Abstract
Endospore-forming bacteria are ubiquitous, and their endospores can be present in food, in domestic animals, and on contaminated surfaces. Many spore-forming bacteria have been used in biotechnological applications, while others are human pathogens responsible for a wide range of critical clinical infections. Due to their resistant properties, it is challenging to eliminate spores and avoid the reactivation of latent spores that may lead to active infections. Furthermore, endospores play an essential role in the survival, transmission, and pathogenesis of some harmful strains that put human and animal health at risk. Thus, different methods have been applied for their eradication. Nevertheless, natural products are still a significant source for discovering and developing new antibiotics. Moreover, targeting the spore for clinical pathogens such as Clostridioides difficile is essential to disease prevention and therapeutics. These strategies could directly aim at the structural components of the spore or their germination process. This work summarizes the current advances in upcoming strategies and the development of natural products against endospores. This review also intends to highlight future perspectives in research and applications.
1. Endospore
Bacteria have evolved a vast arsenal for endurance and spread even under adverse conditions. Self-preservation mechanisms include scavenging, motility, and chemotaxis systems; cannibalism activation; biofilm formation; and endosporulation [1]. Indeed, there are significant advantages brought by each mechanism, and the combination of many of them, particularly in pathogens, is a critical concern for human health.
Endosporulation (indistinctly used as endosporulation or sporulation) is a complex process of morphological differentiation that culminates in the output of a dormant cell type that is exceptionally resistant to severe environmental conditions, surviving for prolonged periods without water or nutrients [2,3]. This process is highly ancient, perhaps arising near the phylogenetic root of bacteria [4,5].
The endospore lacks metabolic activity, allowing cells to survive in various environments for long periods. Wind, water, living animal hosts, and other mechanisms can spread endospores. This ability is advantageous when colonizing, prospering, and enduring a wide range of environmental conditions. Consequently, spore-forming bacteria (SFBs) occur within different ecological niches, including the soil and gastrointestinal tract of invertebrate and vertebrate animals [4,6,7,8]. However, sporulation is restricted to Firmicutes [4,6,7,8], which include the Bacilli (aerobe) and Clostridia (strict anaerobe) classes [9,10,11].
The complete set of environmental stimuli that induce sporulation is unclear, but some characterized signals include starvation [12], a viral attack [13], or abrupt oxygen change [14]. Once compromised, sporulation is irreversible and highly costly, requiring tight control to guarantee its success. In the most well-characterized sporulation model organisms, B. subtilis and C. difficile, it has been demonstrated that sporulation requires spatial and temporal coordination for the expression of hundreds of genes during spore development. The onset of sporulation is governed by the activity of conserved transcription factor Spo0A [15,16,17,18], but additional sigma factors (σE, σF, σG, and σK) orchestrate this process. Since the complete reconfiguration of the cellular architecture and metabolism is required for the formation of a mature spore, many different genes are activated during this process, including signal transduction systems, sigma factors, transcriptional regulators, metabolic enzymes, and structural proteins. For instance, at least in Bacillus subtilis, sporulation affects the expression of more than 500 genes [19].
A mature spore typically consists of several layers surrounding a core where genetic information is protected. (Figure 1). Among a subset of SFB species, both pathogenic and non-pathogenic, the outermost layer of the spore is the exosporium [20]. However, the typical layers in a spore are the coat, the outer membrane, the cortex, the peptidoglycan cell wall (GCW), and the inner membrane. Lastly, surrounded by all the protective layers is the core, which has a low water content and a high level of dipicolinic acid (DPA), which, together with various divalent cations and small acid-soluble spore proteins, stabilizes the DNA [7,21,22,23].

Figure 1.
The spore is composed of a series of concentric layers contributing to its resistance properties. Abbreviations: Ex, exosporium; Ct, coat; OM outer membrane; Cx, cortex; IM, inner membrane; GCM, germ cell wall.
These layers of the spore have different roles in the resistance to environmental aggression and even in pathogenesis. For example, core dehydration appears to play a role in maintaining spore dormancy and resistance to high temperatures [24,25,26], while the inner membrane is a significant barrier against small molecules, possibly even water, and several potentially damaging chemicals (Figure 1) [27].
Unlike the vegetative cell wall and membrane, the inner membrane of spores has lower lateral mobility and higher viscosity [28,29,30]. In B. subtilis, the spore contains three germination receptors in the inner membrane, which are encoded by the tricistronic operons gerA, gerB, and gerK. These receptors form heterocomplexes composed of three subunits [31,32]. Other proteins located in the inner membrane are the operon-spoVA-encoded proteins, which play a role in the uptake and release of DPA during sporulation (Figure 2).
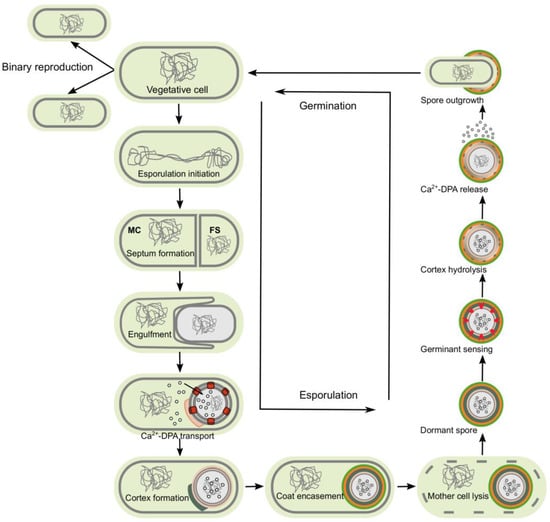
Figure 2.
