Antimicrobial Chemotherapy for Recalcitrant Severe Human Periodontitis
Abstract
1. Introduction
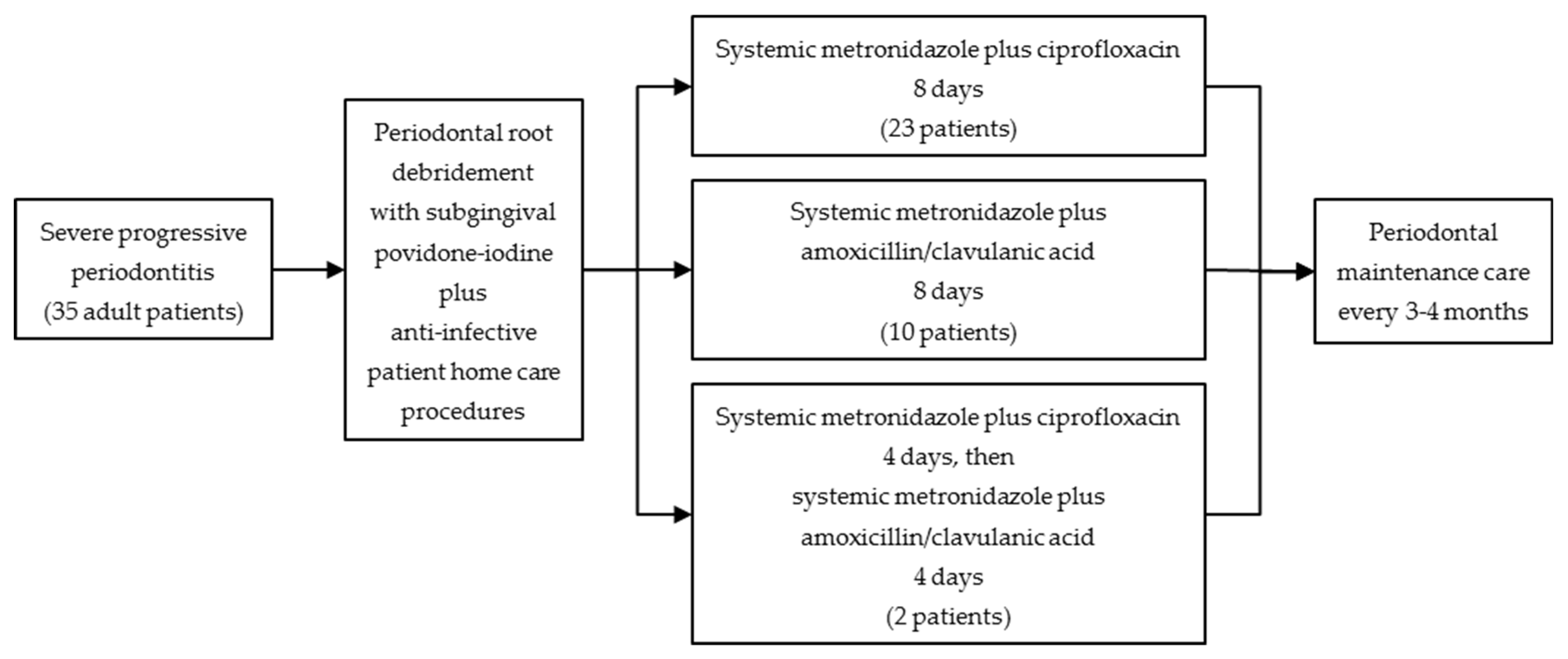
2. Materials and Methods
2.1. Patients
- (1).
- Ongoing periodontal attachment loss and radiographic bone loss on ≥3 teeth despite comprehensive periodontal therapy provided during the previous 1–4 years by periodontists prior to referral to author T.E.R. The previous periodontal therapy included repeated subgingival debridement, surgical flap procedures, oral hygiene instructions, and systematic maintenance care. Eight patients developed periodontal abscesses despite these treatment procedures and were empirically prescribed ≥1 systemic regimens of tetracycline and/or penicillin antibiotics.
- (2).
- (3).
- Persistence, despite previous periodontal therapy, of a high-risk subgingival microbiota associated with progression of periodontitis [57]. This was defined as cultivable detection of either A. actinomycetemcomitans and/or P. gingivalis, and/or cultivable recovery of P. intermedia/nigrescens at ≥2.5%, P. micra at ≥3.0%, C. rectus at ≥2.0%, and/or either Gram-negative enteric rods/pseudomonads, staphylococci, or Candida species at ≥5.0%, of total subgingival viable counts.
- (4).
- Minimal or no supragingival dental plaque or clinically detectable subgingival dental calculus because of previous periodontal therapy.
- (5).
- (6).
- Patient informed consent for periodontal therapy in compliance with the Helsinki Declaration of 1975, as revised in 2000.
- (7).
- Post-treatment outcomes documented at 1 month, 1 year, and 5 years post-treatment.
