.; Garibo, D. Antibacterial and antifungal studies of biosynthesized silver nanoparticles against plant parasitic nematode Meloidogyne incognita, plant pathogens Ralstonia solanacearum and Fusarium oxysporum. Molecules 2021, 26, 2462. [Google Scholar] [CrossRef]
- Shakiba, S.; Astete, C.E.; Paudel, S.; Sabliov, C.M.; Rodrigues, D.F.; Louie, S.M. Emerging investigator series: Polymeric nanocarriers for agricultural applications: Synthesis, characterization, and environmental and biological interactions. Environ. Sci. Nano 2020, 7, 37–67. [Google Scholar] [CrossRef]
- Mosaddik, A.; Ravinayagam, V.; Elaanthikkal, S.; Fessi, H.; Badri, W.; Elaissari, A. Development and Use of Polymeric Nanoparticles for the Encapsulation and Administration of Plant Extracts. In Natural Products as Source of Molecules with Therapeutic Potential; Cechinel Filho, V., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Singh, S. Enhancing phytochemical levels, enzymatic and antioxidant activity of spinach leaves by chitosan treatment and an insight into the metabolic pathway using DART-MS technique. Food Chem. 2016, 199, 176–184. [Google Scholar] [CrossRef]
- Ghasemnezhad, M.; Nezhad, M.A.; Gerailoo, S. Changes in postharvest quality of loquat (Eriobotrya japonica) fruits influenced by chitosan. Hortic. Environ. Biotechnol. 2011, 52, 40–45. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Plant science review: Multiple effects of chitosan on plant systems: Solid science or hype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.-P.; Beaudoin, N. Chitooligosaccharide sensing and downstream signaling: Contrasted outcomes in pathogenic and beneficial plant-microbe interactions. Planta 2010, 232, 787–806. [Google Scholar] [CrossRef]
- Seabra, A.B.; Silveira, N.M.; Ribeiro, R.V.; Pieretti, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M.; Hancock, J.T.; Petřivalský, M.; Gupta, K.J.; et al. Nitric oxide-releasing nanomaterials: From basic research to potential biotechnological applications in agriculture. New Phytol. 2022, 234, 1119–1125. [Google Scholar] [CrossRef]
- Chun, S.-C.; Chandrasekaran, M. Chitosan and chitosan nanoparticles induced expression of pathogenesis-related proteins genes enhances biotic stress tolerance in tomato. Int. J. Biol. Macromol. 2019, 125, 948–954. [Google Scholar] [CrossRef]
- García-Rincón, J.; Vega-Pérez, J.; Guerra-Sánchez, M.G.; Hernández-Lauzardo, A.N.; Peña-Díaz, A.; Valle, V.D. Effect of chitosan on growth and plasma membrane properties of Rhizopus stolonifer (Ehrenb.:Fr.) Vuill. Pestic. Biochem. Physiol. 2010, 97, 275–278. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.F.; Liu, Y.; Yang, Z.; Wu, H.; Cai, Q. Effects of chitosan on control of postharvest blue mold decay of apple fruit and the possible mechanisms involved. Sci. Hortic. 2015, 186, 77–83. [Google Scholar] [CrossRef]
- Grillo, R.; Pereira, A.; Nishisaka, C.; Lima, R.; Oehlke, K.; Greiner, R.; Fraceto, L.F. Chitosan/tripolyphosphate nanoparticles loaded with paraquat herbicide: An environmentally safer alternative for weed control. J. Hazard. Mater. 2014, 278, 163–171. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Indhumathi, M. Chitosan thiamine nanoparticles intervene innate immunomodulation during Chickpea-Fusarium interaction. Int. J. Biol. Macromol. 2022, 198, 11–17. [Google Scholar] [CrossRef]
- Gechev, T.S.; Van Breusegem, F.; Stone, J.M.; Denev, I.; Laloi, C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 2006, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.M.; Omara, R.I.; Mostafa, Y.S.; Alamri, S.A.; Hashem, M.; Alrumman, S.A.; Ahmad, A.A. Mechanism of Wheat Leaf Rust Control Using Chitosan Nanoparticles and Salicylic Acid. J. Fungi 2022, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, V.; Gunasekaran, C.; Paul, C.A.; Dharmaraj, J. Development of encapsulated peppermint essential oil in chitosan nanoparticles: Characterization and biological efficacy against stored-grain pest control. Pestic. Biochem. Physiol. 2020, 170, 104679. [Google Scholar] [CrossRef] [PubMed]