Sporulation begins once the cell senses unfavorable environmental conditions (e.g., starvation). The first structural event is the formation of a polar septum, which divides the cell into two asymmetric compartments, the mother cell (MC) and the forespore (FS). Next, after the completion of DNA segregation, the mother cell engulfs the forespore. Calcium dipicolinic acid (Ca-DPA) is synthesized in the mother cell and transported into the forespore in exchange for water. The forespore is then coated with the cortex and coat layers. Finally, once the spore is mature, the mother cell lyses, releasing the dormant endospore into the environment. Germination is activated upon sensing an appropriate small molecule, such as amino acids. The dormant spore initiates a signaling cascade that activates hydrolases and core hydration, which is necessary for metabolism to resume in the germinating spore.
The cortex, a thick layer of specialized peptidoglycan (PG), is essential to the dehydration of the spore core and heat resistance [33,34]. Although the PG in the spore cortex is similar to that in growing cells, it has two modifications: muramic acid-lactam (MAL) and muramic acid linked only to alanine [35]. These are not present in growing cells. In addition, this layer becomes the bacterial cell wall after endospore germination.
The outer membrane is essential in spore formation but may not be a permeability barrier in mature spores. Instead, this layer is necessary for spore germination. For instance, in B. subtilis, GerP family channels are found in the inner membrane, allowing germinants to have access to their receptors [36,37].
More than 70 proteins compose the coat, a multilayer structure with a unique folding geometry [7,38,39] that acts as a filter, preventing the passage of large molecules (Figure 1). A consequence of this constraint is that structures within the spore are protected from degradation enzymes such as lysozyme, which readily lyses the cortex [21,38,40]. Given its biochemical complexity, it is likely that the coat has other functions, although these are still poorly understood [21]. Interestingly, only 25% of the more than 70 proteins that compose the spore coat are conserved between B. subtilis and C. difficile [7], which suggests that the spore surface may be a significant source of evolutionary adaptation [41].
In some bacteria, such as B. cereus and C. difficile, a layer of protein, the exosporium, surrounds the coat. The exosporium layer is a highly diverse and complex structure, which is not present in all endospore-forming strains. It is a barrier against penetration by large molecules but still permeable to small molecules that are required for germination. Furthermore, the exosporium also confers resistance to chemicals such as ethanol, toluene, chloroform, phenol, and nitric oxide [21,42]. Generally, the exosporium is composed of a paracrystalline basal layer and an external layer consisting of a nap of fine filaments, termed the hairy nap. In C. difficile, this external hair-like nap is principally composed of collagen-like glycoprotein BclA [43], which appears to be involved in adherence and entry into the host [44].
Spores assemble within the mother, where genome segregation and asymmetric cell division occur [45,46,47]. It begins with the formation of a polar septum, resulting in two unequal cells, a mother cell and the forespore. They have identical copies of the chromosome, but express distinct genetic programs controlled by specific sigma factors [45,46,47]. The mutation in all sigma factors abrogates spore formation [41,45].
After polar division, the forespore is engulfed by the mother cell. In late stages of development, it directs the assembly of the cortex, where several multiprotein layers encase it [40,48,49,50,51]. When the spore reaches maturity, the mother cell lyses, freeing it into the environment where it persists until the conditions are suitable for germination (Figure 2) [28,30].
Germination is a complex mechanism by which the metabolism and macromolecular synthesis are restarted to give rise to a new vegetative cell. This process involves, during dormancy, the ability of spores to sense their environmental situation, and once the conditions are suitable for resuming growth (generally, specific nutrients in the environment), germination occurs. During germination, the resistance properties are lost [52,53,54] (Figure 2). The dormant spore is transformed into a metabolically active vegetative cell upon the binding of the germinant and, in some cases, co-germinants to their appropriate receptors. Upon the binding of the germinant to its receptors on the spore inner membrane, the signal is transduced, resulting in the activation of proteins that allow small molecules to move across the membrane and deconstruct the protective layers, restoring regular hydration and active metabolism [54].
Germinants are low-molecular-weight biomolecules found in the environment where the growth of the organism is favored. Common germinants may include amino acids, sugars, purine nucleosides, inorganic salts, or combinations of these molecules [28,55]. It is also possible to induce spore germination through a receptor-independent process, including exogenous CaDPA, lysozyme, dodecylamine, or extremely high pressure or heat. In addition, fragments of vegetative cell peptidoglycan induce spore germination in a protein kinase-dependent manner that is not at all understood [30,54]
Germination is defined as events beginning at the time of germinant recognition and ending when the water content in the spore core increases to 80% of wet weight [30]. This water content allows normal metabolism and macromolecular synthesis for outgrowth to occur. Five main events have been described in spore germination: germinant sensing, commitment to germinate, release of CaDPA, hydrolysis of cortex peptidoglycan by cortex-lytic enzymes, and finally, swelling and growth (Figure 2) [30]. The last stage of germination involves core swelling, germinal cell wall remodeling, internal membrane remodeling, internal membrane lipid mobility, and core protein mobility [30]. At the end of germination, cells take their usual rod shape and get ready to divide (Figure 2).
2. Spore Resistance and Killing Procedures
As previously described, the layers of the spore and core characteristics are responsible for their extensive resistance to varying ranges of temperature and pressure, ultraviolet radiation, and many harmful chemical substances such as hypochlorite and aldehydes. The resistance properties of spores to various agents may differ among strains, species, and genera, which can impact protocols for food sterilization, instrumental and surface sanitization, and even pharmacological treatments.
Despite its resistance properties, some extreme treatments can damage different components of the spore, including DNA, inner membrane, and proteins within the spore core. This can lead to the death of the spore. There are numerous excellent reviews [29,56,57,58,59] on different aspects for spore eradication. Here, we summarize some of the most used techniques (Table 1). In the upcoming sections, we will further analyze novel technologies and their applications. It is important to note that the resistance mechanisms of eradication techniques are mostly studied in B. subtilis. Further information is needed on other genera and species.

Table 1.
Spore eradication techniques.