2.2. Clinical Evaluations
2.3. Microbiological Testing
2.4. Clinical Treatment Protocol
2.5. Data Analysis
3. Results
3.1. Clinical Outcomes
3.2. Microbiological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ting, M.; Contreras, A.; Slots, J. Herpesviruses in localized juvenile periodontitis. J. Periodontal Res. 2000, 35, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Feng, P.; Slots, J. Herpesvirus-bacteria synergistic interaction in periodontitis. Periodontol. 2000 2020, 82, 42–64. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.; Moreno, S.M.; Jaramillo, A.; Pelaez, M.; Duque, A.; Botero, J.E.; Slots, J. Periodontal microbiology in Latin America. Periodontol. 2000 2015, 67, 58–86. [Google Scholar] [CrossRef]
- Rams, T.E.; van Winkelhoff, A.J. Introduction to clinical microbiology for the general dentist. Dent. Clin. N. Am. 2017, 61, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef]
- Colombo, A.P.; Bennet, S.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.E.; et al. Impact of periodontal therapy on the subgingival microbiota of severe periodontitis: Comparison between good responders and individuals with refractory periodontitis using the human oral microbe identification microarray. J. Periodontol. 2012, 83, 1279–1287. [Google Scholar] [CrossRef]
- Nath, S.; Pulikkotil, S.J.; Weyrich, L.; Zilm, P.; Kapellas, K.; Jamieson, L. Effect of periodontal interventions on characteristics of the periodontal microbial profile: A systematic review and meta-analysis. Microorganisms 2022, 10, 1582. [Google Scholar] [CrossRef] [PubMed]
- Christersson, L.A.; Slots, J.; Rosling, B.G.; Genco, R.J. Microbiological and clinical effects of surgical treatment of localized juvenile periodontitis. J. Clin. Periodontol. 1985, 12, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Wolff, L.F.; Liljemark, W.F.; Pihlstrom, B.L.; Schaffer, E.M.; Aeppli, D.M.; Bandt, C.L. Dark-pigmented Bacteroides species in subgingival plaque of adult patients on a rigorous recall program. J. Periodontal Res. 1988, 23, 170–174. [Google Scholar] [CrossRef]
- Gmür, R.; Strub, J.R.; Guggenheim, B. Prevalence of Bacteroides forsythus and Bacteroides gingivalis in subgingival plaque of prosthodontically treated patients on short recall. J. Periodontal Res. 1989, 24, 113–120. [Google Scholar] [CrossRef]
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J. Clin. Periodontol. 1990, 17, 345–350. [Google Scholar] [CrossRef]
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. On the inability of root debridement and periodontal surgery to eliminate Actinobacillus actinomycetemcomitans from periodontal pockets. J. Clin. Periodontol. 1990, 17, 351–355. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Rurenga, P.; Wekema-Mulder, G.J.; Singadji, Z.M.; Rams, T.E. Non-oral gram-negative facultative rods in chronic periodontitis microbiota. Microb. Pathog. 2016, 94, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Feik, D.; Listgarten, M.A.; Slots, J. Peptostreptococcus micros in human periodontitis. Oral Microbiol. Immunol. 1992, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Chaves, E.S.; Jeffcoat, M.K.; Ryerson, C.C.; Snyder, B. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J. Clin. Periodontol. 2000, 27, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Schmid, B.; Rutar, A.; Lang, N.P. Persistence patterns of Porphyromonas gingivalis, Prevotella intermedia/nigrescens, and Actinobacillus actinomycetemcomitans after mechanical therapy of periodontal disease. J. Periodontol. 2000, 71, 14–21. [Google Scholar] [CrossRef]
- Tuan, M.C.; Nowzari, H.; Slots, J. Clinical and microbiologic study of periodontal surgery by means of apically positioned flaps with and without osseous recontouring. Int. J. Periodontics Restor. Dent. 2000, 20, 469–475. [Google Scholar]
- Rhemrev, G.E.; Timmerman, M.F.; Veldkamp, I.; Van Winkelhoff, A.J.; Van der Velden, U. Immediate effect of instrumentation on the subgingival microflora in deep inflamed pockets under strict plaque control. J. Clin. Periodontol. 2006, 33, 42–48. [Google Scholar] [CrossRef]
- Uzel, N.G.; Teles, F.R.; Teles, R.P.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J. Clin. Periodontol. 2011, 38, 612–620. [Google Scholar] [CrossRef]
