Nanoencapsulation Boosts the Copper-Induced Defense Responses of a Susceptible Coffea arabica Cultivar against Hemileia vastatrix
Abstract
1. Introduction
2. Results
2.1. Characterization of the Nanoparticles and Encapsulation Efficiency of Cu2+ Ions into CS NPs
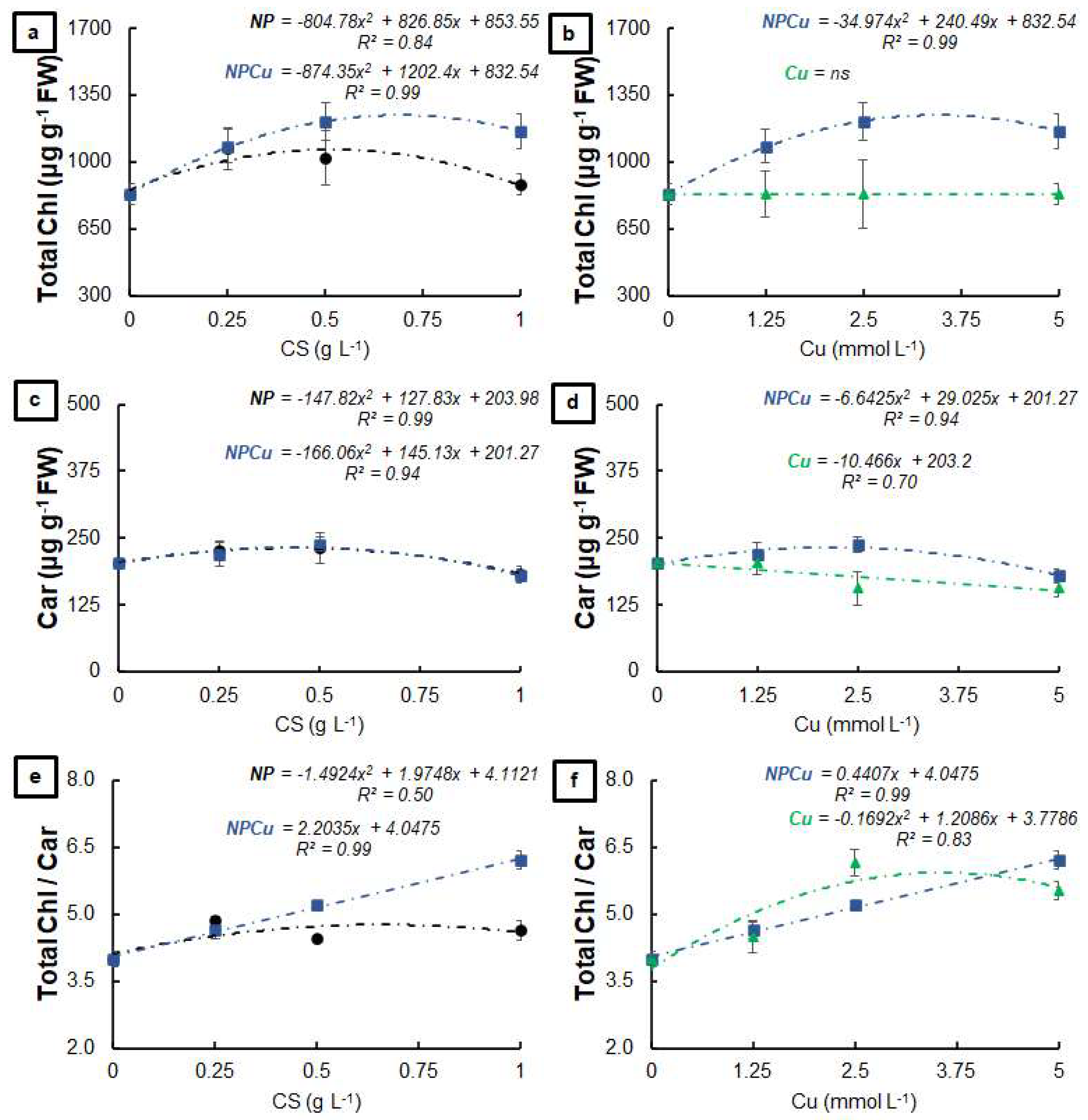
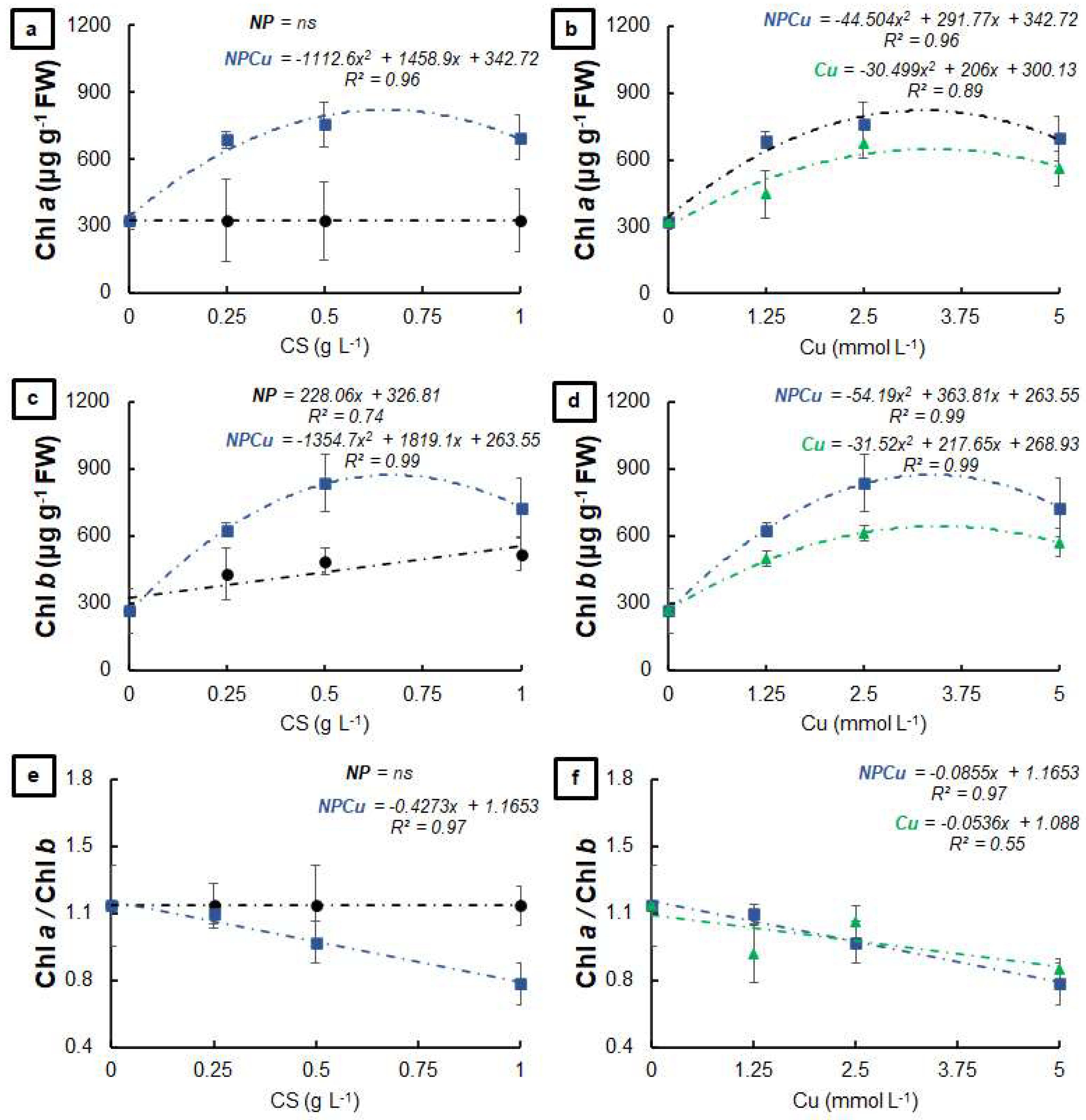
2.2. Experiment to Assess Phytotoxicity
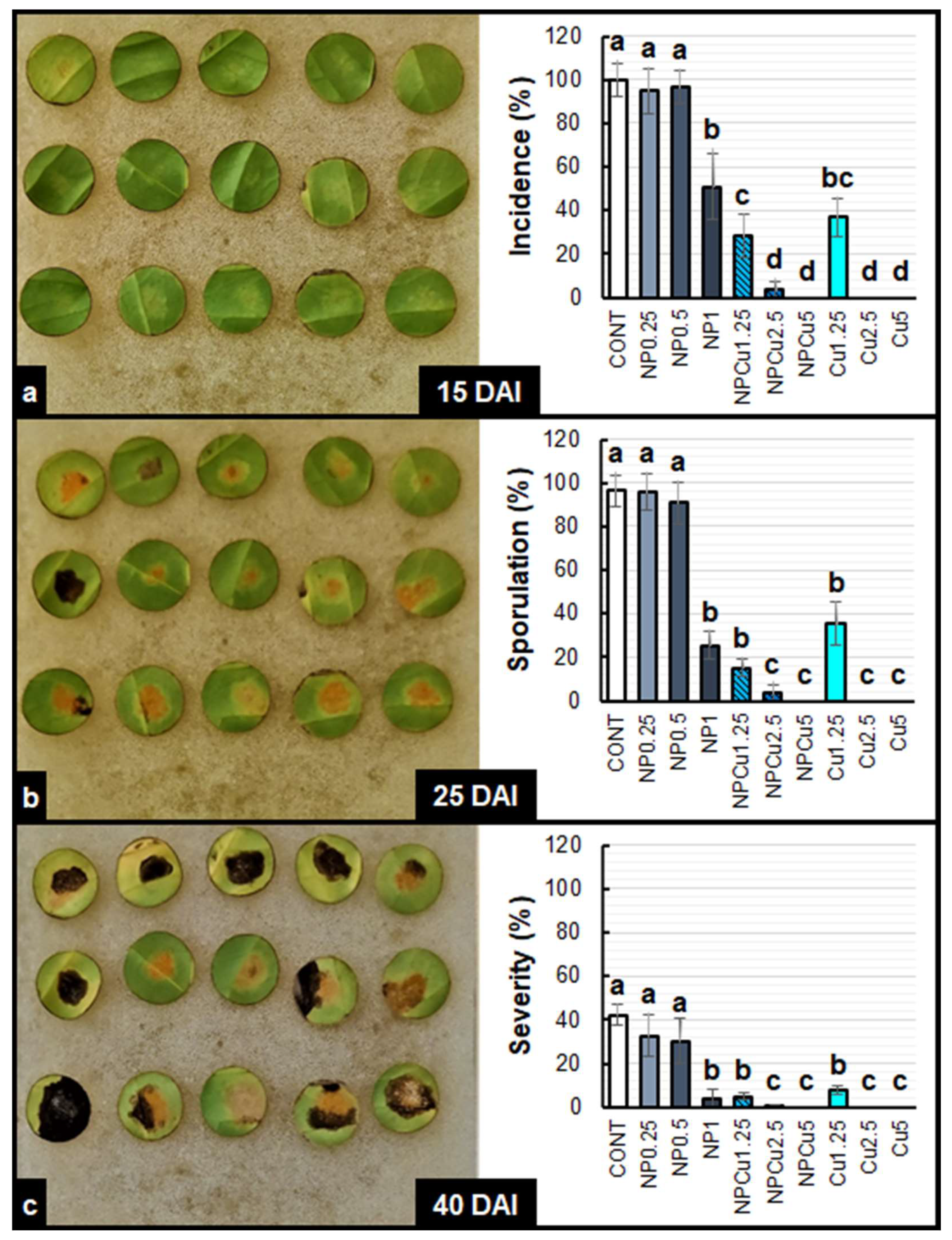
2.3. Evaluation of the Disease Progression
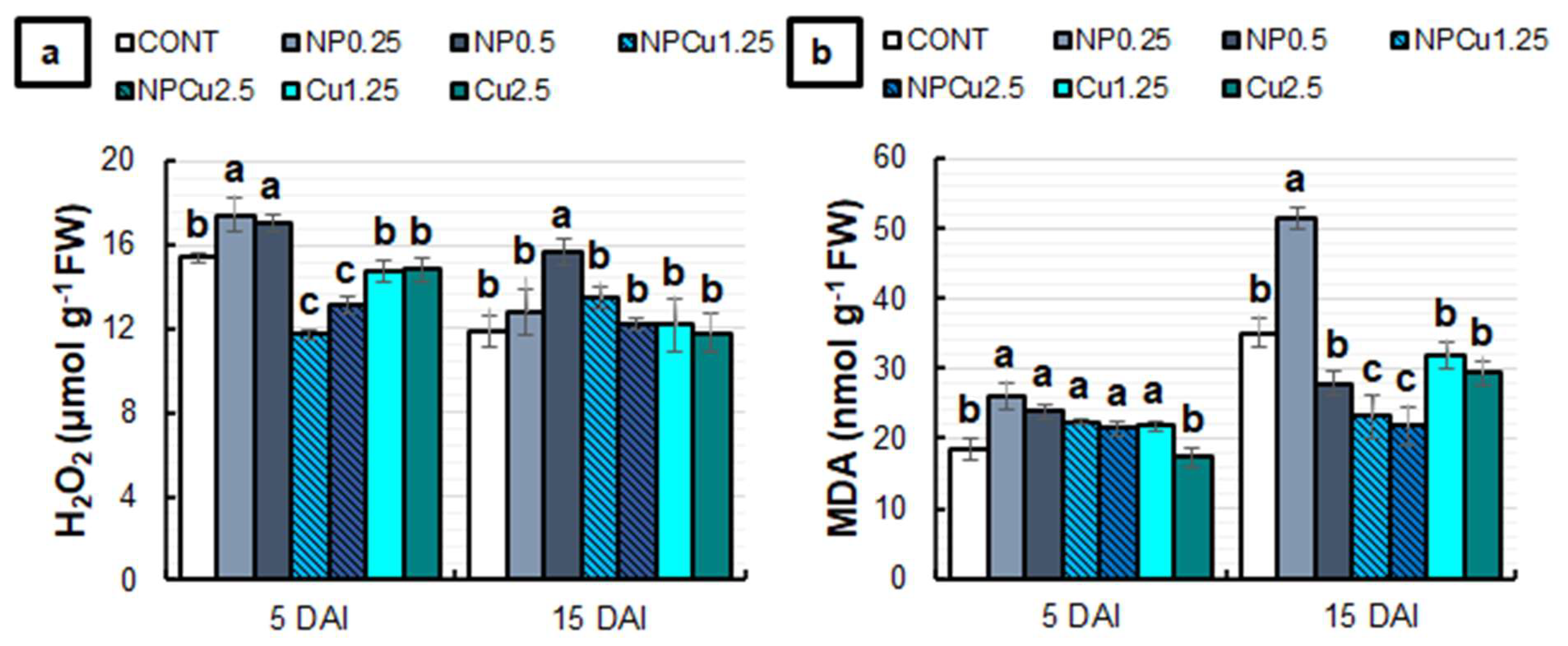
2.4. Evaluation of Oxidative Stress and Antioxidant Response
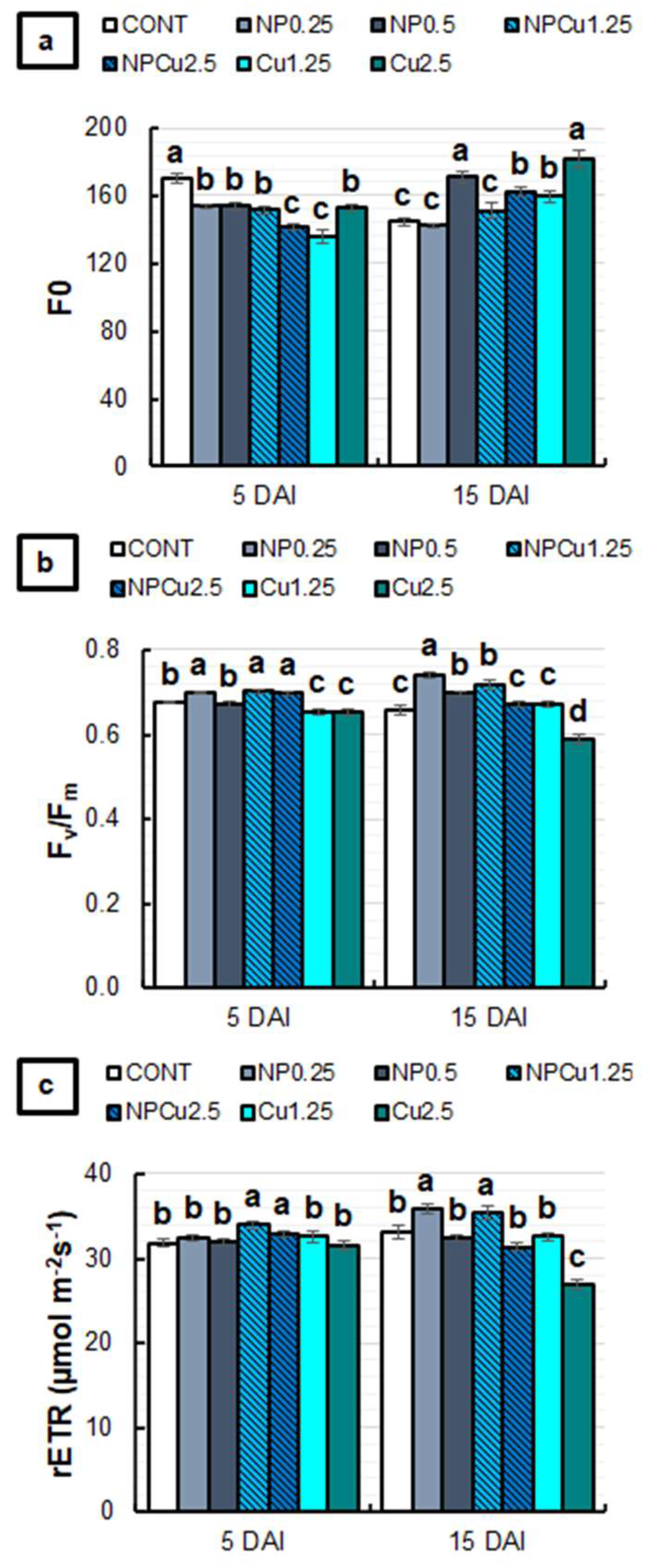
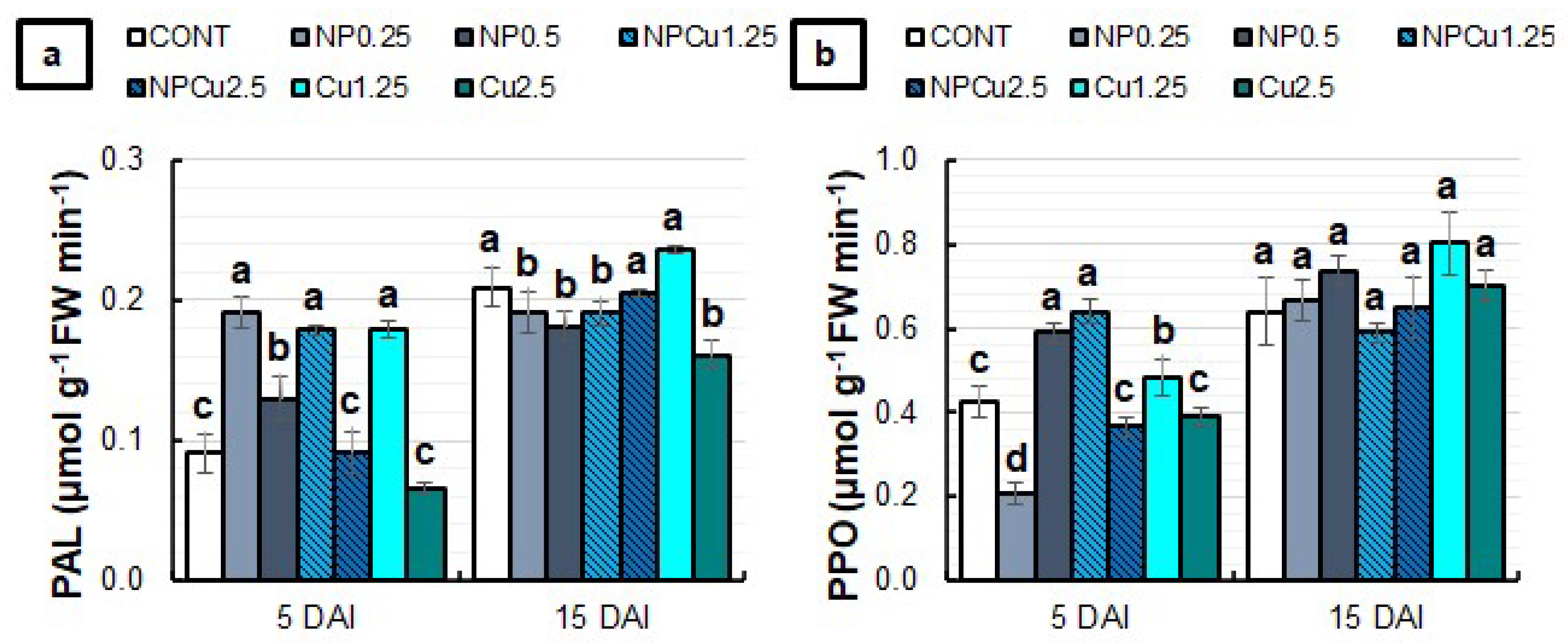
2.5. Evaluation of Chlorophyll a Fluorescence and Activity of PAL and PPO Enzymes
3. Discussion
3.1. Characterization of the Nanoparticles
3.2. Phytotoxicity Assay
3.3. Disease Progression
3.4. Oxidative Stress and Enzymatic Activities
3.5. Photosynthetic Activity
3.6. CS NPs Containing Cu2+ ions as Potential Nanofungicide for Coffee Rust Management
4. Materials and Methods
4.1. Chemicals
4.2. Synthesis of CS NPs Containing Cu2+ Ions
4.3. Characterization of the Nanoparticles and Encapsulation Efficiency of Cu2+ Ions into CS NPs
4.4. Biological Material and Treatments
4.5. Experiment to Assess Phytotoxicity
4.6. Experiment to Evaluate Infection with Hemileia Vastatrix
4.7. Experiment to Evaluate Oxidative Damage and Activity of Antioxidant Enzymes
4.8. Experiment to Evaluate the Fluorescence of Chlorophyll a and the Activity of PAL and PPO Enzymes