3. Pathogen Spore-Forming Bacteria
As mentioned, all SFBs are members of the Firmicutes phylum, but only some members of Firmicutes make spores. SFBs can be found in four different classes of Firmicutes, Bacilli, Clostridia, Erysipelotrichia, and Negativicutes, all of which encode similar sets of core sporulation proteins and low G+C content. It has recently been proposed that sporobiota and sporobiome indicate the members and genomes of these types of bacteria [87]. Since spores are resistant to traditional DNA isolation methods, they may be underrepresented in metagenomic studies. As a result, we have a limited understanding of their types and abundances [88].
Among these SFBs, only a few have been associated with disease. Examples are the Clostridiaceae family, causing severe affections such as tetanus, gas gangrene, and botulism (Table 2) [89], and some Bacilli classes, such as Bacillus anthracis and Bacillus cereus sensu lato. The most common feature of the pathogenic Clostridia and Bacilli is the cell and tissue damage that they cause primarily due to the production of potent extracellular toxins (Table 2). Furthermore, some of these microorganisms have motility by flagella (swimming and swarming, e.g., B. cereus) or by type IV fimbriae (gliding motility, e.g., Clostridium perfringens), which leads to biofilm formation and antibiotic resistance promotion. The ability of these bacteria (e.g., C. perfringens) to form biofilm facilitates gastrointestinal infections and their persistence despite antibiotic treatments [90]. Additionally, it is necessary to avoid human and animal diseases and food contamination by spore formers to avoid prolonged antibiotic treatments. In addition, limiting antibiotics in farms to avoid multiresistant pathogen spore formers (PSFs) in this group of particularly challenging bacteria, considering the inherent resistant properties of the spore and its high rates of transmission and dissemination.
Proper handwashing is a critical step to prevent infections and spore dissemination. In addition, it is a crucial practice for controlling nosocomial infections, including C. difficile and B. cereus infections [91,92,93]. Therefore, hospital staff and the community should be reminded of this and trained in hand hygiene techniques.

Table 2.
Main human spore-forming pathogens.
Table 2.
Main human spore-forming pathogens.
| Disease | SFP | Target | Toxins | Remarks | References |
|---|---|---|---|---|---|
| Anthrax | B. anthracis | Cutaneous, gastrointestinal, and pulmonary infection | Tripartite anthrax-toxin | Anthrax is endemic in several regions around the world, and its epidemiology mainly depends on its dynamics in wildlife, local agriculture, community education on transmission routes, and access to health and vaccination. | [94,95,96,97] |
| Food poisoning | B. cereus | Gastrointestinal system: diarrheal and emetic syndrome | Hbl, Nhe, CytK, and cyclic peptide c cereulide | Both syndromes, gastrointestinal and emetic, are generally mild and self-limiting. However, severe, and even lethal cases of emetic foodborne B. cereus disease have been reported. | [8,98] |
| Botulism | C. botulinum, C. baratii, and C. butyricum | Gastrointestinal, nervous, and muscular systems | Botulinum neurotoxin (BoNT) | Metabolic and biochemical tests have divided the C. botulinum strains into four groups, while antibody neutralization has separated the neurotoxins into seven serotypes. | [99,100,101,102,103,104] |
| Food poisoning, myonecrosis (Gas gangrene), fatal infections in postdelivery women, and necrotizing colitis | C. perfringens | Gastrointestinal system, and wound and extremities | α-, β-, ε-, and ι toxins are the most common toxins | Currently, 23 virulence genes that encode toxins and virulent enzymes have been identified in C. perfringens, making it the most prolific toxin-producing pathogen presently known. | [105,106,107] |
| C. tetani | Tetanus | Nervous and muscular systems: local, cephalic, and neonatal tetanus | Tetanospasmin (also called tetanus neurotoxin; TeNT) | People who have not been vaccinated are more likely to have cases of generalized tetanus, while people who are poorly immunized are more likely to have local cases. With this disease, the most enduring challenge has been the prevention measure of free vaccination campaigns. | [108,109,110,111] |
| Pseudomem-braneous colitis | C. difficile | Gastrointestinal system (colon) | Toxin A (TcdA) and toxin B (TcdB) | The fecal–oral route transmits spores. These bacteria colonize the large intestine when there is dysbiosis in the gut microbiota caused by antibiotic treatments. It is associated with multiple relapses and recurrence. | [112,113,114] |
| Septic shock and necrotizing fasciitis | C. sordelii | Mostly associated with gynecological complications in women | Pathogenic strains of C. sordellii generate up to 7 identified exotoxins; among them, the lethal toxin (LT) and hemorrhagic toxin (HT) are regarded as the major virulence factors | The infection progresses rapidly. Thus, therapeutic interventions are rarely successful. At present, there is no antitoxin available. | [89,115,116] |
4. Approaches in the Food Industry to Control of Spore-Forming Bacteria
For several reasons, aerobic and anaerobic SFBs are a critical concern in the food industry. First, the widespread distribution of spores makes it impossible to prevent their presence in raw food and ingredients. As previously described, spores are extremely resistant to heat, dehydration, and chemical or physical stresses. Typical treatments used in the food industry, including heat treatments, can inactivate vegetative cells but fail to kill spores. Survivors may germinate and proliferate rapidly in the product or in the intestinal tract leading to food spoilage or active infections. Consequently, intensive wet heat treatment, generally at a temperature higher than 100 °C, is usually applied to inactivate spores in food products. Therefore, the biggest challenge in food production is to prevent its contamination, especially by SFBs, while avoiding the loss of organoleptic characteristics. Another critical point is people’s awareness of the proper storage of food. Storage at the wrong temperature can lead to contamination, for example, by C. botulinum, which can lead to poisoning and death. Different types of labeling have been proposed to prevent this [104].