- McCawley, T.K.; McCawley, M.N.; Rams, T.E. Immediate effects of Laser-Assisted New Attachment Procedure (LANAP) on human periodontitis microbiota. J. Int. Acad. Periodontol. 2018, 20, 163–171. [Google Scholar]
- Slots, J.; Rams, T.E. New views on periodontal microbiota in special patient categories. J. Clin. Periodontol. 1991, 18, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Rams, T.E. Antibiotics in periodontal therapy: Advantages and disadvantages. J. Clin. Periodontol. 1990, 17, 479–493. [Google Scholar] [CrossRef]
- Rams, T.E.; Slots, J. Antibiotics in periodontal therapy: An update. Compendium 1992, 13, 1130–1134. [Google Scholar] [PubMed]
- Fine, D.H. Microbial identification and antibiotic sensitivity testing, an aid for patients refractory to periodontal therapy. A report of 3 cases. J. Clin. Periodontol. 1994, 21, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.B.; Pappas, J.D.; Tyler, K.Z.; Cohen, S.; Gordon, J.M. Antibiotic susceptibilities of periodontal bacteria: In vitro susceptibilities to eight antimicrobial agents. J. Periodontol. 1985, 56, 67–74. [Google Scholar] [CrossRef]
- Pajukanta, R.; Asikainen, S.; Saarela, M.; Alaluusua, S.; Jousimies-Somer, H. In vitro antimicrobial susceptibility of different serotypes of Actinobacillus actinomycetemcomitans. Scand. J. Dent. Res. 1993, 101, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Feik, D.; Mortensen, J.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic susceptibility of periodontal Streptococcus constellatus and Streptococcus intermedius clinical isolates. J. Periodontol. 2014, 85, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Feik, D.; Rams, T.E. Prevalence and antimicrobial susceptibility of Enterobacteriaceae, Pseudomonadaceae and Acinetobacter in human periodontitis. Oral Microbiol. Immunol. 1990, 5, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Feik, D.; Slots, J. Staphylococci in human periodontal diseases. Oral Microbiol. Immunol. 1990, 5, 29–32. [Google Scholar] [CrossRef]
- Rams, T.E.; Feik, D.; Young, V.; Hammond, B.F.; Slots, J. Enterococci in human periodontitis. Oral Microbiol. Immunol. 1992, 7, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Feik, D.; Mortensen, J.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic susceptibility of periodontal Enterococcus faecalis. J. Periodontol. 2013, 84, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Sakellari, D.; Goodson, J.M.; Socransky, S.S.; Kolokotronis, A.; Konstantinidis, A. Concentration of 3 tetracyclines in plasma, gingival crevice fluid and saliva. J. Clin. Periodontol. 2000, 27, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Antibiotic resistance of human periodontal pathogen Parvimonas micra over 10 years. Antibiotics 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Babalola, O.O.; Slots, J. Subgingival occurrence of enteric rods, yeasts and staphylococci after systemic doxycycline therapy. Oral Microbiol. Immunol. 1990, 5, 166–168. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J.; Rams, T.E.; Slots, J. Systemic antibiotic therapy in periodontics. Periodontol. 2000 1996, 10, 45–78. [Google Scholar] [CrossRef]
- Wade, W.G. In-vitro activity of ciprofloxacin and other agents against oral bacteria. J. Antimicrob. Chemother. 1989, 24, 683–687. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Feik, D.; Rams, T.E. In vitro antimicrobial sensitivity of enteric rods and pseudomonads from advanced adult periodontitis. Oral Microbiol. Immunol. 1990, 5, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human chronic periodontitis microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef]
- Akrivopoulou, C.; Green, I.M.; Donos, N.; Nair, S.P.; Ready, D. Aggregatibacter actinomycetemcomitans serotype prevalence and antibiotic resistance in a UK population with periodontitis. J. Glob. Antimicrob. Resist. 2017, 10, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Youngs, D.J.; Keighley, M.R. A randomised prospective controlled study of ciprofloxacin with metronidazole versus amoxicillin/clavulanic acid with metronidazole in the treatment of intra-abdominal infection. Infection 1991, 19, 25–29. [Google Scholar] [CrossRef]