- Preisler, A.C.; Pereira, A.E.; Campos, E.V.; Dalazen, G.; Fraceto, L.F.; Oliveira, H.C. Atrazine nanoencapsulation improves pre-emergence herbicidal activity against Bidens pilosa without enhancing long-term residual effect on Glycine max. Pest Manag. Sci. 2020, 76, 141–149. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Grillo, R.; Mello, N.F.S.; Rosa, A.H.; Fraceto, L.F. Application of poly (epsilon-caprolactone) nanoparticles containing atrazine herbicide as an alternative technique to control weeds and reduce damage to the environment. J. Hazard. Mater. 2014, 268, 207–215. [Google Scholar] [CrossRef]
- Vijayamma, R.; Maria, H.J.; Thomas, S.; Shishatskaya, E.I.; Kiselev, E.G.; Nemtsev, I.V.; Sukhanova, A.A.; Volova, T.G. A study of the properties and efficacy of microparticles based on P(3HB) and P(3HB/3HV) loaded with herbicides. J. Appl. Polym. Sci. 2022, 139, 51756. [Google Scholar] [CrossRef]
- Yan, S.; Gu, N.; Peng, M.; Jiang, Q.; Liu, E.; Li, Z.; Yin, M.; Shen, J.; Du, X.; Dong, M. A Preparation Method of Nano-Pesticide Improves the Selective Toxicity toward Natural Enemies. Nanomaterials 2022, 12, 2419. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Fang, X.; Yao, N.; Lei, H.; Yang, G.; Wang, Z.; Dong, Y.; Hua, Z. Rationally designing renewable plant oil-based polymers as efficient nanocarriers for sustained pesticide delivery. Chem. Eng. J. 2022, 450, 138294. [Google Scholar] [CrossRef]
- Artusio, F.; Casà, D.; Granetto, M.; Tosco, T.; Pisano, R. Alginate Nanohydrogels as a Biocompatible Platform for the Controlled Release of a Hydrophilic Herbicide. Processes 2021, 9, 1641. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Hasan, M.A.; Yadav, K.K.; Pinto, M.; Malik, N.; Yadav, V.K.; Khan, A.H.; Islam, S.; Sharma, G.K. Agro-Nanotechnology as an Emerging Field: A Novel Sustainable Approach for Improving Plant Growth by Reducing Biotic Stress. Appl. Sci. 2021, 11, 2282. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Missaoui, W.N.; Arnold, R.D.; Cummings, B.S. Toxicological status of nanoparticles: What we know and what we don’t know. Chem. Biol. Interact. 2018, 295, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Abdelfattah, N.A.H.; Salem, S.S.; Awad, M.F.; Fouda, A. Efficacy Assessment of Biosynthesized Copper Oxide Nanoparticles (CuO-NPs) on Stored Grain Insects and Their Impacts on Morphological and Physiological Traits of Wheat (Triticum aestivum L.) Plant. Biology 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Shahid, M.; Noman, M.; Niazi, M.B.K.; Mahmood, F.; Manzoor, I.; Zhang, Y.; Li, B.; Yang, Y.; Yan, C.; et al. Silver Nanoparticles Synthesized by Using Bacillus cereus SZT1 Ameliorated the Damage of Bacterial Leaf Blight Pathogen in Rice. Pathogens 2020, 9, 160. [Google Scholar] [CrossRef]
- Chen, J.; Wu, L.; Lu, M.; Lu, S.; Li, Z.; Ding, W. Comparative Study on the Fungicidal Activity of Metallic MgO Nanoparticles and Macroscale MgO against Soilborne Fungal Phytopathogens. Front. Microbiol. 2020, 11, 365. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Ikram, M.; Oraby, H.F.; Mashwani, Z.-U.; Mohamed, A.H.; Singh, A.; Omar, A.A. Plant-Based Titanium Dioxide Nanoparticles Trigger Biochemical and Proteome Modifications in Triticum aestivum L. under Biotic Stress of Puccinia striiformis. Molecules 2022, 27, 4274. [Google Scholar] [CrossRef]