Food spoilage SFBs are typically Bacillales, including the Bacillus, Geobacillus, Anoxybacillus, Alicyclobacillus, and Paenibacillus genera, while among Clostridiales, cases of contamination by species of the Clostridium and Desulfutomaculum genera have been reported [117].
SFBs become especially relevant in powdered dairy products (whole and skimmed milk, whey and milk isolates, whey and milk protein concentrate, casein, and caseinates) [118]. These bacteria can be controlled with physical treatments, such as dehydration, low-temperature storage, pasteurization, thermal sterilization, and nonthermal methods, such as irradiation and ultra-high-pressure processing [119]. High-temperature heating followed by microfiltration is a common method for milk treatment [120], but this procedure often leads to the development of psychrotolerant SFBs, mainly due to re-contamination [120].
Another food product marketed in powder form is dry sardine meal in Japan [65]. If the short-time ultra-pasteurization method is followed by rapid cooling to 60 °C, it allows the destruction of spores with a minimum change in the product color to be achieved [65].
Supercritical fluids (SCFs) and cold plasma are other techniques that do not alter the sensory characteristics of food products [78]. Plasma is generated when a gas is subjected to an electric current. It consists of a wide range of molecules and atoms in an excited state, such as ions, electrons, free radicals, reactive species (reactive oxygen and nitrogen species), and UV radiation [79]. The mechanism of action is membrane destruction by free radicals and induction of oxidative stress that produces the oxidation of enzymes and lipids [80]. The disadvantages are lipid oxidation in fish or decomposition of oligosaccharides in juice [79].
A supercritical fluid (SCF) is a substance at a temperature and pressure above its critical point where there is equilibrium between gas and liquid. The SCF shows gas-like viscosity, intermediate diffusivity, and liquid-like density, which provides it with good penetrability [83]. Carbon dioxide has been used for sterilizing food products, since it is considered GRAS (because it is environmentally friendly and non-corrosive), has good solubility, and is easy to remove and recycle. Hart et al., 2022, observed that SCF-CO2 was effective in inactivating bacterial spores without modification of the properties of the food [82]. The proposed mechanism of action is that SCF-CO2 destroys the spore cell wall, coat, cortex, and membrane. In the case of the vegetative cells, SCF-CO2 destroys the cytoplasmic membrane and degrades proteins, and altered pH is observed, since the fluid interacts with the cellular components, producing a loss of enzymatic activity [81]. Even though there are patents for the application of SCF-CO2 to food, there are still no processes at an industrial level.
Germination elimination at high isostatic pressure does not alter the sensory characteristics of food products. This strategy involves artificial induction or germination using chemical or physical media followed by a mild treatment. The spore loses its resistance properties upon germination induction and can be killed with less aggressive techniques. However, a critical disadvantage is that it allows superdormant spores to survive [84]. In various germination elimination processes, superdormancy of spore subpopulations has been observed. Superdormancy is a term that describes a group of spores that is different from the rest of the population in terms of their ability to germinate. These spores remain dormant or germinate extremely slowly [86].
Currently, the exact causes of spore superdormancy are unclear, but it is mainly explained by two factors. One factor is heat activation, which reverses spore germination; the other is related to the spore’s low levels of germinant receptors. Some research groups are studying the characteristics of superdormant spores to design novel strategies that prevent this subpopulation’s presence and reduce the potential spoilage of food products [84].
5. Natural Products against the Spore
Natural products are “small molecules produced by any organism, including primary and secondary metabolites”. Natural products can have simple chemistry, as in the case of urea, or complex structures, as in the case of Taxol. Generally, these natural molecules are only obtained in small quantities and may have a wide range of biological activities (https://www.nature.com/subjects/natural-products, accessed on 24 October 2022). As mentioned before, the pursuit of developing new procedures to combat spores in food products is a very active area. Furthermore, new treatments to combat human infections caused by SFPs are also an active area of research. Various natural products have been studied as promissory strategies to target endospores. Particularly, the microbial natural products bacteriocins have demonstrated activity against germinated spores, i.e., the state in which the spore inner membrane is exposed (Table 3).
The most well-studied bacteriocin is nisin, a 34-amino-acid-residue polypeptide produced by strains of Lactococcus lactis that is inhibitory toward many pathogens [121]. Nisin inhibits Gram-positive vegetative cells by generating membrane pores and interfering with cell wall biosynthesis by interacting with lipid II [71]. Furthermore, recent super-resolution structured illumination microscopy (SR-SIM) analysis showed the condensation of chromosomal DNA in Staphylococcus aureus cells exposed to nisin, suggesting that nisin interferes with chromosome replication or segregation in S. aureus [72].
Although spores differ from vegetative cells, nisin prevents the outgrowth of spores from Bacillus and Clostridium species (Table 3) [85,122]. Studies to understand the mechanism of action of nisin have shown that nisin mutants in the hinge region can bind lipid II but are incapable of forming pores and are active against B. anthracis vegetative cells without affecting spore outgrowth [122]. This evidence suggests that inhibitory mechanism of nisin acting on the outgrowth of B. anthracis spores is a combination of binding to lipid II and membrane disruption (pore formation) [122]. Furthermore, recent observations have shown that nisin treatment of B. subtilis spores caused the inner membrane of germinated spores to appear smaller than that of untreated spores [123].
Due to its broad spectrum against Gram-positive bacteria, including foodborne pathogens, the nisin effect has also been tested on nosocomial pathogen C. difficile. The vegetative form is also susceptible to the nisin effect in vitro [124] and also in an ex vivo model of the colon [125]. One benefit of nisin, and probably many other bacteriocins, is the ability to destroy C. difficile with minimal impact on the microbiota composition [125]. High nisin concentrations (25.6 μg ml− 1) reduced spore viability by 40–50 %, inhibiting C. difficile vegetative growth and its germinated spores [124]. Nisin also affects germinated spores of C. perfringens and other clostridia (Table 2). Interestingly, germinated spores of C. perfringens, depending on their origin (food poisoning (FP) or non-foodborne (NFB)), exhibit differential nisin resistance, with the highest being in NFB isolates [126]. These results may reflect changes in the spore layer composition, as has been observed in the spore resistance of C. botulinum species [127].