- Solomkin, J.S.; Reinhart, H.H.; Dellinger, E.P.; Bohnen, J.M.; Rotstein, O.D.; Vogel, S.B.; Simms, H.H.; Hill, C.S.; Bjornson, H.S.; Haverstock, D.C.; et al. Results of a randomized trial comparing sequential intravenous/oral treatment with ciprofloxacin plus metronidazole to imipenem/cilastatin for intra-abdominal infections. Ann. Surg. 1996, 223, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.M.; Lipsett, P.A.; Buchman, T.G.; Cheadle, W.G.; Milsom, J.W.; O’Marro, S.; Yellin, A.E.; Jungerwirth, S.; Rochefort, E.V.; Haverstock, D.C.; et al. Comparison of intravenous/oral ciprofloxacin plus metronidazole versus piperacillin/tazobactam in the treatment of complicated intraabdominal infections. Ann. Surg. 2000, 232, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Wacha, H.; Warren, B.; Bassaris, H.; Nikolaidis, P.; Intra-Abdominal Infections Study Group. Comparison of sequential intravenous/oral ciprofloxacin plus metronidazole with intravenous ceftriaxone plus metronidazole for treatment of complicated intra-abdominal infections. Surg. Infect. 2006, 7, 341–354. [Google Scholar] [CrossRef]
- Matthaiou, D.K.; Peppas, G.; Bliziotis, I.A.; Falagas, M.E. Ciprofloxacin/metronidazole versus beta-lactam-based treatment of intra-abdominal infections: A meta-analysis of comparative trials. Int. J. Antimicrob. Agents 2006, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Whiting, J.L.; Cheng, N.; Chow, A.W. Interactions of ciprofloxacin with clindamycin, metronidazole, cefoxitin, cefotaxime, and mezlocillin against gram-positive and gram-negative anaerobic bacteria. Antimicrob. Agents Chemother. 1987, 31, 1379–1382. [Google Scholar] [CrossRef]
- Boeckh, M.; Lode, H.; Deppermann, K.M.; Grineisen, S.; Shokry, F.; Held, R.; Wernicke, K.; Koeppe, P.; Wagner, J.; Krasemann, C. Pharmacokinetics and serum bactericidal activities of quinolones in combination with clindamycin, metronidazole, and ornidazole. Antimicrob. Agents Chemother. 1990, 34, 2407–2414. [Google Scholar] [CrossRef]
- Kraseman, C. Sensitivity of Bacteroidaceae, Peptococcaceae, and clostridia to ciprofloxacin with seven other antibiotics. Chemioterapia 1985, 2 (Suppl. 4), 392–393. [Google Scholar]
- Werk, R.; Schneider, L. Ciprofloxacin in combination with metronidazole. Infection 1988, 16, 257–260. [Google Scholar] [CrossRef]
- Pavicić, M.J.; van Winkelhoff, A.J.; de Graaff, J. In vitro susceptibilities of Actinobacillus actinomycetemcomitans to a number of antimicrobial combinations. Antimicrob. Agents Chemother. 1992, 36, 2634–2638. [Google Scholar] [CrossRef]
- Pavicić, M.J.; van Winkelhoff, A.J.; de Graaff, J. Synergistic effects between amoxicillin, metronidazole, and the hydroxymetabolite of metronidazole against Actinobacillus actinomycetemcomitans. Antimicrob. Agents Chemother. 1991, 35, 961–966. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Teles, R.P.; Socransky, S.S. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontol. 2000 2006, 42, 219–258. [Google Scholar] [CrossRef]
- Dakic, A.; Boillot, A.; Colliot, C.; Carra, M.C.; Czernichow, S.; Bouchard, P. Detection of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans after systemic administration of amoxicillin plus metronidazole as an adjunct to non-surgical periodontal therapy: A systematic review and meta-analysis. Front. Microbiol. 2016, 7, 1277. [Google Scholar] [CrossRef]
- Feres, M.; Retamal-Valdes, B.; Fermiano, D.; Faveri, M.; Figueiredo, L.C.; Mayer, M.P.A.; Lee, J.J.; Bittinger, K.; Teles, F. Microbiome changes in young periodontitis patients treated with adjunctive metronidazole and amoxicillin. J. Periodontol. 2021, 92, 467–478. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Rams, T.E.; Listgarten, M.A.; Slots, J. Utility of radiographic crestal lamina dura for predicting periodontitis disease-activity. J. Clin. Periodontol. 1994, 21, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Listgarten, M.A.; Slots, J. Radiographic alveolar bone morphology and progressive periodontitis. J. Periodontol. 2018, 89, 424–430. [Google Scholar] [CrossRef]
- Rams, T.E.; Listgarten, M.A.; Slots, J. Utility of 5 major putative periodontal pathogens and selected clinical parameters to predict periodontal breakdown in patients on maintenance care. J. Clin. Periodontol. 1996, 23, 346–354. [Google Scholar] [CrossRef]
