Abstract
Periodontal disease (PD) is multifactorial oral disease that damages tooth-supporting tissue. PD treatment includes proper oral hygiene, deep cleaning, antibiotics therapy, and surgery. Despite the availability of basic treatments, some of these are rendered undesirable in PD treatment due to side effects and expense. Therefore, the aim of the present study is to develop novel molecules to combat the PD triggering pathogens. The study involved the synthesis of 4-((5-(substituted-phenyl)-1,3,4-oxadiazol-2-yl)methoxy)benzamidine (5a-e), by condensation of 2-(4-carbamimidoylphenoxy)acetohydrazide (3) with different aromatic acids; and synthesis of 4-((4-(substituted benzylideneamino)-4H-1,2,4-triazol-3-yl)methoxy)benzamidine (6a-b) by treatment of compound 3 with CS2 followed by hydrazination and a Schiff reaction with different aromatic aldehydes. Synthesized compounds were characterized based on the NMR, FTIR, and mass spectrometric data. To assess the effectiveness of the newly synthesized compound in PD, new compounds were subjected to antimicrobial evaluation against P. gingivalis and E. coli using the micro-broth dilution method. Synthesized compounds were also subjected to cytotoxicity evaluation against HEK-293 cells using an MTT assay. The present study revealed the successful synthesis of heterocyclic derivatives of benzamidine with significant inhibitory potential against P. gingivalis and E. coli. Synthesized compounds exhibited minimal to the absence of cytotoxicity. Significant antimicrobial potential and least/no cytotoxicity of new heterocyclic analogs of benzamidine against PD-triggering bacteria supports their potential application in PD treatment.
1. Introduction
Periodontal disease (PD) is a noncommunicable oral inflammatory disease that affects the tissue of the teeth, causing severe damage to the periodontal ligament, leading to tooth loss and reducing the quality of life [1]. PD affects around 11% of the global population [2]. Risk factors such as osteoporosis, metabolic disorder, diabetes, and obesity are strongly linked with chronic PD; however, lifestyle (smoking, alcohol consumption, and poor oral hygiene), poor dietary vitamin D, and calcium intake also play a role [3]. Increasing evidence showed that there is a link between PD and other ailments such as respiratory infections, adverse pregnancy outcomes, cardiovascular diseases, chronic kidney disease, diabetes, cancer, Alzheimer’s, and Parkinson’s disease [4,5,6,7,8]. The onset and progression of PD are associated with the synergy among the organisms found in the microbiota, which then interacts with the host immune defense, leading to severe oral inflammation [9]. The oral microbiota is composed of several organisms that are important in the regulation and protection of the oral cavity against the colonization of non-essential organisms. However, dysregulation of these organisms can cause gingivitis which has the potential to progress to PD [10]. PD is known to be associated with diverse species of bacteria, especially P. gingivalis and E. coli [11,12]; that are strongly linked to and implicated in the initial onset, progression, and severity of PD and its associated systemic diseases [13,14]. The strategies for prevention and treatment of PD are relatively simple, yet difficult to apply. The reduction of risk factors, the use of probiotic agents, and antioxidants, along with mechanical treatment (scaling) and/or a combination of antibiotics such as metronidazole and amoxicillin or metronidazole and ciprofloxacin can greatly contribute to the reduction or elimination of periodontal associated pathogens [15,16]. Furthermore, they aid the treatment of PD associated systemic diseases [17,18]. Although the systematic adjuvant use of mechanical and combination of antibiotics is the best strategy now, other evidence showed that P. gingivalis and its related pathogens are developing resistance to commonly available antibiotics and rendering them less effective by degradation using their virulence factors [19,20,21,22]. Concerning antibiotic resistance, an alternative treatment strategy for periodontal pathogens is the use of synthetic inhibitors. Recent evidence showed that synthetic molecules have the potential to ease the burden of oral infections caused by P. gingivalis with no significant cytotoxicity observed [23,24]. Facts suggest that incorporation of heterocyclic groups into the organic moieties enhances their biological potential [25,26]. A study reported that benzamidine and its derivatives displayed inhibition against gingipains, a major virulence factor produced by P. gingivalis [27]. Our previous study showed that benzamidine and its derivatives (ester, hydrazides, and Schiff bases) inhibit P. gingivalis and its associated pathogens [12]. Recent studies showed effectiveness of oxadiazoles and triazoles against periodontitis triggering pathogens [28,29,30]. To combat antibiotic resistance, oxadiazoles have been used due to their sensitive antimicrobial activity [31]. Hence, based on the severity of PD, associated pathogens and their resistance, potential of benzamidine analogs against PD, and the enhancement of inhibitory potential by incorporation of oxadiazoles and triazoles groups in different chemical moieties, researchers are motivated to perform the synthesis, characterization, cytotoxicity analysis, and evaluation of novel heterocyclic derivatives (oxadiazoles and triazoles) of benzamidine against periodontal-disease-triggering bacteria. In the continuation of a previous study, our present study highlights that the synthesis of new oxadiazole and triazole derivatives of benzamidine analogs possess high inhibition potential against triggering bacteria, which makes them the forefront of potential PD treatment.
2. Materials and Methods
2.1. General Information
The reagents, solvents, and chemicals used for the synthesis of compounds in the present study were acquired from Sigma-Aldrich Co. (St. Louis, MO, USA), HmbG® Chemicals, Hamburg, Germany, Friendemann Schmidt Chemical, Washington, DC, USA, Merck KGaA (Darmstadt, Germany), and Qrec Chemicals, Rawang, Malaysia. The Ashless Whattman No. 1 filter paper was used for filtration. To verify compounds’ purity, the open capillary tube method was used. The melting points of all synthesized compounds were determined using SMP11 Analogue apparatus. The compounds’ characterization was recorded by 1H-NMR and 13C-NMR (NMR 700 MHz ASCEND™ spectrometer) using deuterated DMSO solvent, on a δ value scale as the downfield chemical shift in ppm against tetramethylsilane (TMS). The NMR signals are stated as s, single; d, doublet; t, triplet; m, multiplet. The IR of synthesized compounds was recorded using a Jasco ft/ir-6700 instrument in a wavelength range of 400–4000 cm−1. The analysis of mass spectra was recorded from a Direct Infusion IonTrap MS Full Scan (Thermo Scientific Q Exactive HF-X hybrid quadrupole-Orbitrap mass spectrometer, Waltham, MA, USA). Elemental analysis was performed on a Perkin Elmer 240 B and 240 C. Elemental analysis (C, H, N), indicated by employing element symbols, was within ±0.4% of theoretical values. The purity of compounds and monitoring of reactions were assessed by TLC on aluminum sheets with silica gel 60 F254 (0.2 mm) (Merck Millipore, Darmstadt, Germany) using methanol: chloroform (0.3: 1.7) as a solvent system in a UV chamber using a SPRECTROLINE® CM-26 UV viewing chamber. The 4-hydroxybenzenecarboximidamide analogs were synthesized as per the protocol given by previous authors with slight modifications [32,33,34,35,36].
2.2. Synthesis
2.2.1. General Procedure for the Synthesis of 4-((5-(Substituted-phenyl)-1,3,4-oxadiazol-2-yl)methoxy)benzamidine (5a-e)
To synthesize the oxadiazole derivatives of benzamidine (5a-e), an equal molar concentration of compound (3) (0.02 M) and 3-phenoxy benzoic acid was dissolved in 10 mL of phosphoryl chloride and refluxed for 8 h. At the end, the mixture was cooled, washed with ice, filtered, and recrystallized to obtain the pure compound 5a. The synthetic scheme for synthesis of compound 5a-e is given in Figure 1. During the experiment, anhydrous reaction conditions were maintained, and the recrystallization was done using methanol and activated charcoal. The synthesized compound 5a was further characterized based on the spectrometric data (Figures S1–S4). Similarly, other compounds 5b-e were synthesized, purified, and characterized.