Other bacteriocins can also inhibit spore outgrowth and germination or diminish the heat resistance of different spores (Table 3). To be active, most of these peptides require the germination of spores or mixed treatments at high temperatures or pressure, but some molecules seem to be active on the resting spores of some species. For instance, Plantaricin causes critical damage to the morphology of cells and spores of B. cereus [128,129]. This bacteriocin removes the exosporium, and the spores look hollow [128,129]. Enterocin ASK48 affects the endospore structure without any pre-germination treatment. The exposure of Alicyclobacillus acidoterrestris to Enterocin ASK48 results in the disorganization of its endospore structure [130].
As inferred, a single bacteriocin can have heterogeneous effects depending on the origin of the spores (Table 3). Thus, some spores appear more “fragile” or susceptible to specific bacteriocins. Furthermore, these heterogeneous outcomes seem to depend on the bacterial species involved, since the same bacteriocin can affect spore ultrastructural characteristics differently.
One critical observation about these natural products is that even when the majority do not kill spores, these peptides have the sporostatic effect of preventing spore outgrowth; therefore, the proliferation of the vegetative forms and production of lantibiotics and other peptides do not confer resistance as conventional antibiotics do. However, nisin-resistant mutants of C. botulinum appeared through continuous exposure to the lantibiotic, causing their ability to germinate at levels of nisin that typically reduced their parental strain [131,132]. Although the mechanism that can bypass nisin action in these resistant mutants is unknown, it is not a nisin-specific phenomenon, as resistance can also be observed in various bacteriocins across a range of classes [131]. The bacteriostatic effects of nisin and other bacteriocins are a huge disadvantage for massive utilization in products where sterility is critical.

Table 3.
Bacteriocins against endospores.
Table 3.
Bacteriocins against endospores.
| Bacteriocin | Producer | Spore Tested | Remarks |
|---|---|---|---|
| Haloduracin | Bacillus halodurans | B. anthracis | It inhibited spore outgrowth [133] |
| Nisin | Lactococcus lactis | C. perfringens | It arrested outgrowth of germinated spores in rich medium, but it did not affect a meat model system [126] |
| C. sporogenes | Effective when spores germinated but not on spores themselves [134] | ||
| C. difficile | At high concentrations, nisin appeared to cause a statistically significant decrease in the viability of non-germinated spores [124] | ||
| C. beijerinckii | Active with previous high temperature [124] | ||
| C. botulinum | Heated spores were very sensitive to nisin, and nisin-treated spores became more heat sensitive; nisin acted as a pro-germinant [135] | ||
| B. subtilis | Sporicidal on germinated spores [123] | ||
| A. acidoterrestris | At high concentrations ranging from 0.1 to 1.5 mg liter−1, nisin had an inhibitory effect without the application of any previous thermal treatment [136]. | ||
| Enterocin ASK48 | Enterococcus faecalis | A. acidoterrestris | Active on resting spores [137] |
| Enterocin EJ9 | Enterococcus faecalis EJ97 | Geobacillus stearothermophilus | Heat-activated endospores became sensitive [138] |
| Bificin C6165 | Bifidobacterium animalis subsp. animalis CICC 6165 | A. acidoterrestris | In commercial diluted apple juice, no significant activity of bificin was observed against the endospores, but its addition contributed to the reduction in thermal resistance [139] |
| Lacticin | L. lactis IFPL 3593 two-peptide lantibiotic | C. tyrobutyricum | It inhibited the germination of clostridia spores and decreased the number of clostridia spores [140] |
| Plantaricin | Lactobacillus plantarum TF711 | C. sporogenes | Clostridia spore count was significantly lower in the experimental cheese model [141] |
| Plantaricin JY22 | Lactobacillus plantarum JY22 | B. cereus | It affected spore integrity; the exosporium was peeled out, and leaking spores were hollow [129] |
| Plantaricin YKX | L. plantarum | Alicyclobacillus spp. | Bacteriocins could induce the germination of A. acidoterrestris spores [142]; in endospore suspensions, cell viability decreased in proportion to the bacteriocin concentration added [130] |
| Thurincin H | Bacillus thuringiensis | B. cereus | Decrease in viable counts only when germination was induced by BHI (p < 0.05) [143] |
6. Conclusions and Future Directions
Spores are associated with persistence and resistance, and they are difficult to eliminate from food, surfaces, or even human bodies. The eradication of spores from surfaces and food is critical, since spores not only can affect the quality of food products, but they can also serve as vectors for different diseases, including potentially fatal ones. It is also difficult to eradicate an infection when one occurs in a person. Infections are associated with multiple relapses, reinfections, and further pathogen dissemination. Therefore, it is critical to prevent infection by assuring food security and by breaking the contagious chain with the proper sanitation of surfaces and handwashing. Particularly relevant for health workers dealing with opportunistic infections, such as C. difficile, is to prevent pathogen dissemination. Although vaccines have been developed to counteract infections by spore formers, some countries are still behind in their vaccination programs. This makes people living in those countries more vulnerable to infections. As seen in the COVID-19 pandemic, health is a concern of all countries. As the One Health program declares, all is interconnected. Therefore, it is crucial to ensure food safety and global health, especially considering that spore formers are less restricted by physical and temporal barriers and are thus easier to disperse.