4.9. Statistical Analyzes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, L.; Hanika, K.; Visser, R.G.F.; Bai, Y. Improving Pathogen Resistance by Exploiting Plant Susceptibility Genes in Coffee (Coffea spp.). Agronomy 2020, 10, 1928. [Google Scholar] [CrossRef]
- Gichuru, E.; Alwora, G.; Gimase, J.; Kathurima, C. Coffee leaf rust (Hemileia vastatrix) in Kenya—A Review. Agronomy 2021, 11, 2590. [Google Scholar] [CrossRef]
- Soares, A.D.S.; Viera, B.S.; Bezerra, T.A.; Martins, G.D.; Siquieroli, A.C.S. Early detection of coffee leaf rust caused by Hemileia vastatrix using multispectral images. Agronomy 2022, 12, 2911. [Google Scholar] [CrossRef]
- Silva, M.D.C.; Guerra-Guimarães, L.; Diniz, I.; Loureiro, A.; Azinheira, H.; Pereira, A.P.; Tavares, S.; Batista, D.; Várzea, V. An overview of the mechanisms involved in coffee-Hemileia vastatrix interactions: Plant and pathogen perspectives. Agronomy 2022, 12, 326. [Google Scholar] [CrossRef]
- Júnior, J.H.; Zambolim, L.; Aucique-Pérez, C.E.; Resende, R.S.; Rodrigues, F.A. Photosynthetic and antioxidative alterations in coffee leaves caused by epoxiconazole and pyraclostrobin sprays and Hemileia vastatrix infection. Pestic. Biochem. Phys. 2015, 123, 31–39. [Google Scholar] [CrossRef]
- Zambolim, L. Current status and management of coffee leaf rust in Brazil. Trop. Plant Pathol. 2016, 41, 1–8. [Google Scholar] [CrossRef]
- Sera, G.H.; de Carvalho, C.H.S.; Abrahão, J.C.R.; Pozza, E.A.; Matiello, J.B.; de Almeida, S.R.; Bartelega, L.; Botelho, D.M.S. Coffee leaf rust in brazil: Historical events, current situation, and control measures. Agronomy 2022, 12, 496. [Google Scholar] [CrossRef]