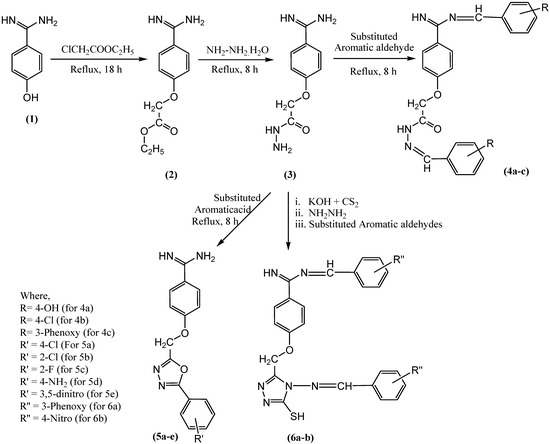
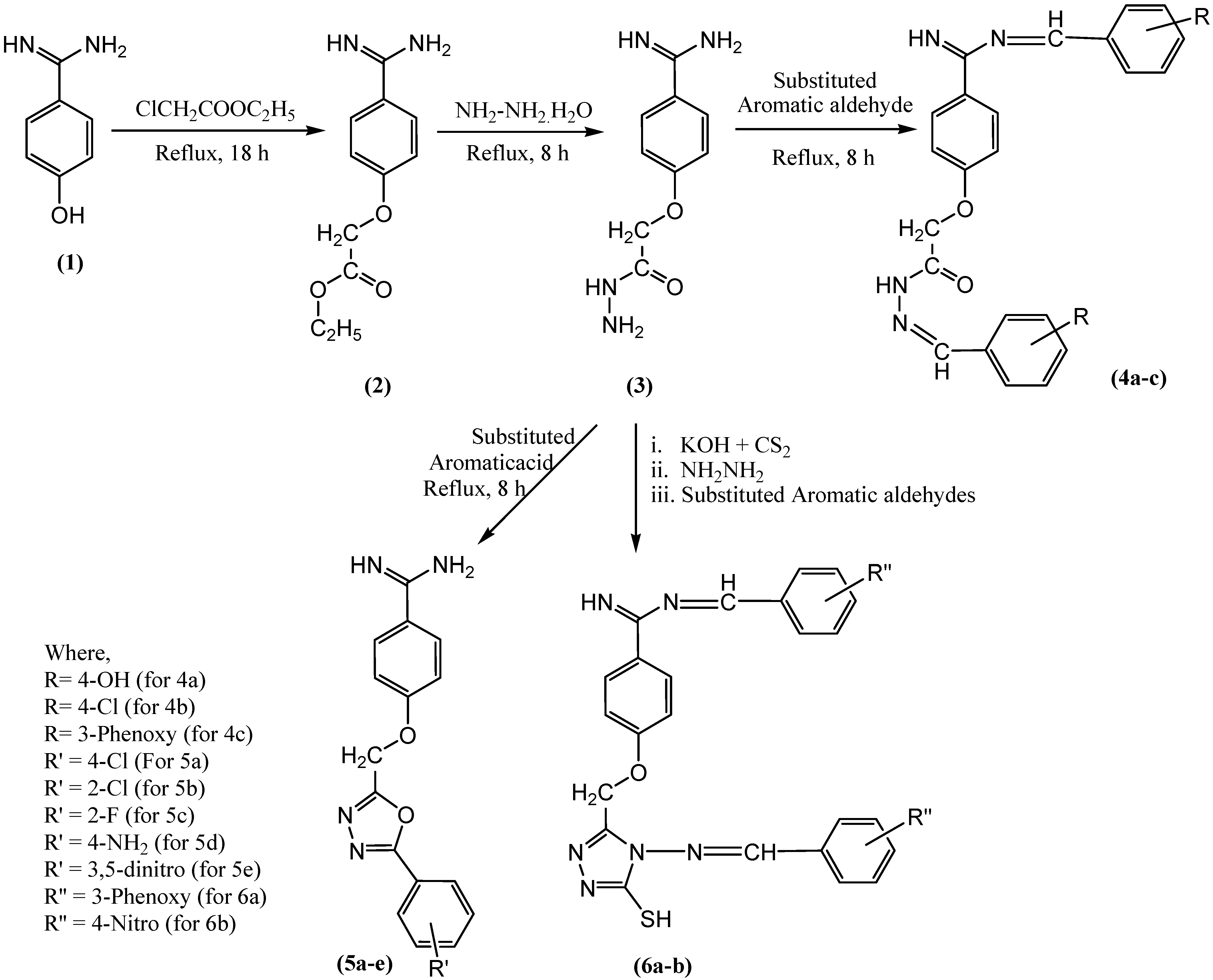
Figure 1.
Scheme for synthesis of novel benzamidine analogues.
4-((5-(4-Chlorophenyl)-1,3,4-oxadiazol-2-yl)methoxy)benzamidine (5a)
White crystalline (Yield 73%, m.p. 184 °C); IR (KBr, cm−1): 3051 (Aromatic C–H), 2978 (Aliphatic H–C), 1681 (C=N), 1591, 1425 (Aromatic C=C), 1238, 1066 (C–O–C of oxadiazole ring); 1H-NMR (DMSO-d6, ppm) δ: 3.21 (s, 2H, O–CH2), 3.71 (brs, 2H, NH2), 7.25–7.79 (m, 8H, Ar–H), 8.41 (s, 1H, C=NH); and 13C–NMR (DMSO, ppm) δ: 68.17 (CH2), 122.93, 128.35, 128.88, 129.29, 130.79, 130.95, 131.432, 133.62, 138.86 (Ar-C), 167.47 (C=N); Mass (m/z): Calcd. 328.75, found 328.4; Anal. Calcd. for C16H13ClN4O2: C, 58.45; H, 3.99; N, 17.04%, Found: C, 58.53; H, 3.91; N, 17.12%.
4-((5-(2-Chlorophenyl)-1,3,4-oxadiazol-2-yl)methoxy)benzamidine (5b)
Beige crystalline (Yield 70%, m.p. 124 °C); IR (KBr, cm−1): 3065 (Aromatic C–H), 2998, 2888 (Aliphatic C–H), 1682 (C=N), 1569, 1474 (Aromatic C=C), 1265, 1043 (C–O–C of oxadiazole ring); 1H-NMR (DMSO-d6, ppm) δ: 3.21 (s, 2H, O–CH2), 3.72 (brs, 2H, NH2), 7.46–7.83 (m, 8H, Ar-H), 8.12 (s, 1H, C=NH); 13C-NMR (DMSO, ppm) δ: δ 68.18 (O–CH2), 122.51, 127.64, 131.01, 131.17, 131.50, 131.97, 132.07, 132.93 (Ar-C), 167.24 (C=N); Mass (m/z): Calcd. 328.75, found 328.5; Anal. Calcd. for C16H13ClN4O2: C, 58.45; H, 3.99; N, 17.04%, Found: C, 58.58; H, 3.93; N, 17.16%.
4-((5-(2-Fluorophenyl)-1,3,4-oxadiazol-2-yl)methoxy)benzamidine (5c)
Light brown crystalline (Yield 75%, m.p. 138 °C); IR (KBr, cm−1): 3051 (Aromatic C–H), 2917 (Aliphatic C–H), 1675 (C=N), 1508, 1426 (Aromatic C=C), 1292, 1067 (C–O–C of oxadiazole ring); 1H-NMR (DMSO-d6, ppm) δ: 3.20 (s, 2H, CH2), 3.49 (brs, 2H, NH2), 7.30–8.01 (m, 8H, Ar–H), 8.01 (s, 1H, C=NH); 13C-NMR (DMSO, ppm) δ: 68.44 (O-CH2), 115.99, 117.20, 122.63, 123.90, 127.79, 128.77, 129.87, 131.40, 132.35, 134.56 (Ar–C), 164.67 (C=N); Mass (m/z): Calcd. 312.3, found 312.1; Anal. Calcd. for C16H13FN4O2: C, 61.53; H, 4.20; N, 17.94%, Found: C, 61.61; H, 4.25; N, 17.88%.