Heat and chemicals have been used extensively for spore eradication, particularly for material and surface decontamination. However, when dealing with delicate substances and materials, such as food products, there are fewer options available for spore eradication. Furthermore, treatments for infections by spore-forming pathogens are very limited, and now, the global problem of antimicrobial resistance makes these infections even more challenging to treat. Traditional methods for spore elimination in food products have the great disadvantage of affecting the organoleptic properties or have raised concerns about biosecurity, as the g-radiation method has. Plasma and supercritical fluids are promising methods for eliminating spores from food without affecting the organoleptic properties, but these methods are expensive and limited to small-scale procedures. The use of small peptides such as bacteriocins is not only possible in food but also as pharmacological treatment for infections. Unfortunately, most of the cases require pre-activation (induction of germination) treatment. Besides the high cost associated with germination procedures, heterogeneous spore germination and superdormancy are concerns when using germination eradication procedures. Consequently, the disruption of the formed spores existing in delicate materials or causing infection in human beings remains a considerable challenge. With a better understand of endospore physiology, especially the properties of endospore layers, we could design more specific strategies for their control and eradication.
Author Contributions
A.R.-R., conceptualization, literature search and organization, and original draft preparation; B.R.-V., literature search, writing, editing and figure design, S.S., manuscript editing and writing; C.F.M.-d.l.P., literature search and writing. All authors have read and agreed to the published version of the manuscript.
Funding
A.R.R. was funded by UNAM/PAPIIT grant IA206823. The support of NUATEI Program from Instituto de Investigaciones Biomédicas Institutional Program is greatly acknowledged.
Acknowledgments
We are indebted to Marco A. Ortíz Jiménez for his support during this review elaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dworkin, J.; Losick, R. Linking nutritional status to gene activation and development. Genes Dev. 2001, 15, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Munakata, N.; Horneck, G.; Melosh, H.J.; Setlow, P. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. MMBR 2000, 64, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, E.M.; Boekhorst, J.; Kuipers, O.P.; Wells-Bennik, M.H. A mobile genetic element profoundly increases heat resistance of bacterial spores. ISME J. 2016, 10, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Zeigler, D.R.; Nicholson, W.L. Experimental evolution of Bacillus subtilis. Environ. Microbiol. 2017, 19, 3415–3422. [Google Scholar] [CrossRef] [PubMed]
- Tocheva, E.I.; Ortega, D.R.; Jensen, G.J. Sporulation, bacterial cell envelopes and the origin of life. Nat. Rev. Microbiol. 2016, 14, 535–542. [Google Scholar] [CrossRef]
- Bressuire-Isoard, C.; Bornard, I.; Henriques, A.O.; Carlin, F.; Broussolle, V. Sporulation Temperature Reveals a Requirement for CotE in the Assembly of both the Coat and Exosporium Layers of Bacillus cereus Spores. Appl. Environ. Microbiol. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Henriques, A.O.; Moran, C.P., Jr. Structure, assembly, and function of the spore surface layers. Annu. Rev. Microbiol. 2007, 61, 555–588. [Google Scholar] [CrossRef]
- Stenfors Arnesen, L.P.; Fagerlund, A.; Granum, P.E. From soil to gut: Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Rev. 2008, 32, 579–606. [Google Scholar] [CrossRef]
- Traag, B.A.; Driks, A.; Stragier, P.; Bitter, W.; Broussard, G.; Hatfull, G.; Chu, F.; Adams, K.N.; Ramakrishnan, L.; Losick, R. Do mycobacteria produce endospores? Proc. Natl. Acad. Sci. USA 2010, 107, 878–881. [Google Scholar] [CrossRef]
- Hutchison, E.A.; Miller, D.A.; Angert, E.R. Sporulation in Bacteria: Beyond the Standard Model. Microbiol. Spectr. 2014, 2, 87–102. [Google Scholar] [CrossRef]
- Higgins, D.; Dworkin, J. Recent progress in Bacillus subtilis sporulation. FEMS Microbiol. Rev. 2012, 36, 131–148. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.L.; Reis, A.; Kent, C.A.; Kosseva, M.; Roseiro, J.C.; Hewitt, C.J. Stress-induced physiological responses to starvation periods as well as glucose and lactose pulses in Bacillus licheniformis CCMI 1034 continuous aerobic fermentation processes as measured by multi-parameter flow cytometry. Biochem. Eng. J. 2005, 24, 31–41. [Google Scholar] [CrossRef]
- Makarova, K.S.; Anantharaman, V.; Aravind, L.; Koonin, E.V. Live virus-free or die: Coupling of antivirus immunity and programmed suicide or dormancy in prokaryotes. Biol. Direct 2012, 7, 40. [Google Scholar] [CrossRef]
- Mearls, E.B.; Izquierdo, J.A.; Lynd, L.R. Formation and characterization of non-growth states in Clostridium thermocellum: Spores and L-forms. BMC Microbiol. 2012, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.N.; Nawrocki, K.L.; McBride, S.M. Conserved oligopeptide permeases modulate sporulation initiation in Clostridium difficile. Infect. Immun. 2014, 82, 4276–4291. [Google Scholar] [CrossRef] [PubMed]