- Cunha, R.L.; Mendes, A.N.G.; Chalfoun, S.M. Controle químico da ferrugem do cafeeiro (Coffea arabica L.) e seus efeitos na produção e preservação do enfolhamento. Ciênc. Agrotec. 2004, 28, 990–996. [Google Scholar] [CrossRef]
- Silva, L.F.; Zambolim, L.; Vidigal, A.E.C.; Rubinger, M.M.M. Potencial dos fungicidas do grupo dos ditiocarbimatos no controle da ferrugem do cafeeiro. In Simpósio de Pesquisa dos Cafés do Brasil; Consórcio Pesquisa Café: Vitória, Brazil, 2019; pp. 1–6. [Google Scholar]
- Alves, V.M.; Juliatti, F.C. Fungicidas no manejo da ferrugem da soja, processos fisiológicos e produtividade da cultura. Summa Phytopathol. 2018, 44, 245–251. [Google Scholar] [CrossRef]
- Possa, K.F.; Silva, J.A.G.; Resende, M.L.V.; Tenente, R.; Pinheiro, C.; Chaves, I.; Planchon, S.; Monteiro, A.C.A.; Renaut, J.; Carvalho, M.A.F.; et al. Primary metabolism is distinctly modulated by plant resistance inducers in Coffea arabica leaves infected by Hemileia vastatrix. Front. Plant Sci. 2020, 11, 309. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered chitosan-based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Pereira, A.D.E.S.; Oliveira, H.C.; Fraceto, L.F. Polymeric nanoparticles as an alternative for application of gibberellic acid in sustainable agriculture: A feld study. Sci. Rep. 2019, 9, 7135. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.C.; Gomes, B.C.R.; Pelegrino, M.T.; Seabra, A.B. Nitric oxide-releasing chitosan nanoparticles alleviate the effects of salt stress in maize plants. Nitric Oxide 2016, 61, 10–19. [Google Scholar] [CrossRef]