- Farhana; Munis, M.F.H.; Alamer, K.H.; Althobaiti, A.T.; Kamal, A.; Liaquat, F.; Haroon, U.; Ahmed, J.; Chaudhary, H.J.; Attia, H. ZnO Nanoparticle-Mediated Seed Priming Induces Biochemical and Antioxidant Changes in Chickpea to Alleviate Fusarium Wilt. J. Fungi 2022, 8, 753. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO nanoparticles from a promising Trichoderma harzianum strain and their antifungal potential against important phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of Action and Probable Bio-Application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaka, M.; Abbasi, B.H.; Rahman, L.; Shah, A. Seed germination and biochemical profile of Silybum marianum exposed to monometallic and bimetallic alloy nanoparticles. IET Nanobiotechnol. 2016, 10, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jia, X.; Zhang, Z.; Chen, K.; Wang, L.; Chen, H.; Yang, Z.; Li, C.; Zhao, L. AgNPs seed priming accelerated germination speed and altered nutritional profile of Chinese cabbage. Sci. Total Environ. 2022, 808, 151896. [Google Scholar] [CrossRef] [PubMed]
- Kanhed, P.; Birla, S.; Gaikwad, S.; Gade, A.; Seabra, A.B.; Rubilar, O.; Duran, N.; Rai, M. In vitro antifungal efficacy of copper nanoparticles against selected crop pathogenic fungi. Mater. Lett. 2014, 115, 13–17. [Google Scholar] [CrossRef]
- Shende, S.S.; Gaikwad, N.D.; Bansod, S.D. Synthesis and evaluation of antimicrobial potential of copper nanoparticle against agriculturally important Phytopathogens. Int. J. Bio. Res. 2016, 4, 41–47. [Google Scholar]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar Application of Copper Nanoparticles Increases the Fruit Quality and the Content of Bioactive Compounds in Tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef]
- Irshad, M.A.; Nawaz, R.; Rehman, M.Z.U.; Imran, M.; Ahmad, J.; Ahmad, S.; Inam, A.; Razzaq, A.; Rizwan, M.; Ali, S. Synthesis and characterization of titanium dioxide nanoparticles by chemical and green methods and their antifungal activities against wheat rust. Chemosphere 2020, 258, 127352. [Google Scholar] [CrossRef]
- Satti, S.H.; Raja, N.I.; Javed, B.; Akram, A.; Mashwani, Z.-U.; Ahmad, M.S.; Ikram, M. Titanium dioxide nanoparticles elicited agro-morphological and physicochemical modifications in wheat plants to control Bipolaris sorokiniana. PLoS ONE 2021, 16, e0246880. [Google Scholar] [CrossRef]
- Luksiene, Z.; Rasiukeviciute, N.; Zudyte, B.; Uselis, N. Innovative approach to sunlight activated biofungicides for strawberry crop protection: ZnO nanoparticles. J. Photochem. Photobiol. B Biol. 2020, 203, 111656. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Tang, Y.; Naz, I.; Alam, S.S.; Wang, W.; Ahmad, M.; Najeeb, S.; Rao, C.; Li, Y.; Xie, B.; et al. Management of Ralstonia solanacearum in Tomato Using ZnO Nanoparticles Synthesized through Matricaria chamomilla. Plant Dis. 2021, 105, 3224–3230. [Google Scholar] [CrossRef]
- Giorgetti, L. Effects of Nanoparticles in Plants. In Nanomaterials in Plants, Algae and Microorganisms; Academic Press: Cambridge, MA, USA, 2018; pp. 65–87. [Google Scholar] [CrossRef]
- Ji, X.; Wang, H.; Song, B.; Chu, B.; He, Y. Silicon Nanomaterials for Biosensing and Bioimaging Analysis. Front. Chem. 2018, 6, 38. [Google Scholar] [CrossRef]
- Mathur, P.; Roy, S. Nanosilica facilitates silica uptake, growth and stress tolerance in plants. Plant Physiol. Biochem. 2020, 157, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Jeelani, P.G.; Mulay, P.; Venkat, R.; Ramalingam, C. Multifaceted application of silica nanoparticles. A review. Silicon 2020, 12, 1337–1354. [Google Scholar] [CrossRef]
- Fan, N.; Zhao, C.; Yue, L.; Ji, H.; Wang, X.; Xiao, Z.; Rasmann, S.; Wang, Z. Nanosilicon alters oxidative stress and defence reactions in plants: A meta-analysis, mechanism and perspective. Environ. Sci. Nano 2022, 9, 3742–3755. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Dubey, N.K. Silicon nanoparticles (SiNP) alleviate chromium (VI) phytotoxicity in Pisum sativum (L.) seedlings. Plant Physiol. Biochem. 2015, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Haliem, M.E.; Hegazy, H.S.; Hassan, N.S.; Naguib, D.M. Effect of silica ions and nano silica on rice plants under salinity stress. Ecol. Eng. 2017, 99, 282–289. [Google Scholar] [CrossRef]