- Deakin, L.J.; Clare, S.; Fagan, R.P.; Dawson, L.F.; Pickard, D.J.; West, M.R.; Wren, B.W.; Fairweather, N.F.; Dougan, G.; Lawley, T.D. The Clostridium difficile spo0A gene is a persistence and transmission factor. Infect. Immun. 2012, 80, 2704–2711. [Google Scholar] [CrossRef]
- Underwood, S.; Guan, S.; Vijayasubhash, V.; Baines, S.D.; Graham, L.; Lewis, R.J.; Wilcox, M.H.; Stephenson, K. Characterization of the sporulation initiation pathway of Clostridium difficile and its role in toxin production. J. Bacteriol. 2009, 191, 7296–7305. [Google Scholar] [CrossRef]
- Pettit, L.J.; Browne, H.P.; Yu, L.; Smits, W.K.; Fagan, R.P.; Barquist, L.; Martin, M.J.; Goulding, D.; Duncan, S.H.; Flint, H.J.; et al. Functional genomics reveals that Clostridium difficile Spo0A coordinates sporulation, virulence and metabolism. BMC Genom. 2014, 15, 160. [Google Scholar] [CrossRef]
- Meeske, A.J.; Rodrigues, C.D.; Brady, J.; Lim, H.C.; Bernhardt, T.G.; Rudner, D.Z. High-Throughput Genetic Screens Identify a Large and Diverse Collection of New Sporulation Genes in Bacillus subtilis. PLoS Biol. 2016, 14, e1002341. [Google Scholar] [CrossRef]
- Boone, T.J.; Mallozzi, M.; Nelson, A.; Thompson, B.; Khemmani, M.; Lehmann, D.; Dunkle, A.; Hoeprich, P.; Rasley, A.; Stewart, G.; et al. Coordinated Assembly of the Bacillus anthracis Coat and Exosporium during Bacterial Spore Outer Layer Formation. mBio 2018, 9, e01166-18. [Google Scholar] [CrossRef]
- Swick, M.C.; Koehler, T.M.; Driks, A. Surviving Between Hosts: Sporulation and Transmission. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Tan, I.S.; Ramamurthi, K.S. Spore formation in Bacillus subtilis. Environ. Microbiol. Rep. 2014, 6, 212–225. [Google Scholar] [CrossRef]
- Carrera, M.; Zandomeni, R.O.; Fitzgibbon, J.; Sagripanti, J.L. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 2007, 102, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. I will survive: DNA protection in bacterial spores. Trends Microbiol. 2007, 15, 172–180. [Google Scholar] [CrossRef]
- Setlow, P. Mechanisms which contribute to the long-term survival of spores of Bacillus species. Soc. Appl. Bacteriol. Symp. Ser. 1994, 23, 49S–60S. [Google Scholar]
- Atrih, A.; Foster, S.J. The role of peptidoglycan structure and structural dynamics during endospore dormancy and germination. Antonie Van Leeuwenhoek 1999, 75, 299–307. [Google Scholar] [CrossRef]
- Westphal, A.J.; Price, P.B.; Leighton, T.J.; Wheeler, K.E. Kinetics of size changes of individual Bacillus thuringiensis spores in response to changes in relative humidity. Proc. Natl. Acad. Sci. USA 2003, 100, 3461–3466. [Google Scholar] [CrossRef]
- Setlow, P. Germination of spores of Bacillus species: What we know and do not know. J. Bacteriol. 2014, 196, 1297–1305. [Google Scholar] [CrossRef]
- Setlow, P. Spore Resistance Properties. Microbiol. Spectr. 2014, 2, 201–215. [Google Scholar] [CrossRef]
- Setlow, P.; Wang, S.; Li, Y.Q. Germination of Spores of the Orders Bacillales and Clostridiales. Annu. Rev. Microbiol. 2017, 71, 459–477. [Google Scholar] [CrossRef]
- Zheng, L.; Abhyankar, W.; Ouwerling, N.; Dekker, H.L.; van Veen, H.; van der Wel, N.N.; Roseboom, W.; de Koning, L.J.; Brul, S.; de Koster, C.G. Bacillus subtilis Spore Inner Membrane Proteome. J. Proteome Res. 2016, 15, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Abel-Santos, E. The Ger receptor family from sporulating bacteria. Curr. Issues Mol. Biol. 2010, 12, 147–158. [Google Scholar]
- Alabdali, Y.A.J.; Oatley, P.; Kirk, J.A.; Fagan, R.P. A cortex-specific penicillin-binding protein contributes to heat resistance in Clostridioides difficile spores. Anaerobe 2021, 70, 102379. [Google Scholar] [CrossRef]
- Rao, L.; Liao, X.; Setlow, P. Bacillus spore wet heat resistance and evidence for the role of an expanded osmoregulatory spore cortex. Lett. Appl. Microbiol. 2016, 63, 247–253. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Butzin, X.Y.; Troiano, A.J.; Coleman, W.H.; Griffiths, K.K.; Doona, C.J.; Feeherry, F.E.; Wang, G.; Li, Y.Q.; Setlow, P. Analysis of the effects of a gerP mutation on the germination of spores of Bacillus subtilis. J. Bacteriol. 2012, 194, 5749–5758. [Google Scholar] [CrossRef]
- Behravan, J.; Chirakkal, H.; Masson, A.; Moir, A. Mutations in the gerP locus of Bacillus subtilis and Bacillus cereus affect access of germinants to their targets in spores. J. Bacteriol. 2000, 182, 1987–1994. [Google Scholar] [CrossRef]
- Aronson, A. Regulation of expression of a select group of Bacillus anthracis spore coat proteins. FEMS Microbiol. Lett. 2018, 365, fny063. [Google Scholar] [CrossRef]
- McKenney, P.T.; Driks, A.; Eichenberger, P. The Bacillus subtilis endospore: Assembly and functions of the multilayered coat. Nat. Rev. Microbiol. 2013, 11, 33–44. [Google Scholar] [CrossRef]
- Driks, A.; Eichenberger, P. The Spore Coat. Microbiol. Spectr. 2016, 4, 179–200. [Google Scholar] [CrossRef]