- Hoang, T.; Jorgensen, M.G.; Keim, R.G.; Pattison, A.M.; Slots, J. Povidone-iodine as a periodontal pocket disinfectant. J. Periodontal Res. 2003, 38, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Slots, J. Comparison of two pressure-sensitive periodontal probes and a manual periodontal probe in shallow and deep pockets. Int. J. Periodontics Restor. Dent. 1993, 13, 520–529. [Google Scholar]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Rams, T.E.; Roberts, T.W.; Slots, J. Evaluation of peri-implant sulcular temperature. J. Clin. Periodontol. 1993, 20, 465–468. [Google Scholar] [CrossRef]
- Kung, R.T.; Ochs, B.; Goodson, J.M. Temperature as a periodontal diagnostic. J. Clin. Periodontol. 1990, 17, 557–563. [Google Scholar]
- Maiden, M.F.; Tanner, A.C.; Macuch, P.J.; Murray, L.; Kent, R.L., Jr. Subgingival temperature and microbiota in initial periodontitis. J. Clin. Periodontol. 1998, 25, 786–793. [Google Scholar] [CrossRef]
- Dahlén, G.; Pipattanagovit, P.; Rosling, B.; Möller, Å.J.R. A comparison of two transport media for saliva and subgingival samples. Oral Microbiol. Immunol. 1993, 8, 375–382. [Google Scholar] [CrossRef]
- Rauch, C.A.; Nichols, J.H. Laboratory accreditation and inspection. Clin. Lab. Med. 2007, 27, 845–858. [Google Scholar] [CrossRef]
- Möller, Å.J.R. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol. Tidskr. 1966, 74, 1–380. [Google Scholar]
- Slots, J.; Rams, T.E.; Listgarten, M.A. Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adult periodontitis. Oral Microbiol. Immunol. 1988, 3, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J. Clin. Microbiol. 1982, 15, 606–609. [Google Scholar] [CrossRef]
- Rams, T.E.; Feik, D.; Slots, J. Campylobacter rectus in human periodontitis. Oral Microbiol. Immunol. 1993, 8, 230–235. [Google Scholar] [CrossRef]
- Slots, J. Rapid identification of important periodontal microorganisms by cultivation. Oral Microbiol. Immunol. 1986, 1, 48–55. [Google Scholar] [CrossRef]
- Rams, T.E.; Sautter, J.D.; Getreu, A.; van Winkelhoff, A.J. Phenotypic identification of Porphyromonas gingivalis validated with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Microb. Pathog. 2016, 94, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Sautter, J.D.; Hsiao, C.Y.; van Winkelhoff, A.J. Phenotypic identification of periodontal Prevotella intermedia/nigrescens group isolates validated by MALDI-TOF mass spectrometry. Anaerobe 2018, 54, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J. Presence of various types of non-haemolytic streptococci in dental plaque and in other sites of the oral cavity in man. Odontol. Revy 1967, 18, 55–74. [Google Scholar] [PubMed]
- Rams, T.E.; Slots, J. Effects of supragingival air-polishing on subgingival periodontitis microbiota. Can. J. Dent. Hyg. 2023, 57. in press. [Google Scholar]
- Rams, T.E.; Lopes, J.A.; Crowley, M.J.; Chialastri, S.M. Comparative in vitro performance of an ODU 11/12 dental explorer and differential reflectometry for detection of subgingival dental calculus. J. Oral Biol. 2017, 4, 5. [Google Scholar]
- Rams, T.E.; Manos, M.P. Comparative in vitro evaluation of WHO periodontal probe and #11/12 dental explorer for subgingival calculus detection. J. Contemp. Dent. Pract. 2021, 22, 13–17. [Google Scholar]
- Rackur, H. New aspects of mechanism of action of povidone-iodine. J. Hosp. Infect. 1985, 6, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Rosling, B.; Hellström, M.K.; Ramberg, P.; Socransky, S.S.; Lindhe, J. The use of PVP-iodine as an adjunct to non-surgical treatment of chronic periodontitis. J. Clin. Periodontol. 2001, 28, 1023–1031. [Google Scholar] [CrossRef]
- Rams, T.E.; Keyes, P.H.; Wright, W.E.; Howard, S.A. Long-term effects of microbiologically modulated periodontal therapy on advanced adult periodontitis. J. Am. Dent. Assoc. 1985, 111, 429–441. [Google Scholar] [CrossRef]
- Keyes, P.H.; Rams, T.E. A rationale for management of periodontal diseases: Rapid identification of microbial “therapeutic targets” with phase-contrast microscopy. J. Am. Dent. Assoc. 1983, 106, 803–812. [Google Scholar] [CrossRef]