- El-Ashry, R.M.; El-Saadony, M.T.; El-Sobki, A.E.A.; El-Tahan, A.M.; Al-Otaibi, S.; El-Shehawi, A.M.; Saad, A.M.; Elshaer, N. Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi J. Biol. Sci. 2022, 29, 920–932. [Google Scholar] [CrossRef]
- Kandhol, N.; Singh, V.P.; Peralta-Videa, J.; Corpas, F.J.; Tripathi, D.K. Silica nanoparticles: The rising star in plant disease protection. Trends Plant Sci. 2022, 27, 7–9. [Google Scholar] [CrossRef]
- Bapat, G.; Zinjarde, S.; Tamhane, V. Evaluation of silica nanoparticle mediated delivery of protease inhibitor in tomato plants and its effect on insect pest Helicoverpa armigera. Colloids Surf. B Biointerfaces 2020, 193, 111079. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pan, T.; Gao, X.; An, J.; Ning, C.; Li, S.; Cai, K. Silica nanoparticles activate defense responses by reducing reactive oxygen species under Ralstonia solanacearum infection in tomato plants. Nanoimpact 2022, 28, 100418. [Google Scholar] [CrossRef]
- Zhang, J.; Kothalawala, S.; Yu, C. Engineered silica nanomaterials in pesticide delivery: Challenges and perspectives. Environ. Pollut. 2023, 320, 121045. [Google Scholar] [CrossRef]
- Albalawi, M.A.; Abdelaziz, A.M.; Attia, M.S.; Saied, E.; Elganzory, H.H.; Hashem, A.H. Mycosynthesis of Silica Nanoparticles Using Aspergillus niger: Control of Alternaria solani Causing Early Blight Disease, Induction of Innate Immunity and Reducing of Oxidative Stress in Eggplant. Antioxidants 2022, 11, 2323. [Google Scholar] [CrossRef] [PubMed]
- Udalova, Z.V.; Zinovieva, S.V. Effects of Silicon Nanoparticles on the Activity of Antioxidant Enzymes in Tomato Roots Invaded by Meloidogyne incognita (Kofoid et White, 1919) Chitwood, 1949. Dokl. Biochem. Biophys. 2022, 506, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Hersanti; Hidayat, S.; Susanto, A.; Virgiawan, R.; Joni, M. The effectiveness of Penicillium sp. mixed with silica nanoparticles in controlling Myzus persicae. AIP Conf. Proc. 2018, 1927, 030029. [Google Scholar]
- Baka, Z.A.; El-Zahed, M.M. Antifungal activity of silver/silicon dioxide nanocomposite on the response of faba bean plants (Vicia faba L.) infected by Botrytis cinerea. Bioresour. Bioprocess. 2022, 9, 102. [Google Scholar] [CrossRef]
- Zohra, E.; Ikram, M.; Omar, A.A.; Hussain, M.; Satti, S.H.; Raja, N.I.; Mashwani, Z.U.R.; Ehsan, M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Process. Synth. 2021, 10, 456–475. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Hu, D.; Yu, S.; Yu, D.; Liu, N.; Tang, Y.; Fan, Y.; Wang, C.; Wu, A. Biogenic Trichoderma harzianum-derived selenium nanoparticles with control functionalities originating from diverse recognition metabolites against phytopathogens and mycotoxins. Food Control. 2019, 106, 106748. [Google Scholar] [CrossRef]
- González-García, Y.; Cadenas-Pliego, G.; Alpuche-Solís, Á.; Cabrera, R.; Juárez-Maldonado, A. Carbon Nanotubes Decrease the Negative Impact of Alternaria solani in Tomato Crop. Nanomaterials 2021, 11, 1080. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Song, Y.; Li, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon dots promote the growth and photosynthesis of mung bean sprouts. Carbon 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Fincheira, P.; Tortella, G.; Seabra, A.B.; Quiroz, A.; Diez, M.C.; Rubilar, O. Nanotechnology advances for sustainable agriculture: Current knowledge and prospects in plant growth modulation and nutrition. Planta 2021, 254, 66. [Google Scholar] [CrossRef]