- Lopes-Oliveira, P.J.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Pimenta, J.A.; Stolf-Moreira, R.; Seabra, A.B.; Oliveira, H.C. Effects of nitric oxide-releasing nanoparticles on neotropical tree seedlings submitted to acclimation under full sun in the nursery. Sci. Rep. 2019, 9, 17371. [Google Scholar] [CrossRef]
- do Carmo, G.C.; Iastrenski, L.F.; Debiasi, T.V.; da Silva, R.C.; Gomes, D.G.; Pelegrino, M.T.; Bianchini, E.; Stolf-Moreira, R.; Pimenta, J.A.; Seabra, A.B.; et al. Nanoencapsulation improves the protective effects of a nitric oxide donor on drought-stressed Heliocarpus popayanensis seedlings. Ecotoxicol. Environ. Saf. 2021, 225, 112713. [Google Scholar] [CrossRef]
- Seabra, A.B.; Silveira, N.M.; Ribeiro, R.V.; Pieretti, J.C.; Barroso, J.B.; Corpas, F.J.; Palma, J.M.; Hancock, J.T.; Petřivalský, M.; Gupta, K.J.; et al. Nitric oxide-releasing nanomaterials: From basic research to potential biotechnological applications in agriculture. New Phytol. 2022, 234, 1119–1125. [Google Scholar] [CrossRef]
- Gomes, D.G.; Debiasi, T.V.; Pelegrino, M.T.; Pereira, R.M.; Ondrasek, G.; Batista, B.L.; Seabra, A.B.; Oliveira, H.C. Soil treatment with nitric oxide-releasing chitosan nanoparticles protects the root system and promotes the growth of soybean plants under copper stress. Plants 2022, 11, 3245. [Google Scholar] [CrossRef] [PubMed]
- Gomes, D.G.; Pelegrino, M.T.; Ferreira, A.S.; Bazzo, J.H.B.; Zucareli, C.; Seabra, A.B.; Oliveira, H.C. Seed priming with copper-loaded chitosan nanoparticles promotes early growth and enzymatic antioxidant defense of maize (Zea mays L.) seedlings. J. Chem. Technol. Biotechnol. 