4-((5-(4-Aminophenyl)-1,3,4-oxadiazol-2-yl)methoxy)benzamidine (5d)
Brown crystalline (Yield 70%, m.p. 134 °C); IR (KBr, cm−1): 3229 (N–H), 3065 (Aromatic C–H), 2918 (Aliphatic C–H), 1653 (C=N), 1593, 1407 (Aromatic C=C), 1243, 1062 (C–O–C of oxadiazole ring); 1H-NMR (DMSO-d6, ppm) δ: 3.22 (s, 2H, O-CH2), 3.70–4.20 (brs, 4H, NH2 and Ar–NH2), 7.03–8.11 (m, 8H, Ar-H), 8.12 (s, 1H, C=NH); 13C-NMR (DMSO, ppm) δ: 68.11 (O–CH2), 113.06, 116.17, 119.70, 120.03, 123.24, 130.09, 130.62, 131.82 (Ar–C), 167.19 (C=N); Mass (m/z): Calcd. 384.3, found 384.10; Anal. Calcd. for C16H15N5O2: C, 62.13; H, 4.89; N, 22.64%, Found: C, 62.22; H, 4.93; N, 22.71%.
4-((5-(3,5-Dinitrophenyl)-1,3,4-oxadiazol-2-yl)methoxy)benzamidine (5e)
Yellowish brown crystalline (Yield 75%, m.p. 130 °C); IR (KBr, cm−1): 3051 (Aromatic C–H), 2919 (Aliphatic C–H), 1680 (C=N), 1589, 1474 (Aromatic C=C), 1310 (N-O), 1242, 1042 (C–O–C of oxadiazole ring); 1H-NMR (DMSO-d6, ppm) δ: 3.16 (s, 2H, O–CH2), 3.71 (brs, 2H, NH2), 7.41–7.78 (m, 7H, Ar–H), 8.02 (s, 1H, C=NH); 13C-NMR (DMSO, ppm) δ: 67.98 (O–CH2), 122.12, 123.26, 127.7, 128.07, 129.21, 130.56, 131.06, 132.01, 133.28, 134.55 (Ar–C), 167.23 (C=N); Mass (m/z): Calcd. 309.32, Found 309.20; Anal. Calcd. for C16H12N6O6: C, 50.01; H, 3.15; N, 21.87%, Found: C, 50.12; H, 3.11; N, 21.79%.
2.2.2. General Procedure for the Synthesis of 4-((4-3-Phenoxybenzylideneamino)4-4-nitrobenzylideneamino)-4H-1,2,4-triazole-3-yl methoxy)benzamidine (6a-b)
To synthesize the compound 6a,b, a mixture of compound 3 (0.1 M), potassium hydroxide (0.15 M), and CS2 (0.15 M) in absolute ethanol was stirred for 18 h. To the resulting solution, 250 mL of anhydrous ether was added to precipitate potassium dithiocarbazinate. The 0.02 M of dithiocarbazinate was hydrazinated with 0.04 M of hydrazine hydrate. The hydrazinated product was treated with different aromatic aldehydes separately in equimolar concentration. The synthesized compounds were recrystallized using absolute ethanol to offer pure compounds 6a,b. The synthetic scheme for synthesis of compound 6a-b is given in Figure 1. During the experiment, anhydrous reaction conditions were maintained, and the recrystallization was done using methanol and activated charcoal. The synthesized compound 6a was further characterized based on spectrometric data (Figures S5, S6, S7a–c and S8). Similarly, compound 6b was also synthesized, purified, and characterized.
4-((4-(3-Phenoxybenzylideneamino)-4H-1,2,4-triazole-3-yl)methoxy)benzamidine (6a)
Yellow crystalline (Yield 85%, m.p 193 °C); IR (KBr, cm−1): 3034 (Aromatic C–H), 2918 (Aliphatic C–H), 1687 (C=N), 1481, 1447 (Aromatic C=C); 1H-NMR (DMSO-d6, ppm) δ: 3.35 (s, 2H, O–CH2), 7.06 (s, 1H, S–H), 7.17–7.71 (m, 22H, Ar–H), 9.29 (s, 1H, N=CH), 9.99 (s, 1H, C=NH); 13C-NMR (DMSO, ppm) δ: 67.89 (O–CH2), 117.82, 118.59, 119.75, 119.81, 123.23, 124.52, 124.58, 124.75, 124.89, 125.22, 130.71, 130.77, 130.83, 131.51, 133.30, 138.42, 163.33 (Ar–C), 157.75 (C=N), 167.38 (1H, C=NH), 193.01 (N=C-S); Mass (m/z): Calcd. 624.71, Found 624.20; Anal. Calcd. for C36H28N6O3S: C, 69.21; H, 4.52; N, 13.45%, Found: C, 69.18; H, 4.59; N, 13.51%.
4-((4-(4-Nitrobenzylideneamino)-4H-1,2,4-triazole-3-yl)methoxy)benzamidine (6b)
Yellow crystalline (Yield 88%, m.p 180 °C); IR (KBr, cm−1): 3052 (Aromatic C–H), 2919 (Aliphatic C–H), 1703 (C=N), 1537, 1444 (Aromatic C=C); 1H-NMR (DMSO-d6, ppm) δ: 3.36 (s, 2H, O–CH2), 7.06 (s, 1H, S–H), 8.16–8.42 (m, 12H, Ar–H), 9.29 (s, 1H, N=CH), 9.98 (s, 1H, C=NH); 13C-NMR (DMSO, ppm) δ: 68.01 (O–CH2), 124.73, 125.32, 127.12, 131.11, 131.57, 135.28, 133.67, 136.59, 138.45, 139.17, 139.82, 140.54 (Ar–C), 151.09 (C=N), 167.82 (C=NH), 192.79 (N=C-S); Mass (m/z): Calcd. 530.52, Found 530.20. Anal. Calcd. for C24H18N8O5S: C, 54.34; H, 3.42; N, 21.12%, Found: C, 54.29; H, 3.39; N, 21.08%.
2.3. Determination of Antimicrobial Activity
In the present study, the micro-broth dilution method was used to determine the inhibition susceptibility of synthesized compounds against P. gingivalis (ATCC 33277) and E. coli (ATCC 25922). The strain of P. gingivalis and E. coli were obtained from ATCC. The bacteria were cultured in blood-enriched tryptic soy agar (eTSA) (Merck KGaA, Darmstadt, Germany), supplemented with sterile filtered 5% L-cysteine (Bio-Basic, Markham, ON, Canada), 1% dithiothreitol (Sigma Life Sciences, Burlington, MA, USA), and 0.5 mg/mL vitamin K (Sigma Life Sciences, Burlington, MA, USA) with an adjusted pH of 7.4 [37]. As per CLSI guidelines, the micro broth dilution method was used to determine the minimum inhibition concentration (MIC) of P. gingivalis. The synthesized compounds were diluted in two-fold serial dilution, starting with the highest concentration at 500 µg/mL, and the lowest concentration at 7.8125 µg/mL. Ampicillin was used as a control (Akum Drugs and Pharmaceuticals, New Delhi, India), with final concentrations of 250 µg/mL to 1.6 µg/mL. All of these dilutions were carried out aseptically. To inoculate bacterial culture for MIC, the 0.5 McFarland standard was used (1.5 × 108 CFU/mL) [38,39]. To the microtiter plate, an equal volume of 1.5 × 108 CFU/mL of P. gingivalis was added, excluding only the negative control. The microtiter plates were incubated in an anaerobic jar (Oxoid, Winchester, UK) supplemented with a gas pack (Merck KGaA, Darmstadt, Germany) that generate 90% N2, 5% CO2, and H2 and a gas indicator (Thermo Fisher Scientific, Waltham, MA, USA) for 46 h at 37 °C.
Cation-adjusted Mueller–Hinton broth (CAMHB) and agar (CAMHA) (HiMedia, Mumbai, India) were used for E. coli MIC evaluation. The MIC of E. coli was determined using the same method as that of P. gingivalis. The final concentration of the ampicillin was 250 µg/mL to 1.6 µg/mL (CSC Pharmaceuticals, Mumbai, India). The microtiter plates were incubated at 37 °C in aerobic conditions for 18 h.
To determine P. gingivalis minimum bactericidal concentration, MIC results of the clear wells of samples where there was no visible bacterial growth were aseptically plated on eTSB agar and incubated in an anaerobic jar at 37 °C with a gas indicator and gas pack for 46 h. The MBC of E. coli was determined by plating the MIC results of the clear wells on CAMHA and incubating for 18 h at 37 °C according to the guidelines given by CLSI. After incubation, MBC was recorded as the lowest concentration of a compound with no visible growth of bacteria with agar clarity, the same as that of the negative control. All experiments were performed in triplicate.