- El-Samad, L.M.; Hassan, M.A.; Bakr, N.R.; El-Ashram, S.; Radwan, E.H.; Aziz, K.K.A.; Hussein, H.K.; El Wakil, A. Insights into Ag-NPs-mediated Pathophysiology and Ultrastructural Aberrations in Ovarian Tissues of Darkling Beetles. Sci. Rep. 2022, 12, 13899. [Google Scholar] [CrossRef] [PubMed]
- El-Samad, L.M.; Bakr, N.R.; El-Ashram, S.; Radwan, E.H.; Aziz, K.K.A.; Hussein, H.K.; El Wakil, A.; Hassan, M.A. Silver Nanoparticles Instigate Physiological, Genotoxicity, and Ultrastructural Anomalies in Midgut Tissues of Beetles. Chem. Biol. Interact. 2022, 367, 110166. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, E.; Drobne, D. Nanomaterials in Plants: A Review of Hazard and Applications in the Agri-Food Sector. Nanomaterials 2019, 9, 1094. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Roy, A.; Bhasin, S.; Bin Emran, T.; Khusro, A.; Eftekhari, A.; Moradi, O.; Rokni, H.; Karimi, F. Nanomaterials: An alternative source for biodegradation of toxic dyes. Food Chem. Toxicol. 2022, 164, 112996. [Google Scholar] [CrossRef] [PubMed]
| NP | Size (nm) | Crop Stress | Impact | Mechanism | Ref. |
|---|---|---|---|---|---|
| Chitosan | Not provided | Wilt disease caused by Fusariumandiyazi in tomato | In vitro studies showed that, among different tested concentrations (0.1–5.0 mg/mL), 5.0 mg/mL concentration of chitosan NPs produced the maximum inhibition of radial mycelial growth (73.8%). | By inducing the up-regulation of PR-proteins and antioxidant Genes, which play a role in plant defense against pathogen attack. | [70] |
| Chitosan loaded with paraquat (herbicide) | 300 | Control of weeds in agriculture | Cytotoxicity and genotoxicity assays showed that the nanoencapsulated herbicide was less toxic than the pure compound. | Lower cytotoxicity and genotoxicity effects of the encapsulated herbicide, compared to its free form, were attributed to the encapsulation effect and the sustained paraquat release. | [73] |
| Chitosan with and without combination with salicylic acid | Not provided | Rust disease caused by Puccinia striiformis (obligate fungal parasite) inoculated in wheat leaf | Infected wheat plants treated with the nanoparticles showed reduction in pustule size and leaf rust when compared to untreated plants. | Increased the activity of antioxidant enzymes, reduction of ROS formation, activation of transcription levels of PR1-PR5 and PR10 genes | [76] |
| Chitosan loaded with the essential oil peppermint | 563 | To promote the control of stored food pest for the insets Sitophilus oryzae and Tribolium castaneum | Significant efficacy of the NPs against both stored product pest compared to control group (untreated) | Inhibition of AChE, which is an essential detoxification enzyme of insect organization. | [77] |
| Poly(ε-caprolactone) loaded with the herbicide atrazine | 483 | Bidens pilosa (weed species) on soybean plants | Enhancement of herbicide activity and decrease of its toxicity, upon atrazine encapsulation. | Nanoencapsulation of atrazine reduced the levels of applied herbicide applied, due to the sustained release. | [79] |
| Polyhydroxyalkanoates (PHAs)–of two types– poly-3-hydroxybutyrate [P(3HB)] and poly(3-hydroxybutyrate-co-3-hydroxyvalerate [P(3HB/3HV)] loaded with commercial herbicides | 430–750 | Elsholtzia ciliata weed plants | At the end of the experiment (30 days), the herbicidal activity of encapsulated metribuzin was comparable to the positive control, and all plants were killed. The application of encapsulated herbicides led to the death of weeds, whereas the herbicides remained biologically active, without being prematurely degraded in soil. | Enhancement of herbicide stability upon its encapsulation, which led to a sustained release. | [80] |
| NP | Size (nm) | Crop Stress | Impact | Mechanism | Ref. |
|---|---|---|---|---|---|