2021, 96, 2176–2184. [Google Scholar] [CrossRef]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Saharan, V.; Kumaraswamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved Food. J. Agric. Food. Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Joshi, A.; Kumari, S.; Kumaraswamy, R.V.; Saharan, V. Preparation of Cu-chitosan nanoparticle and its effect on growth and enzyme activity during seed germination in maize. J. Pharmacogn. Phytochem. 2017, 6, 669–673. [Google Scholar]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Manikandan, A. Application of copper-chitosan nanoparticles stimulate growth and induce resistance in finger millet (Eleusine coracana Gaertn.) plants against blast disease. J. Agric. Food. Chem. 2018, 66, 1784–1790. [Google Scholar] [CrossRef]
- Ruberti, I.; Sessa, G.; Ciolfi, A.; Possenti, M.; Carabelli, M.; Morelli, G. Plant adaptation to dynamically changing environment: The shade avoidance response. Biotechnol. Adv. 2012, 30, 1047–1058. [Google Scholar] [CrossRef]
- Husak, V. Copper and copper-containing pesticides: Metabolism, toxicity and oxidative stress. J. Vasyl Stefanyk Precarpathian Natl. Univ. 2015, 2, 38–50. [Google Scholar] [CrossRef]
- Saharan, V.; Mehrotra, A.; Khatika, R.; Rawal, P.; Sharma, S.S.; Palc, A. Synthesis of chitosan based nanoparticles and their in vitro evaluation against phytopathogenic fungi. Int. J. Biol. Macromol. 2013, 62, 677–683. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The application of selenium and copper nanoparticles modifies the biochemical responses of tomato plants under stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Silva, H.S.A.; Terrasan, C.R.F.; Tozzi, J.P.L.; Melo, I.S.; Bettiol, W. Bactérias endófitas do cafeeiro e a indução de enzimas relacionadas com o controle da ferrugem (Hemileia vastatrix). Trop. Plant Pathol. 2008, 33, 49–54. [Google Scholar] [CrossRef]
- Silva, M.C.; Guerra-Guimarães, L.; Loureiro, A.; Nicole, M.R. Involvement of peroxidases in the coffee resistance to orange rust (Hemileia vastatrix). Physiol. Mol. Plant Pathol. 2008, 72, 29–38. [Google Scholar] [CrossRef]
- Monteiro, A.C.A.; Resende, M.L.V.; Valente, T.C.T.; Junior, P.M.R.; Pereira, V.F.; Costa, J.R.; Silva, J.A.G. Manganese phosphite in coffee defence against Hemileia vastatrix, the coffee rust fungus: Biochemical and molecular analyses. J. Phytopathol. 2016, 164, 1043–1053. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a c3 or c4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef]
- Konrad, M.L.F.; Silva, J.A.B.; Furlani, P.R.; Machado, E.C. Trocas gasosas e fluorescência da clorofila em seis cultivares de cafeeiro sob estresse de alumínio. Bragantia 2005, 64, 339–347. [Google Scholar] [CrossRef]
- Cintra, P.H.N.; Melo, O.F.P.; Menezes, J.O.S.; Padilha, R.C.; Rezende, A.G.; Matos, E.R. Analysis of chlorophyll a fluorescence in coffee seedlings under water stress. Braz. J. Dev. 2020, 6, 27006–27014. [Google Scholar] [CrossRef]
- Silveira, N.M.; Prataviera, P.J.C.; Pieretti, J.C.; Seabra, A.B.; Almeida, R.L.; Machado, E.C.; Ribeiro, R.V. Chitosan-encapsulated nitric oxide donors enhance physiological recovery of sugarcane plants after water deficit. Environ. Exp. Bot. 2021, 190, 104593. [Google Scholar] [CrossRef]