2.3.1. Cell Viability Assay
MTT is the most common cell viability assay used and it depends on the conversion of substrate to a chromogenic product by live cells. This assay involves the conversion of the water-soluble MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] to an insoluble formazan by the action of mitochondrial reductase. The solubilized formazan concentration is then determined by an optical density at 570 nm [40]. To determine cell viability, HEK 293 cells obtained from ATCC were revived and cultured using DMEM (Dulbecco’s modified Eagle medium) supplemented with 5% fetal bovine serum (FBS) (Sigma life science, Burlington, MA, USA) and 1% antibiotic (GIBCO, Waltham, MA, USA) and incubated at 37 °C, with 5% CO2, and relative humidity of about 95% (Heal Force/HF90, Hong Kong, China).
2.3.2. Cell Counting
Cells were counted using the hemocytometer (Hirschmann Laborgerate, Darmstadt, Germany) counting technique. Here, cells were washed with PBS (First base, Axil Scientific, Singapore), treated with trypsin (Sigma life science, Burlington, MA, USA), and incubated at 37 °C to detach them from the flask surface. After trypsinization, 0.1 mL of cells were added to 0.9 mL of 0.2% trypan blue (Sigma life science, Burlington, MA, USA) in a sterile microcentrifuge tube. A 10 μL sample of stained cells were loaded into both sides of the chamber of the hemocytometer, covered with microscopic cover glass, and cells were viewed under the inverted microscope (Olympus/CK40-F200, Shinjuku, Japan). The viable cells were not stained with trypan blue, whereas the dead cells were stained [41]. Using Equations (1) and (2), the total viable cells were calculated:
2.3.3. Cell Treatment
After 24 h of incubation, the counted cells were treated with different concentrations (50–7.8125 μg/mL) of the synthesized compounds. All seeded cells were treated with synthesized compounds except the controls, and cells were further incubated until MTT analysis [42].
2.3.4. 3-[4,5-Dimethylthiazol-2-yl]2,5-diphenyl Tetrazolium Bromide (MTT) Assay
A 20 µL sample of MTT reagent (0.5 mg/mL) (Sigma life science, Burlington, MA, USA) in PBS was added to each well, including the controls, and the plates were covered with aluminum foil paper and incubated at 37 °C for 4 h. After incubation, the cells were treated with MTT detergent (DMSO) (Sigma life science, Burlington, MA, USA) and further incubated for 1 h. After 1 h of incubation, the sample OD was measured at 570 nm with the reference of 630 nm using Infinite 200 PRO (Tecan Microplate Reader, Mannedorf, Switzerland). A triplicate experiment was carried out for all synthesized compounds. Below is the formula used for calculating the percentage (%) of cell viability:
2.3.5. Statistical Analysis
GraphPad Prism software version 5 (GraphPad Software, Inc., San Diego, CA, USA) was used to analyze cytotoxicity statistical data. One-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc test to determine the source of significant difference between the groups using SPSS software (IBM SPSS Statistics, Version 25). Results are presented as mean ± standard error of experiment performed in triplicate.
3. Results and Discussion
3.1. Chemistry
In a previous study, the authors of the present study, described the synthesis of compounds 2, 3, and 4a-c, that involved preparation of ethyl-2-(4-carbamimidoylphenoxy)acetate 2, by esterification of 4-hydroxybenzenecarboximidamide (1), followed by hydrazination to form 2-(4-carbamimidoylphenoxy)acetohydrazide (3), which was further treated with different aromatic aldehydes to offer N-(substituted benzylidene)-2-(4-(N-(4-ydroxybenzylidene)carbamimidoyl)phenoxy)acetohydrazide (4a-c) [12].
In the current study, compound 3 was subjected to different types of reactions. In one part of the experiment, compound (3) was treated with different aromatic acids (4-chlorobenzoic acid, 2-chlorobenzoic acid, 4-flurobenzoic acid, 4-aminobenzoic acid, and 3,5-dinitrobenzoic acid) in the presence of POCl3 to offer new oxadiazoles derivatives of benzamidine (5a-e). The stated experiment involved cyclo-condensation reaction of aromatic acids with hydrazide (3) in the presence of POCl3 to form compound 5a-e. The physical and chemical properties of newly synthesized compounds in the present study are also supported by other investigations [43,44]. Whereas, in another part of the experiment, compound (3) was treated with carbon disulfide in the presence of potassium hydroxide to offer potassium dithiocarbazinate, which was further subjected to hydrazination followed by treatment with different aromatic aldehydes to offer compounds (6a,b) [45,46,47]. The synthetic scheme for all new compounds 5a-e and 6a-b is given in Figure 1.
Figure 1 shows synthetic route of novel analogs obtained in this study. The purity of synthesized compounds was determined based on melting point, single spot TLC (thin-layer chromatography) pattern, and CHN analysis. In the present study, the spectrometric analysis of synthesized compounds using mass spectrometry, FTIR, 1H, and 13C-NMR confirmed the structure of compounds 5a-e and 6a-b. The successful synthesis of compound 5a-e was confirmed based on the presence of the characteristic IR bands at 1042–1295 (C–O–C of oxadiazole ring), disappearance of 1H-NMR signals at 8.27, appearance of extra 13C-NMR signals for aromatic carbons raging between 113 and 166, and the appearance of a mass spectrum ion peak ranging between 309 and 384 confirmed the structure of the synthesized compounds 5a-e. The successful synthesis of the compound (6a-b) was confirmed based on the appearance of the characteristic IR bands at 1687 and 1703 (C=N), 1H-NMR signals at 7.06 (1H, s, S-H), 9.2 (N=CH), 9.9–10.17 (C=NH), 13C-NMR signals at 193 (N=C–S), and the appearance of mass signals at 624 and 530.
3.2. Biological Activity
3.2.1. In Vitro Antibacterial Activity of Synthesized Compounds
In vitro antibacterial assay consists of numerous biological assays such as agar dilution, well-diffusion, disk-diffusion, and broth dilution methods [48]. The antibacterial screening of all synthesized compounds 5a-e and 6a,b against P. gingivalis and E. coli resulted in a minimum inhibition concentration (MIC) between 31 μg/mL and 250 μg/mL (Table 1). However, not all the synthesized compounds yielded a result for minimum bactericidal concentration (MBC). The synthesized compounds 5b, 5d, 5e, and 6b have all displayed an MBC against P. gingivalis with a range of 250 μg/mL to 125 μg/mL. While for E. coli, only compounds 5c, 5e, and 6b have yielded MBC results with a range of 125 μg/mL to 250 ug/mL (Table 2). Moreover, both compounds 5a and 6a displayed no MBC activity against P. gingivalis and E. coli, respectively. On the contrary, compounds 5e and 6b are the only two compounds to yield MBC against both P. gingivalis and E. coli.

Table 1.
MIC values of synthesized compounds.

Table 2.
MBC values of synthesized compounds.
The synthesis of oxadiazole compounds in recent years has spiked up tremendously, solely due to their biological activities. Their antibacterial activity against pathogenic microorganisms has exceeded some of the known antibiotics, making them an alternative to combat drug resistance organisms [31]. It was previously reported by [49], that evaluation of scaffold oxadiazoles has resulted in MIC against gram-positive and gram-negative organisms. In addition, multiple other studies have shown that oxadiazoles have suppressed bacterial growth at low concentrations [50,51,52,53], similar to the observation that was noted in this study. Although oxadiazoles have broad-spectrum antibacterial activities [54], and their activities have been evaluated in both gram-positive and gram-negatives some of which are associated with oral diseases [55,56], there is no data on its evaluation and inhibition activity against P. gingivalis. This study may be the first one to report the ability of oxadiazoles to inhibit P gingivalis growth, a putative organism that promotes PD.