| CuO | 14–47 | Sitophilus granarius and Rhyzopertha dominica insects that damage wheat grains. | Increased insect mortality by 55–94%; Morphological attributes (lengths, fresh weight, and dry weight of root and shoot, as well as leaves number) and leaf pigments (chlorophylls and carotenoids) were increased. | Stimulating the activity of the enzymes SOD, POD, and APX (antioxidant system) as well as increased concentration of leaf pigments, which have a significant role in scavenging ROS and protecting the plant from stress. | [88] |
| Ag | 23 | Bacterial leaf blight (BLB) disease caused by Xanthomonas oryzae on rice crops. | Decrease in lesion length of ~31–72% according to Ag NP concentration; decrease in antibacterial activity by 24%; Growth-promoting effect by Ag NPs | Increasing the antioxidant enzyme levels to modulate the adverse effects of reactive oxygen species; promoting nutrient uptake and cellular antioxidative system. | [89] |
| MgO | 20–200 | Black shank and black root rot diseases caused by Phytophthora nicotianae and Thielaviopsis basicola, respectively. | 36 and 42% decrease in tobacco black shank and black root rot disease incidence, respectively. Higher inhibitory effect on spore germination, sporangium formation, and hyphal development | Induced ROS production destroys membrane integrity and alters morphological characteristics through pathogen cell uptake. Mg is an essential mineral that participates in numerous physiological and biological processes, playing a crucial role in plant defense. | [90] |
| TiO2 | 10–100 | Yellow stripe rust disease caused by Puccinia striiformis on wheat crops. | Inhibition of growth and proliferation of the fungal pathogen resulted in decreased disease incidence and percent disease index when treated TiO2 NPs; Promotion of photosynthesis. | Up and downregulation of proteins triggering defense-related responses, such as 6-phosphogluconate dehydrogenase, involved in various reactions of the pentose-phosphate pathways to produce NADPH, which in turn is involved in facilitating the activity of NADPH-oxidase, the main ROS-producing enzyme during infection by pathogens. | [91] |
| ZnO | 13 | Fusarium wilt caused by Fusarium oxysporum on chickpea crops. | Increase of antioxidant activity and reduction of 90% in disease incidence; Improve photosynthetic rate and fresh and dry weight of roots. | Seed priming with ZnO NPs helped plants accumulate higher quantities of sugars, phenol, total proteins, and activation of defense enzymes such as SOD, PO and CAT, creating resistance against the pathogen. | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortella, G.; Rubilar, O.; Pieretti, J.C.; Fincheira, P.; de Melo Santana, B.; Fernández-Baldo, M.A.; Benavides-Mendoza, A.; Seabra, A.B. Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture. Antibiotics 2023, 12, 338. https://doi.org/10.3390/antibiotics12020338
Tortella G, Rubilar O, Pieretti JC, Fincheira P, de Melo Santana B, Fernández-Baldo MA, Benavides-Mendoza A, Seabra AB. Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture. Antibiotics. 2023; 12(2):338. https://doi.org/10.3390/antibiotics12020338
Chicago/Turabian StyleTortella, Gonzalo, Olga Rubilar, Joana C. Pieretti, Paola Fincheira, Bianca de Melo Santana, Martín A. Fernández-Baldo, Adalberto Benavides-Mendoza, and Amedea B. Seabra. 2023. "Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture" Antibiotics 12, no. 2: 338. https://doi.org/10.3390/antibiotics12020338
APA StyleTortella, G., Rubilar, O., Pieretti, J. C., Fincheira, P., de Melo Santana, B., Fernández-Baldo, M. A., Benavides-Mendoza, A., & Seabra, A. B. (2023). Nanoparticles as a Promising Strategy to Mitigate Biotic Stress in Agriculture. Antibiotics, 12(2), 338. https://doi.org/10.3390/antibiotics12020338