- Eskes, A.B.; Toma-Braghini, M. Assessment methods for resistance to coffee leaf rust (Hemilea vastatrix Berk. & Br.). FAO Plant Prot. Bull. 1981, 29, 56–66. [Google Scholar]
- Sera, T.; Sera, G.H.; Fazuoli, L.C.; Machado, A.C.Z.; Ito, D.S.; Shigueoka, L.H.; Silva, S.A. IPR 100–Rustic dwarf Arabica coffee cultivars with resistance to nematodes Meloidogyne paranaensis e M. incognita. Crop Breed. Appl. Biotechnol. 2017, 17, 175–179. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, 1–8. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Camejo, G.; Wallin, B.; Enojärvi, M. Analyses of oxidation and antioxidants using microtiter plates. In Free Radical and Antioxidants Protocols; Amstrong, D., Ed.; Humana Press: Mölndal, Sweden, 1998; pp. 377–387. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Methods in enzymology. In Catalase In Vitro; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1984; pp. 114–121. [Google Scholar]
- Anderson, M.D.; Prasad, T.K.; Stewart, C.R. Changes in isozyme profiles of catalase, peroxidase and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol. 1995, 109, 1247–1257. [Google Scholar] [CrossRef]
- Peixoto, H.P.P.; Cambraia, J.; San’t ana, R.; Mosquim, P.R.; Moreira, A.M. Aluminum effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Rev. Bras. Fisiol. Veg. 1999, 11, 137–143. [Google Scholar]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant. Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.R-project.org/ (accessed on 21 October 2022).



| Experimental Group | CS (g L−1) | Cu2+ (mmol L−1) |