In the continuous search for alternatives to antibiotics, triazole Schiff bases derivatives possess the biological properties that inhibit the growth of drug-resistant organisms [57]. In the present study, it is worth knowing that compound 6b has yielded MIC and MBC against both P. gingivalis and E. coli compared to other synthesized compounds (Table 1 and Table 2). But this is not a surprise considering its known activity against pathogenic organisms [58], with some studies reporting that it is twice as active as ciprofloxacin [59], whereas other studies reported that it has activity comparable to that of chloramphenicol [60]. Furthermore, it was reported by [61,62], that triazole Schiff bases have an inhibition activity against multi-drug resistance organisms. Despite triazole Schiff base’s diverse antibacterial activity, there’s less evaluation of its activities against P. gingivalis. Nevertheless, a study showed that synthesized triazoles have the activity to inhibit adherence of P. gingivalis [30]. Another study showed that triazole has an inhibitory influence against HmuY and fimA gene expression (hemin binding proteins) which are responsible for P. gingivalis growth [63]. In the present study, all synthesized compounds have yielded antibacterial effects against tested pathogens with some having higher potential than others, making them a promising therapeutic alternative. Although all synthesized compounds exhibited significant activity against P. gingivalis and E. coli; however, among all, compound 6b was found to be most active, as it exhibited the best MIC and MBC values against P. gingivalis and E. coli.
3.2.2. Cytotoxicity Analysis of Synthesized Compounds
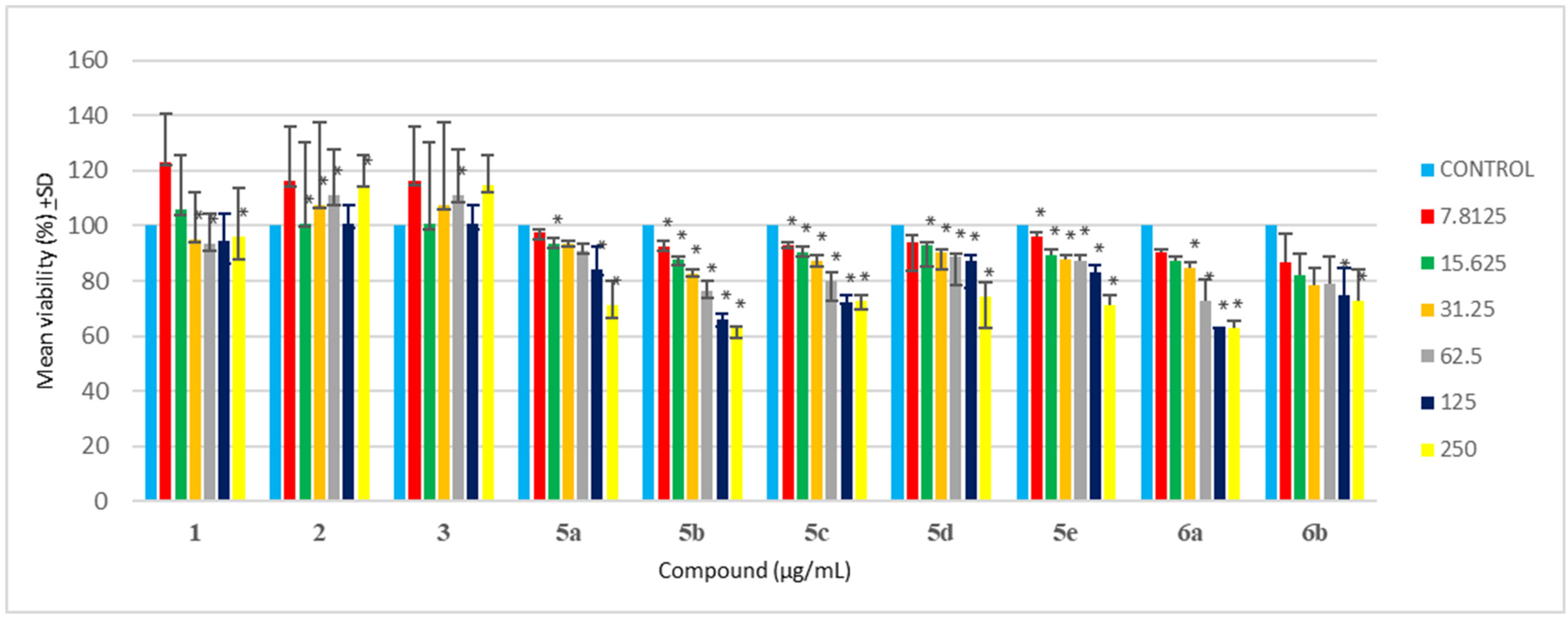
In drug development, cytotoxicity is an important aspect of biological evaluation. In the present study, the MTT assay of all synthesized compounds was evaluated against HEK 293 cells. A previous study suggested that oxadiazole compounds yielded no to less toxicity when tested against NIH/3T3 cells due to cell viability greater than 75% [64]. Testing synthesized compounds of oxadiazoles against A549, L929, and HpG2 cells [65], showed that most synthesized compounds yielded no cytotoxicity against tested cell lines with cell viability greater than 75%, with only a few compounds resulting in cell death. Satisfactory results and minimal cytotoxicity of oxadizoles evaluation were previously described [66,67], which is in agreement with this study. In the current study, it was observed that all synthesized oxadiazoles (5a-e) yielded more than 70% cell viability when tested against HEK 293 cells at 62.5 μg/mL (Table 3 and Figure 1). At 125 μg/mL, all synthesized oxadiazoles showed no sign of cytotoxicity, except for 5b, having only 66% cell viability. At the maximum concentration tested (500 μg/mL), only compound 5d-e showed no signs of cytotoxicity compared to other synthesized compounds (5a-c). Hence the minimal cytotoxicity at high concentrations and high antibacterial at low concentrations of these synthesized compounds are their major advantages.

Table 3.
Cytotoxicity values of synthesized compounds.
Triazole has exemplary biological activity and due to its minimal cytotoxicity, it is ideal for many biological studies. Triazole Schiff bases have shown to be safe and have relatively less cytotoxicity when tested against HEK-293 and WI-38, respectively [68,69,70]. Triazole Schiff bases analysis against kidney, red blood cells, and lung cells all yielded no cytotoxicity, hence supporting their minimal cytotoxicity, and it is even suggested to be safer than cisplatin [71,72,73]. Here in this study, it was observed that 6a-b resulted in more than 70% cell viability when tested against HEK-293 cells at 62.5 μg/mL (Table 3 and Figure 1). However, at 250 μg/mL, only 6b resulted in more than 70% cell viability. Among 6a and 6b, the compound 6b is found to be much safer with 68% cell viability (Table 3) at the maximum tested concentration (500 μg/mL).
Based on the resultant MIC data of synthesized compounds 5a-e and 6a,b given in Table 1, the structure of benzamidine and its analogues synthesized in the present study, were related to their inhibitory potential (MIC) against PD triggering bacteria. The study revealed that incorporation of heterocyclic ring (oxadiazole and triazole) increases their inhibitory potential by twofold against P. gingivalis in comparison to parent benzamidine compound 1. It is observed that incorporation of Cl at ortho, NO2 at ortho and meta, and NH2 group at para position of benzene ring that is directly attached to oxadiazole ring containing benzamidine analogues 5b, 5d, and 5e, enhances their inhibitory potential against P. gingivalis in comparison to compound 1. However, incorporation of Cl at para position of benzene ring in compound 5a offers activity similar to compound 1. Whereas incorporation of NO2 group at para position on benzene ring attached to triazole containing benzamidine analogue 6b further enhances their inhibitory potential against P. gingivalis in comparison to compound 1. The resultant MBC data of compound 5b, 5d, 5e, and 6b given in Table 2, revealed their equipotent MBC value when compared with parent compound 1. As per the resultant cyto-toxicity study data given in Table 3 and Figure 2, the compounds 5a, 5c-e, and 6b can be considered as nontoxic and safer alternatives for the treatment of PD. However, compound 5a containing para substituted Cl on benzene and compound 6a containing phenoxy group at meta position of benzene offers lesser safety when compared with other synthesized compounds. Free hydroxy group is not essential for the activity, conversion into ether linkage further enhances the inhibitory activity. Based on the MIC, MBC, and cytotoxicity data it is recommended that these synthesized compounds should be further subjected to preclinical and clinical evaluation.