- Keyes, P.H.; Rams, T.E. Subgingival microbial and inflammatory cell morphotypes associated with chronic periodontitis progression in treated adults. J. Int. Acad. Periodontol. 2015, 17, 49–57. [Google Scholar] [PubMed]
- Loesche, W.J.; Grossman, N.; Giordano, J. Metronidazole in periodontitis: (IV). The effect of patient compliance on treatment parameters. J. Clin. Periodontol. 1993, 20, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Matuliene, G.; Pjetursson, B.E.; Salvi, G.E.; Schmidlin, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. Influence of residual pockets on progression of periodontitis and tooth loss: Results after 11 years of maintenance. J. Clin. Periodontol. 2008, 35, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Almaghlouth, A.; Cionca, N.; Courvoisier, D.S.; Giannopoulou, C. Differential benefits of amoxicillin-metronidazole in different phases of periodontal therapy in a randomized controlled crossover clinical trial. J. Periodontol. 2015, 86, 367–375. [Google Scholar] [CrossRef]
- Lindhe, J.; Haffaiee, A.D.; Socransky, S.S. Progression of periodontal disease in adult subjects in the absence of periodontal therapy. J. Clin. Periodontol. 1983, 10, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Loos, B.G.; Needleman, I. Endpoints of active periodontal therapy. J. Clin. Periodontol. 2020, 47, 61–71. [Google Scholar] [CrossRef]
- Slots, J. Primer on etiology and treatment of progressive/severe periodontitis: A systemic health perspective. Periodontol. 2000 2020, 83, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Teles, R.P.; Haffajee, A.D.; Socransky, S.S. Microbiological goals of periodontal therapy. Periodontol. 2000 2006, 42, 180–218. [Google Scholar] [CrossRef]
- Slots, J. Concise evaluation and therapeutic guidelines for severe periodontitis: A public health perspective. Periodontol. 2000 2022, 90, 262–265. [Google Scholar] [CrossRef]
- Redanz, S.; Cheng, X.; Giacaman, R.A.; Pfeifer, C.S.; Merritt, J.; Kreth, J. Live and let die: Hydrogen peroxide production by the commensal flora and its role in maintaining a symbiotic microbiome. Mol. Oral Microbiol. 2018, 33, 337–352. [Google Scholar] [CrossRef]
- Tang, Y.L.; Sim, T.S.; Tan, K.S. Oral streptococci subvert the host innate immune response through hydrogen peroxide. Sci. Rep. 2022, 12, 656. [Google Scholar] [CrossRef] [PubMed]
- Graumann, S.J.; Sensat, M.L.; Stoltenberg, J.L. Air polishing: A review of current literature. J. Dent. Hyg. 2013, 87, 173–180. [Google Scholar] [PubMed]
- Checchi, L.; Forteleoni, G.; Pelliccioni, G.A.; Loriga, G. Plaque removal with variable instrumentation. J. Clin. Periodontol. 1997, 24, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Slots, J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol. 2000 1996, 10, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Kornman, K.S.; Newman, M.G.; Moore, D.J.; Singer, R.E. The influence of supragingival plaque control on clinical and microbial outcomes following the use of antibiotics for the treatment of periodontitis. J. Periodontol. 1994, 65, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Mdala, I.; Olsen, I.; Haffajee, A.D.; Socransky, S.S.; de Blasio, B.F.; Thoresen, M. Multilevel analysis of bacterial counts from chronic periodontitis after root planing/scaling, surgery, and systemic and local antibiotics: 2-year results. J. Oral Microbiol. 2013, 5, 20939. [Google Scholar] [CrossRef] [PubMed]
- Lobene, R.R.; Soparkar, P.M.; Hein, J.W.; Quigley, G.A. A study of the effects of antiseptic agents and a pulsating irrigating device on plaque and gingivitis. J. Periodontol. 1972, 43, 564–568. [Google Scholar] [CrossRef]
- Jurczyk, K.; Nietzsche, S.; Ender, C.; Sculean, A.; Eick, S. In-vitro activity of sodium-hypochlorite gel on bacteria associated with periodontitis. Clin. Oral Investig. 2016, 20, 2165–2173. [Google Scholar] [CrossRef]
- Pardo-Castaño, C.; Vásquez, D.; Bolaños, G.; Contreras, A. Strong antimicrobial activity of collinin and isocollinin against periodontal and superinfectant pathogens in vitro. Anaerobe 2020, 62, 102163. [Google Scholar] [CrossRef]
- Galván, M.; Gonzalez, S.; Cohen, C.L.; Alonaizan, F.A.; Chen, C.T.; Rich, S.K.; Slots, J. Periodontal effects of 0.25% sodium hypochlorite twice-weekly oral rinse. A pilot study. J. Periodontal Res. 2014, 49, 696–702. [Google Scholar] [CrossRef]