|---|---|---|
| CONT (Control) | 0.00 | 0.00 |
| NP0.25 | 0.25 | 0.00 |
| NP0.5 | 0.50 | 0.00 |
| NP1 | 1.00 | 0.00 |
| NPCu1.25 | 0.25 | 1.25 |
| NPCu2.5 | 0.50 | 2.50 |
| NPCu5 | 1.00 | 5.00 |
| Cu1.25 | 0.00 | 1.25 |
| Cu2.5 | 0.00 | 2.50 |
| Cu5 | 0.00 | 5.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, D.G.; Sanada, K.; Pieretti, J.C.; Shigueoka, L.H.; Sera, G.H.; Seabra, A.B.; Oliveira, H.C. Nanoencapsulation Boosts the Copper-Induced Defense Responses of a Susceptible Coffea arabica Cultivar against Hemileia vastatrix. Antibiotics 2023, 12, 249. https://doi.org/10.3390/antibiotics12020249
Gomes DG, Sanada K, Pieretti JC, Shigueoka LH, Sera GH, Seabra AB, Oliveira HC. Nanoencapsulation Boosts the Copper-Induced Defense Responses of a Susceptible Coffea arabica Cultivar against Hemileia vastatrix. Antibiotics. 2023; 12(2):249. https://doi.org/10.3390/antibiotics12020249
Chicago/Turabian StyleGomes, Diego G., Karina Sanada, Joana C. Pieretti, Luciana H. Shigueoka, Gustavo H. Sera, Amedea B. Seabra, and Halley C. Oliveira. 2023. "Nanoencapsulation Boosts the Copper-Induced Defense Responses of a Susceptible Coffea arabica Cultivar against Hemileia vastatrix" Antibiotics 12, no. 2: 249. https://doi.org/10.3390/antibiotics12020249
APA StyleGomes, D. G., Sanada, K., Pieretti, J. C., Shigueoka, L. H., Sera, G. H., Seabra, A. B., & Oliveira, H. C. (2023). Nanoencapsulation Boosts the Copper-Induced Defense Responses of a Susceptible Coffea arabica Cultivar against Hemileia vastatrix. Antibiotics, 12(2), 249. https://doi.org/10.3390/antibiotics12020249