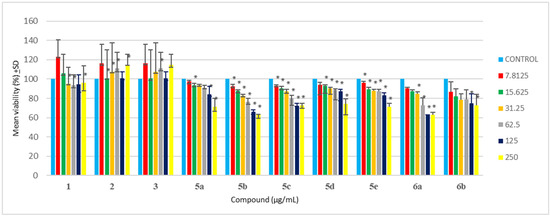
Figure 2.
Cytotoxicity analysis of synthesized compounds against HEK-293 cells (Where, * p < 0.05).
4. Conclusions
In conclusion, compounds were successfully synthesized by the condensation of hydrazides with different aromatic benzoic acids, and cyclo-condensation of a triazole with imine Schiff bases. The synthesized compounds were further confirmed based on sharp melting point, single spot TLC pattern, and spectral data. All synthesized compounds displayed minimum to high inhibition activity against tested pathogens. Addiontally, all the synthesized compounds showed less cytotoxicity when tested against HEK-293 cells. Despite the present study showing the ability of benzamidine derivatives to inhibit the growth of periodontal pathogens with an absence of cytotoxicity of some of these derivatives, additional in-vivo and clinical studies are required to establish their safety and efficacy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12020306/s1, Figure S1: FTIR spectrum of compound 5a; Figure S2: 1H-NMR spectrum of compound 5a; Figure S3: 13C-NMR spectrum of compound 5a; Figure S4: MASS spectrum of compound 5a; Figure S5: FTIR spectrum of compound 6a; Figure S6: 1H-NMR spectrum of compound 6a; Figure S7a: 13C-NMR spectrum of compound 6a; Figure S7b: 13C-NMR spectrum of compound 6a; Figure S7c: 13C-NMR spectrum of compound 6a; Figure S4: MASS spectrum of compound 6a.
Author Contributions
Conceptualization, N.K.F., S.F., M.R. and P.L.; methodology, M.A.S., R.K., N.K.F., S.F., M.R. and P.L.; investigation, M.A.S., R.K., N.K.F., S.F., M.R. and P.L.; resources, M.A.S., R.K., N.K.F., S.F., M.R. and P.L.; data curation, M.A.S., R.K., N.K.F., S.F., M.R. and P.L.; writing—original draft preparation, M.A.S., R.K., N.K.F., S.F., M.R. and P.L.; writing—review and editing, M.A.S., N.K.F., S.F., M.R. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education (MOHE) Malaysia, grant number FRGS/1/2018/SKK14/AIMST/01/1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank the Ministry of Higher Education (Ref: FRGS/1/2018/SKK14/AIMST/01/1) and AIMST University for financial support and assistance to successfully complete this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dannewitz, B.; Holtfreter, B.; Eickholz, P. Periodontitis-therapy of a widespread disease. Bundesgesundheitsblatt 2021, 64, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. J. Periodontol. 2000, 62, 59–94. [Google Scholar] [CrossRef]
- Bui, F.Q.; Almeida-da-Silva, L.C.; Huynh, B.; Trinh, A.; Liu, J.; Woodward, J.; Asadi, H.; Ojcius, D.M. Association between periodontal pathogens and systemic disease. Biomed. J. 2019, 42, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Lyra, P.; Botelho, J.; Machado, V.; Rota, S.; Walker, R.; Staunton, J.; Proença, L.; Chaudhuri, K.R.; Mendes, J.J. Self-reported periodontitis and C-reactive protein in Parkinson’s disease: A cross-sectional study of two American cohorts. NPJ Park. Dis. 2022, 8, 40. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia. 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Parra-Torres, V.; Melgar-Rodríguez, S.; Muñoz-Manríquez, C.; Sanhueza, B.; Cafferata, E.A.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Periodontal bacteria in the brain-Implication for Alzheimer’s disease: A systematic review. Oral Dis. 2023, 29, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Serni, L.; Caroti, L.; Barbato, L.; Nieri, M.; Serni, S.; Cirami, C.L.; Cairo, F. Association between chronic kidney disease and periodontitis. A systematic review and metanalysis. Oral Dis. 2023, 29, 40–50. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Prim. 2017, 3, 17038. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar]
- Chigasaki, O.; Aoyama, N.; Sasaki, Y.; Takeuchi, Y.; Mizutani, K.; Ikeda, Y.; Gokyu, M.; Umeda, M.; Izumi, Y.; Iwata, T.; et al. Porphyromonas gingivalis, the most influential pathogen in red-complex bacteria: A cross-sectional study on the relationship between bacterial count and clinical periodontal status in Japan. J. Periodontol. 2021, 92, 1719–1729. [Google Scholar] [CrossRef]
- Sa’ad, M.A.; Kavitha, R.; Fuloria, S.; Fuloria, N.K.; Ravichandran, M.; Lalitha, P. Synthesis, Characterization and Biological Evaluation of Novel Benzamidine Derivatives: Newer Antibiotics for Periodontitis Treatment. Antibiotics 2022, 11, 207. [Google Scholar] [CrossRef]
- Chopra, A.; Radhakrishnan, R.; Sharma, M. Porphyromonas gingivalis and adverse pregnancy outcomes: A review on its intricate pathogenic mechanisms. Crit. Rev. Microbiol. 2020, 46, 213–236. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Zhang, C.; Yu, N.; Lu, Z.; Zhang, S.; Li, Y.; Li, Q.; Liu, J.; Liu, D.; et al. Porphyromonas gingivalis exacerbates ulcerative colitis via Porphyromonas gingivalis peptidylarginine deiminase. Int. J. Oral Sci. 2021, 13, 31. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Gershovich, E. The prevention of periodontal disease-An overview. Periodontol. 2000. 2020, 84, 9–13. [Google Scholar] [CrossRef]
- Slots, J. Primer on etiology and treatment of progressive/severe periodontitis: A systemic health perspective. Periodontology 2000 2020, 83, 272–276. [Google Scholar] [CrossRef]
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; Figueiredo, L.C.; de Malheiros, Z.; Stewart, B.; Feres, M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz. Oral Res. 2020, 34 (Suppl. 1), e026. [Google Scholar] [CrossRef]
- Di Domenico, G.L.; Minoli, M.; Discepoli, N.; Ambrosi, A.; de Sanctis, M. Effectiveness of periodontal treatment to improve glycemic control: An umbrella review. Acta Diabetol. 2023, 60, 101–113. [Google Scholar] [CrossRef]
- Pretzl, B.; Sälzer, S.; Ehmke, B.; Schlagenhauf, U.; Dannewitz, B.; Dommisch, H.; Eickholz, P.; Jockel-Schneider, Y. Administration of systemic antibiotics during non-surgical periodontal therapy-a consensus report. Clin. Oral Investig. 2019, 23, 3073–3085. [Google Scholar] [CrossRef]
- Kaufmann, M.; Lenherr, P.; Walter, C.; Thurnheer, T.; Attin, T.; Wiedemeier, D.B.; Schmidlin, P.R. Comparing the Antimicrobial In Vitro Efficacy of Amoxicillin/Metronidazole against Azithromycin—A Systematic Review. Dent. J. 2018, 6, 59. [Google Scholar] [CrossRef]