- Califf, K.J.; Schwarzberg-Lipson, K.; Garg, N.; Gibbons, S.M.; Caporaso, J.G.; Slots, J.; Cohen, C.; Dorrestein, P.C.; Kelley, S.T. Multi-omics analysis of periodontal pocket microbial communities pre- and posttreatment. mSystems 2017, 2, e00016-17. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, S.; Cohen, C.L.; Galván, M.; Alonaizan, F.A.; Rich, S.K.; Slots, J. Gingival bleeding on probing: Relationship to change in periodontal pocket depth and effect of sodium hypochlorite oral rinse. J. Periodontal Res. 2015, 50, 397–402. [Google Scholar] [CrossRef]
- American Dental Association. Accepted Dental Therapeutics, 40th ed.; American Dental Association: Chicago, IL, USA, 1984; p. 326. [Google Scholar]
- Rams, T.E.; Keyes, P.H.; Jenson, A.B. Morphological effects of inorganic salts, chloramine-T, and citric acid on subgingival plaque bacteria. Quintessence Int. Dent. Dig. 1984, 15, 835–844. [Google Scholar]
- Newbrun, E.; Hoover, C.I.; Ryder, M.I. Bactericidal action of bicarbonate ion on selected periodontal pathogenic microorganisms. J. Periodontol. 1984, 55, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, K.T.; Genco, R.J.; Wilson, M.E. Antimicrobial properties of hydrogen peroxide and sodium bicarbonate individually and in combination against selected oral, gram-negative, facultative bacteria. J. Dent. Res. 1986, 65, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Drake, D. Antibacterial activity of baking soda. Compend. Contin. Educ. Dent. 1997, 17, S17–S21. [Google Scholar]
- Pratten, J.; Wiecek, J.; Mordan, N.; Lomax, A.; Patel, N.; Spratt, D.; Middleton, A.M. Physical disruption of oral biofilms by sodium bicarbonate: An in vitro study. Int. J. Dent. Hyg. 2016, 14, 209–214. [Google Scholar] [CrossRef]
- Valkenburg, C.; Kashmour, Y.; Dao, A.; Fridus Van der Weijden, G.A.; Slot, D.E. The efficacy of baking soda dentifrice in controlling plaque and gingivitis: A systematic review. Int. J. Dent. Hyg. 2019, 17, 99–116. [Google Scholar] [CrossRef]
- Taschieri, S.; Tumedei, M.; Francetti, L.; Corbella, S.; Del Fabbro, M. Efficacy of 67% sodium bicarbonate toothpaste for plaque and gingivitis control: A systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2022, 22, 101709. [Google Scholar] [CrossRef]
- Sunde, P.T.; Olsen, I.; Enersen, M.; Grinde, B. Patient with severe periodontitis and subgingival Epstein-Barr virus treated with antiviral therapy. J. Clin. Virol. 2008, 42, 176–178. [Google Scholar] [CrossRef]
- Sabeti, M.; Zhong, J.; Hildebrandt, K.; Slots, J. Valacyclovir in pain management of acute apical abscesses: A randomized placebo-controlled double-blind pilot study. J. Endod. 2021, 47, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Low-cost periodontal therapy. Periodontol. 2000 2012, 60, 110–137. [Google Scholar] [CrossRef]
| Treatment Group | |||
|---|---|---|---|
| Clinical Parameter † | I § | II ‡ | III ‖ |
| No. of patients | 23 | 10 | 2 |
| Plaque Index scores: | |||
| Baseline | 0.6 (0.4) | 0.5 (0.3) | 0.6 (0.4) |
| 1 year post-treatment | 0.4 (0.3) | 0.3 (0.2) | 0.4 (0.3) |
| 5 years post-treatment | 0.4 (0.3) | 0.3 (0.2) | 0.4 (0.4) |
| % teeth with PD ≥ 6 mm: | |||
| Baseline | 39.0 (18.9) | 29.6 (23.3) | 40.8 (6.2) |
| 1 year post-treatment | 11.5 (9.9) * | 8.9 (11.3) * | 0 |
| 5 years post-treatment | 9.6 (9.5) * | 9.4 (13.8) * | 0 |
| % sites with BOP: | |||
| Baseline | 30.5 (18.1) | 21.2 (13.8) | 48.5 (16.3) |
| 1 year post-treatment | 2.5 (2.4) * | 2.7 (3.3) * | 1.7 (2.4) |
| 5 years post-treatment | 2.1 (1.8) * | 1.7 (2.3) * | 3.5 (4.9) |
| No. of sites with PD ≥ 5 mm and BOP: | |||
| Baseline | 33.3 (23.4) | 29.0 (21.9) | 22.0 (7.1) |
| 1 year post-treatment | 1.4 (2.4) * | 2.0 (1.5) * | 0.5 (0.7) |
| 5 years post-treatment | 1.2 (1.5) * | 2.5 (2.3) * | 0.5 (0.7) |
| % sites with CAL gain > 2 mm: | |||
| 1 year post-treatment | 4.8 (4.6) | 10.2 (10.7) | 9.9 (0.2) |
| 5 years post-treatment | 5.9 (4.4) | 6.3 (5.4) | 6.8 (0.8) |
| % sites with CAL loss > 2 mm: | |||
| 1 year post-treatment | 0 | 0 | 0 |
| 5 years post-treatment | 0 | 0 | 0 |
| Temperature indicator score | 1.3 (0.4) | 1.9 (0.6) | 1.4 (0.2) |
| Subgingival temperature, °C | 35.6 (0.7) | 36.1 (0.5) | 36.2 (0.4) |