- Ardila, C.-M.; Bedoya-García, J.A. Antimicrobial resistance of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia in periodontitis patients. J. Glob. Antimicrob. Resist. 2020, 22, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Kulik, E.M.; Thurnheer, T.; Karygianni, L.; Walter, C.; Sculean, A.; Eick, S. Antibiotic Susceptibility Patterns of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis Strains from Different Decades. Antibiotics 2019, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Miao, Y.S.; Fu, Y.; Li, X.T.; Yu, S.J. Attenuation of Porphyromonas gingivalis oral infection by α-amylase and pentamidine. Mol. Med. Rep. 2015, 12, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Patil, P.C.; Luzzio, F.A.; Demuth, D.R. In Vitro and In Vivo Activity of Peptidomimetic Compounds That Target the Periodontal Pathogen Porphyromonas gingivalis. Antimicrob. Agents Chemother. 2018, 62, e00400–e00418. [Google Scholar] [CrossRef]
- Obradović, D.; Nikolić, S.; Milenković, I.; Milenković, M.; Jovanović, P.; Savić, V.; Roller, A.; Crnogorac, M.Đ.; Stanojković, T.; Grgurić-Šipka, S. Synthesis, characterization, antimicrobial and cytotoxic activity of novel half-sandwich Ru (II) arene complexes with benzoylthiourea derivatives. J. Inorg. Biochem. 2020, 210, 111164. [Google Scholar] [CrossRef]
- Tipparaju, S.K.; Joyasawal, S.; Pieroni, M.; Kaiser, M.; Brun, R.; Kozikowski, A.P. In Pursuit of Natural Product Leads: Synthesis and Biological Evaluation of 2-[3-hydroxy-2-[(3-hydroxypyridine-2-carbonyl) amino] phenyl] benzoxazole-4-carboxylic acid (A-33853) and Its Analogues: Discovery of N-(2-Benzoxazol-2-ylphenyl) benzamides as Novel Antileishmanial Chemotypes. J. Med. Chem. 2008, 51, 7344–7347. [Google Scholar]
- Fröhlich, E.; Kantyka, T.; Plaza, K.; Schmidt, K.H.; Pfister, W.; Potempa, J.; Eick, S. Benzamidine derivatives inhibit the virulence of Porphyromonas gingivalis. Mol. Oral Microbiol. 2013, 28, 192–203. [Google Scholar] [CrossRef]
- Desai, N.; Monapara, J.; Jethawa, A.; Khedkar, V.; Shingate, B. Oxadiazole: A highly versatile scaffold in drug discovery. Arch. Pharm. 2022, 355, 2200123. [Google Scholar] [CrossRef]
- Patil, P.C.; Tan, J.; Demuth, D.R.; Luzzio, F.A. 1,2,3-Triazole-based inhibitors of Porphyromonas gingivalis adherence to oral streptococci and biofilm formation. Bioorg. Med. Chem. 2016, 24, 5410–5417. [Google Scholar] [CrossRef]
- Patil, P.C.; Tan, J.; Demuth, D.R.; Luzzio, F.A. “Second-generation” 1,2,3-triazole-based inhibitors of Porphyromonas gingivalis adherence to oral streptococci and biofilm formation. MedChemComm 2019, 10, 268–279. [Google Scholar] [CrossRef]
- Glomb, T.; Świątek, P. Antimicrobial Activity of 1,3,4-Oxadiazole Derivatives. Int. J. Mol. Sci. 2021, 22, 6979. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, N.K.; Fuloria, S.; Sathasivam, K.; Karupiah, S. Synthesis and discerning of antimicrobial potential of novel oxadiazole derivatives of chloroxylenol moiety. Acta Pol. Pharm. 2017, 74, 1125-30. [Google Scholar]
- Husain, A.; Varshney, M.M.; Parcha, V.; Ahmad, A.; Khan, S.A. Synthesis and biological evaluation of new hydrazide-Schiff bases. Bangladesh J. Pharmacol. 2015, 10, 23381. [Google Scholar] [CrossRef]
- Taha, M.; Imran, S.; Alomari, M.; Rahim, F.; Wadood, A.; Mosaddik, A.; Uddin, N.; Gollapalli, M.; Alqahtani, M.A.; Bamarouf, Y.A. Synthesis of oxadiazole-coupled-thiadiazole derivatives as a potent β-glucuronidase inhibitors and their molecular docking study. Bioorg. Med. Chem. 2019, 27, 3145–3155. [Google Scholar] [CrossRef]
- Fuloria, N.K.; Singh, V.; Shaharyar, M.; Ali, M. Synthesis and Antimicrobial Evaluation of Some New Oxadiazoles Derived from Phenylpropionohydrazides. Molecules 2009, 14, 1898–1903. [Google Scholar] [CrossRef]
- Yang, S.; Ren, C.-L.; Ma, T.-Y.; Zou, W.-Q.; Dai, L.; Tian, X.-Y.; Liu, X.-H.; Tan, C.-X. 1,2,4-Oxadiazole-Based Bio-Isosteres of Benzamides: Synthesis, Biological Activity and Toxicity to Zebrafish Embryo. Int. J. Mol. Sci. 2021, 22, 2367. [Google Scholar] [CrossRef] [PubMed]
- Potempa, J.; Nguyen, K. Purification and Characterization of Gingipains. Curr. Protoc. Protein Sci. 2017, 49, 21.20.1–21.20.27. [Google Scholar] [CrossRef]
- Herrera, H.A.; Franco, O.L.; Fang, L.; Díaz, C.A. Susceptibility of Porphyromonas gingivalis and Streptococcus mutans to Antibacterial Effect from Mammea americana. Adv. Pharmacol. Sci. 2014, 384815. [Google Scholar]
- Shetty, S.; Shetty, R.M.; Rahman, B.; Vannala, V.; Desai, V.; Shetty, S.R. Efficacy of Psidium guajava and Allium sativum Extracts as Antimicrobial Agents against Periodontal Pathogens. J. Pharm. Bioallied Sci. 2020, 12 (Suppl. 1), S589–S594. [Google Scholar] [CrossRef]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 6, 95505. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Estimation of Cell Number by Hemocytometry Counting. Cold Spring Harb. Protoc. 2019, 2019, 97980. [Google Scholar] [CrossRef] [PubMed]
- Ishteyaque, S.; Mishra, A.; Mohapatra, S.; Singh, A.; Bhatta, R.S.; Tadigoppula, N.; Mugale, M.N. In Vitro: Cytotoxicity, Apoptosis and Ameliorative Potential of Lawsonia inermis Extract in Human Lung, Colon and Liver Cancer Cell Line. Cancer Investig. 2020, 38, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Mousa, E.F.; Jassim, I.K. Synthesis and characterization of oxadiazole compounds derived from naproxen. J. Pharm. Sci. Res. 2018, 10, 3036–3040. [Google Scholar]
- Romeo, R.; Giofrè, S.V.; Chiacchio, M.A.; Veltri, L.; Celesti, C.; Iannazzo, D. Synthesis and Biological Evaluation of 2,3,4-Triaryl-1,2,4-oxadiazol-5-ones as p38 MAPK Inhibitors. Molecules 2021, 26, 1745. [Google Scholar] [CrossRef] [PubMed]
- Ayah, A.; Hameed, F.; Hassan, F.X. Synthesis, Characterization and Antioxidant Activity of Some 4-Amino-5-Phenyl-4h-1, 2, 4-Triazole-3-Thiol Derivatives. KMUTNB Int. J. Appl. Sci. Technol. 2014, 4, 202–211. [Google Scholar]
- Beyzaei, H.; Bahabadi, S.E.; Najafi, S.; Sadegh, F.H. Synthesis and Antimicrobial Evaluation of the Potassium Salts of Benzhydrazine Dithiocarbamates. J. Microbiol. Immunol. Infect. 2020, 7, 15–21. [Google Scholar] [CrossRef]
- Jawahar, J.; Sikdar, P.; Antony, S.R.; Byran, G.; Subramanian, G.; Elango, K. Synthesis and biological evaluation of some Schiff bases of [4-(amino)-5-phenyl-4H-1,2,4-triazole-3-thiol]. Pak. J. Pharm. Sci. 2011, 24, 109–112. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for In Vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Bordei, A.T.; Nuță, D.C.; Căproiu, M.T.; Dumitrascu, F.; Zarafu, I.; Ioniță, P.; Bădiceanu, C.D.; Avram, S.; Chifiriuc, M.C.; Bleotu, C.; et al. Design, Synthesis and In Vitro Characterization of Novel Antimicrobial Agents Based on 6-Chloro-9H-carbazol Derivatives and 1,3,4-Oxadiazole Scaffolds. Molecules 2020, 25, 266. [Google Scholar]