| Subgingival–sublingual temperature differential, °C | −0.4 (0.6) | −0.7 (0.2) | −0.8 (0.3) |
| Post-Systemic Metronidazole Plus Ciprofloxacin | |||
|---|---|---|---|
| Microbial Species | Baseline | 1 Month Post-Treatment | 1 Year Post-Treatment |
| Periodontal Pathogens: | |||
| Aggregatibacter actinomycetemcomitans | 6 (0.2) § | 0 § | 1 (0.1) § |
| Porphyromonas gingivalis | 5 (6.8) | 0 | 1 (4.4) |
| Prevotella intermedia/nigrescens | 9 (11.1) | 4 (3.1) | 4 (3.7) |
| Parvimonas micra | 16 (12.0) | 4 (2.4) | 6 (8.6) |
| Campylobacter rectus | 21 (3.7) | 12 (0.6) | 9 (1.1) |
| Superinfecting Species: | |||
| enteric rods/pseudomonads | 17 (20.3) § | 2 (0.5) § | 3 (0.5) § |
| staphylococci | 0 | 3 (1.4) | 1 (1.1) |
| Enterococcus faecalis | 0 | 0 | 0 |
| Candida species | 4 (0.1) | 4 (3.4) | 3 (0.8) |
| Mean % total recovery of test periodontal pathogens and superinfecting species (SD) † | 22.9 (18.2) | 0.9 (1.7) * | 2.4 (3.2) * |
| No. (%) of patients with high-risk subgingival microbiota ‡ | 23 (100) | 3 (13.0) * | 3 (13.0) * |
| Mean % recovery of viridans streptococci (SD) | 2.6 (1.5) | 24.2 (21.7) * | 27.9 (22.2) * |
| Post-Systemic Metronidazole Plus Amoxicillin/Clavulanic Acid | |||
|---|---|---|---|
| Microbial Species | Baseline | 1 Month Post-Treatment | 1 Year Post-Treatment |
| Periodontal Pathogens: | |||
| Aggregatibacter actinomycetemcomitans | 7 (2.9) § | 0 § | 1 (0.01) § |
| Porphyromonas gingivalis | 1 (14.3) | 0 | 0 |
| Prevotella intermedia/nigrescens | 8 (6.2) | 0 | 3 (2.3) |
| Parvimonas micra | 7 (9.6) | 4 (3.2) | 1 (21.6) |
| Campylobacter rectus | 9 (2.7) | 4 (0.4) | 4 (2.5) |
| Superinfecting Species: | |||
| enteric rods/pseudomonads | 1 (0.5) § | 2 (18.7) § | 2 (7.2) § |
| staphylococci | 0 | 0 | 1 (2.7) |
| Enterococcus faecalis | 0 | 0 | 0 |
| Candida species | 0 | 2 (0.03) | 3 (0.02) |
| Mean % total recovery of test periodontal pathogens and superinfecting species (SD) † | 18.2 (10.6) | 1.3 (2.4) * | 3.5 (3.1) * |
| No. (%) of patients with high-risk subgingival microbiota ‡ | 10 (100) | 2 (20.0) * | 4 (40.0) * |
| Mean % recovery of viridans streptococci (SD) | 5.7 (3.4) | 18.5 (14.4) * | 19.1 (22.7) * |
| Post-Systemic Metronidazole Plus Ciprofloxacin, and Metronidazole Plus Amoxicillin/Clavulanic Acid | |||
|---|---|---|---|
| Microbial Species | Baseline | 1 Month Post-Treatment | 1 Year Post-Treatment |
| Periodontal Pathogens: | |||
| Aggregatibacter actinomycetemcomitans | 2 (7.2) § | 0 § | 0 § |
| Porphyromonas gingivalis | 0 | 0 | 0 |
| Prevotella intermedia/nigrescens | 2 (6.8) | 0 | 0 |
| Parvimonas micra | 2 (7.4) | 1 (1.6) | 0 |
| Campylobacter rectus | 2 (18.7) | 1 (0.1) | 1 (1.3) |
| Superinfecting Species: | |||
| enteric rods/pseudomonads | 2 (19.7) § | 0 § | 0 § |
| staphylococci | 0 | 0 | 0 |
| Enterococcus faecalis | 0 | 0 | 0 |
| Candida species | 0 | 0 | 1 (0.01) |
| Mean % total recovery of test periodontal pathogens and superinfecting species (SD) † | 50.7 (19.9) | 0.9 (1.1) | 0.7 (0.9) |
| No. (%) of patients with high-risk subgingival microbiota ‡ | 2 (100) | 0 (0) | 0 (0) |
| Mean % recovery of viridans streptococci (SD) | 6.5 (5.7) | 16.6 (8.4) | 31.0 (19.0) |
| Mean No. of Periodontal Sites/Patient with PD ≥ 5 mm and BOP (SD) | |||
|---|---|---|---|
| High-Risk Subgingival Microbiota ‡ Detected at 1 Month and/or 1 Year Post-Treatment | No. of Patients | 1 Year Post-Treatment | 5 Years Post-Treatment |
| Yes | 12 | 3.2 (2.8) | 2.9 (2.1) |
| No | 23 | 0.7 (0.9) * | 0.8 (1.1) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rams, T.E.; Slots, J. Antimicrobial Chemotherapy for Recalcitrant Severe Human Periodontitis. Antibiotics 2023, 12, 265. https://doi.org/10.3390/antibiotics12020265
Rams TE, Slots J. Antimicrobial Chemotherapy for Recalcitrant Severe Human Periodontitis. Antibiotics. 2023; 12(2):265. https://doi.org/10.3390/antibiotics12020265
Chicago/Turabian StyleRams, Thomas E., and Jørgen Slots. 2023. "Antimicrobial Chemotherapy for Recalcitrant Severe Human Periodontitis" Antibiotics 12, no. 2: 265. https://doi.org/10.3390/antibiotics12020265
APA StyleRams, T. E., & Slots, J. (2023). Antimicrobial Chemotherapy for Recalcitrant Severe Human Periodontitis. Antibiotics, 12(2), 265. https://doi.org/10.3390/antibiotics12020265