- Mansoori, M.H.; Khatik, G.L.; Mishra, V. Synthesis and pharmacological evaluation of pyridinyl-1,3,4-oxadiazolyl-ethanone derivatives as antimicrobial, antifungal and antitubercular agents. Med. Chem. Res. 2018, 27, 744–755. [Google Scholar] [CrossRef]
- Peraman, R.; Varma, R.V.; Reddy, Y.P. Re-engineering nalidixic acid’s chemical scaffold: A step towards the development of novel anti-tubercular and anti-bacterial leads for resistant pathogens. Bioorg. Med. Chem. Lett. 2015, 25, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, Q.; Kim, W.; Tharmalingam, N.; Fuchs, B.B.; Mylonakis, E. Antimicrobial activity of 1,3,4-oxadiazole derivatives against planktonic cells and biofilm of Staphylococcus aureus. Future Med. Chem. 2018, 10, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Zoumpoulakis, P.; Camoutsis, C.; Pairas, G.; Soković, M.; Glamočlija, J.; Potamitis, C.; Pitsas, A. Synthesis of novel sulfonamide-1,2,4-triazoles, 1,3,4-thiadiazoles and 1,3,4-oxadiazoles, as potential antibacterial and antifungal agents. Biological evaluation and conformational analysis studies. Bioorg. Med. Chem. 2012, 20, 1569–1583. [Google Scholar] [CrossRef]
- Tresse, C.; Radigue, R.; Gomes Von Borowski, R.; Thepaut, M.; Hanh Le, H.; Demay, F.; Georgeault, S.; Dhalluin, A.; Trautwetter, A.; Ermel, G.; et al. Synthesis and evaluation of 1,3,4-oxadiazole derivatives for development as broad-spectrum antibiotics. Bioorg. Med. Chem. 2019, 27, 115097. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.H.; Mohamed, A.A.B.; Tawfik, S.S.; Hassan, H.M.; El-Emam, A.A. 1,3,4-Oxadiazole N-Mannich Bases: Synthesis, Antimicrobial, and Anti-Proliferative Activities. Molecules 2021, 26, 2110. [Google Scholar] [CrossRef]
- Aljamali, N.J.; Al-Jammali, Z.S.; Ali, S. Microbial Studying Of (Thiazole, Oxadiazole, Thiadiazole)-Derivatives on Mouth and Teeth Bacteria. Int. J. Med. Res. Pharm. Sci. 2016, 3, 30–39. [Google Scholar]
- Gao, F.; Wang, T.; Xiao, J.; Huang, G. Antibacterial activity study of 1,2,4-triazole derivatives. Eur. J. Med. Chem. 2019, 173, 274–281. [Google Scholar] [CrossRef]
- Deodware, S.A.; Barache, U.B.; Chanshetti, U.B.; Sathe, D.J.; Panchsheela, A.U.; Gaikwad, S.H.; Prasad, K.S. Newly synthesized triazole-based Schiff base ligands and their Co (II) complexes as antimicrobial and anticancer agents: Chemical synthesis, structure and biological investigations. Results Chem. 2021, 3, 100162. [Google Scholar] [CrossRef]
- Login, C.C.; Bâldea, I.; Tiperciuc, B.; Benedec, D.; Vodnar, D.C.; Decea, N.; Suciu, Ş. A Novel Thiazolyl Schiff Base: Antibacterial and Antifungal Effects and In Vitro Oxidative Stress Modulation on Human Endothelial Cells. Oxidative Med. Cell. Longev. 2019, 2019, 1607903. [Google Scholar] [CrossRef]
- Jin, R.Y.; Zeng, C.Y.; Liang, X.H.; Sun, X.H.; Liu, Y.F.; Wang, Y.Y.; Zhou, S. Design, synthesis, biological activities and DFT calculation of novel 1,2,4-triazole Schiff base derivatives. Bioorg. Chem. 2018, 80, 253–260. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Kandeel, M.; Pillay, M.; Deb, P.K.; Abdallah, H.H.; Mahomoodally, M.F.; Chopra, D. Anti-Tubercular Properties of 4-Amino-5-(4-Fluoro-3-Phenoxyphenyl)-4H-1,2,4-Triazole-3-Thiol and Its Schiff Bases: Computational Input and Molecular Dynamics. Antibiotics 2020, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Gavara, L.; Verdirosa, F.; Legru, A.; Mercuri, P.S.; Nauton, L.; Sevaille, L.; Feller, G.; Berthomieu, D.; Sannio, F.; Marcoccia, F.; et al. 4-(N-Alkyl- and -Acyl-amino)-1,2,4-triazole-3-thione Analogs as Metallo-β-Lactamase Inhibitors: Impact of 4-Linker on Potency and Spectrum of Inhibition. Biomolecules 2020, 10, 1094. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Zhang, X.; Hao, W.; Wang, S. Two Transition Metal Coordination Polymers: Luminescent Sensing Properties and Treatment Effect on Chronic Periodontitis by Reducing IL-6 and TNF-α Content. J. Fluoresc. 2021, 31, 165–173. [Google Scholar] [CrossRef]
- Levent, S.; Kaya, Ç.B.; Sağlık, B.N.; Osmaniye, D.; Acar, Ç.U.; Atlı, Ö.; Özkay, Y.; Kaplancıklı, Z.A. Synthesis of Oxadiazole-Thiadiazole Hybrids and Their Anticandidal Activity. Molecules 2017, 22, 2004. [Google Scholar] [CrossRef]
- Paruch, K.; Biernasiuk, A.; Berecka-Rycerz, A.; Hordyjewska, A.; Popiołek, Ł. Biological Activity, Lipophilicity and Cytotoxicity of Novel 3-Acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Int. J. Mol. Sci. 2021, 22, 13669. [Google Scholar] [CrossRef]
- Mamatha, S.V.; Belagali, S.L.; Bhat, M. Synthesis, characterisation and evaluation of oxadiazole as promising anticancer agent. SN Appl. Sci. 2020, 2, 882. [Google Scholar] [CrossRef]
- Tiwari, A.; Gopalan Kutty, N.; Kumar, N.; Chaudhary, A.; Vasanth, R.P.; Shenoy, R.; Mallikarjuna, R.C. Synthesis and evaluation of selected 1,3,4-oxadiazole derivatives for In Vitro cytotoxicity and In Vivo anti-tumor activity. Cytotechnology 2016, 68, 2553–2565. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Awolade, P.; Kisten, P.; Cele, N.; Pillay, A.S.; Saha, S.; Kaur, M.; Jonnalagadda, S.B.; Singh, P. Synthesis, Cytotoxicity and Antimicrobial Evaluation of New Coumarin-Tagged β-Lactam Triazole Hybrid. Chem. Biodivers. 2020, 17, 201900462. [Google Scholar] [CrossRef]
- Palakhachane, S.; Ketkaew, Y.; Chuaypen, N.; Sirirak, J.; Boonsombat, J.; Ruchirawat, S.; Tangkijvanich, P.; Suksamrarn, A.; Limpachayaporn, P. Synthesis of sorafenib analogues incorporating a 1,2,3-triazole ring and cytotoxicity towards hepatocellular carcinoma cell lines. Bioorg. Chem. 2021, 112, 104831. [Google Scholar] [CrossRef]
- Zampieri, D.; Cateni, F.; Moneghini, M.; Zacchigna, M.; Laurini, E.; Marson, D.; De Logu, A.; Sanna, A.; Mamolo, M.G. Imidazole and 1,2,4-Triazole-based Derivatives Gifted with Antitubercular Activity: Cytotoxicity and Computational Assessment. Curr. Top. Med. Chem. 2019, 19, 620–632. [Google Scholar] [CrossRef]
- Magalhães, T.F.F.; da Silva, C.M.; Dos Santos, L.B.F.; Santos, D.A.; Silva, L.M.; Fuchs, B.B.; Mylonakis, E.; Martins, C.V.B.; de Resende-Stoianoff, M.A.; de Fátima, Â. Cinnamyl Schiff bases: Synthesis, cytotoxic effects and antifungal activity of clinical interest. Lett. Appl. Microbiol. 2020, 71, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Sumrra, S.H. Synthesis, characterization and biological properties of thienyl derived triazole Schiff bases and their oxovanadium (IV) complexes. J. Enzym. Inhib. Med. Chem. 2012, 27, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lv, Y.F.; Zhang, H.; Hu, J.M.; Li, H.M.; Liu, S.J. Synthesis and Antitumor Activity of 1-Substituted 1,2,3-Triazole-Mollugin Derivatives. Molecules 2021, 26, 3249. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).