- de Hoon, M.J.; Eichenberger, P.; Vitkup, D. Hierarchical evolution of the bacterial sporulation network. Curr. Biol. CB 2010, 20, R735–R745. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Todd, S.J.; Ball, D.A.; Shepherd, A.M.; Sylvestre, P.; Moir, A. ExsY and CotY Are Required for the Correct Assembly of the Exosporium and Spore Coat of Bacillus cereus. J. Bacteriol. 2006, 188, 7905–7913. [Google Scholar] [CrossRef]
- Stewart, G.C. The Exosporium Layer of Bacterial Spores: A Connection to the Environment and the Infected Host. Microbiol. Mol. Biol. Rev. MMBR 2015, 79, 437–457. [Google Scholar] [CrossRef]
- Castro-Cordova, P.; Mora-Uribe, P.; Reyes-Ramirez, R.; Cofre-Araneda, G.; Orozco-Aguilar, J.; Brito-Silva, C.; Mendoza-Leon, M.J.; Kuehne, S.A.; Minton, N.P.; Pizarro-Guajardo, M.; et al. Entry of spores into intestinal epithelial cells contributes to recurrence of Clostridioides difficile infection. Nat. Commun. 2021, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Fimlaid, K.A.; Bond, J.P.; Schutz, K.C.; Putnam, E.E.; Leung, J.M.; Lawley, T.D.; Shen, A. Global analysis of the sporulation pathway of Clostridium difficile. PLoS Genet. 2013, 9, e1003660. [Google Scholar] [CrossRef]
- Fimlaid, K.A.; Shen, A. Diverse mechanisms regulate sporulation sigma factor activity in the Firmicutes. Curr. Opin. Microbiol. 2015, 24, 88–95. [Google Scholar] [CrossRef]
- Pereira, F.C.; Saujet, L.; Tome, A.R.; Serrano, M.; Monot, M.; Couture-Tosi, E.; Martin-Verstraete, I.; Dupuy, B.; Henriques, A.O. The spore differentiation pathway in the enteric pathogen Clostridium difficile. PLoS Genet. 2013, 9, e1003782. [Google Scholar] [CrossRef]
- Setlow, P. Dynamics of the assembly of a complex macromolecular structure the coat of spores of the bacterium Bacillus subtilis. Mol. Microbiol. 2012, 83, 241–244. [Google Scholar] [CrossRef]
- Imamura, D.; Kuwana, R.; Takamatsu, H.; Watabe, K. Proteins involved in formation of the outermost layer of Bacillus subtilis spores. J. Bacteriol. 2011, 193, 4075–4080. [Google Scholar] [CrossRef]
- Imamura, D.; Kuwana, R.; Takamatsu, H.; Watabe, K. Localization of proteins to different layers and regions of Bacillus subtilis spore coats. J. Bacteriol. 2010, 192, 518–524. [Google Scholar] [CrossRef]
- McKenney, P.T.; Eichenberger, P. Dynamics of spore coat morphogenesis in Bacillus subtilis. Mol. Microbiol. 2012, 83, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Niu, S.; Yankova, M.; Mecklenburg, M.; King, S.M.; Ravichandran, J.; Kalia, R.K.; Nakano, A.; Vashishta, P.; Setlow, P. Analysis of killing of growing cells and dormant and germinated spores of Bacillus species by black silicon nanopillars. Sci. Rep. 2017, 7, 17768. [Google Scholar] [CrossRef] [PubMed]
- Kochan, T.J.; Foley, M.H.; Shoshiev, M.S.; Somers, M.J.; Carlson, P.E.; Hanna, P.C. Updates to Clostridium difficile Spore Germination. J. Bacteriol. 2018, 200, e00218-18. [Google Scholar] [CrossRef] [PubMed]
- Moir, A.; Cooper, G. Spore Germination. Microbiol. Spectr. 2015, 3, 217–236. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, D.; McAllister, K.N.; Sorg, J.A. Germinants and Their Receptors in Clostridia. J. Bacteriol. 2016, 198, 2767–2775. [Google Scholar] [CrossRef] [PubMed]
- Shen, A. Clostridioides difficile Spore Formation and Germination: New Insights and Opportunities for Intervention. Annu. Rev. Micro. 2020, 74, 545–566. [Google Scholar] [CrossRef]
- Wood, J.P.; Adrion, A.C. Review of Decontamination Techniques for the Inactivation of Bacillus anthracis and Other Spore-Forming Bacteria Associated with Building or Outdoor Materials. Environ. Sci. Technol. 2019, 53, 4045–4062. [Google Scholar] [CrossRef]
- Reineke, K.; Mathys, A. Endospore Inactivation by Emerging Technologies: A Review of Target Structures and Inactivation Mechanisms. Annu. Rev. Food Sci. Technol. 2020, 11, 255–274. [Google Scholar] [CrossRef]
- Lv, R.L.; Liu, D.H.; Zhou, J.W. Bacterial spore inactivation by non-thermal technologies: Resistance and inactivation mechanisms. Curr. Opin. Food Sci. 2021, 42, 31–36. [Google Scholar] [CrossRef]
- Taylor, W.; Camilleri, E.; Craft, D.L.; Korza, G.; Granados, M.R.; Peterson, J.; Szczpaniak, R.; Weller, S.K.; Moeller, R.; Douki, T.; et al. DNA Damage Kills Bacterial Spores and Cells Exposed to 222-Nanometer UV Radiation. Appl. Environ. Microbiol. 2020, 86, e03039-19. [Google Scholar] [CrossRef]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef] [PubMed]
- Setlow, P. Spore Resistance Properties. Microbiol Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Clair, G.; Esbelin, J.; Mallea, S.; Bornard, I.; Carlin, F. The spore coat is essential for Bacillus subtilis spore resistance to pulsed light, and pulsed light treatment eliminates some spore coat proteins. Int. J. Food Microbiol. 2020, 323, 108592. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Mosher, W.; Wiertzema, J.; Peng, P.; Min, M.; Cheng, Y.; An, J.; Ma, Y.; Fan, X.; Niemira, B.A.; et al. Effects of intense pulsed light and gamma irradiation on Bacillus cereus spores in mesquite pod flour. Food Chem. 2021, 344, 128675. [Google Scholar] [CrossRef]
- Ortatatli, M.; Canitez, K.; Sezigen, S.; Eyison, R.K.; Kenar, L. Evaluation of Gamma-Radiation Inactivation of a Bioterrorism Agent, Bacillus anthracis Spores, on Different Materials. Indian J. Microbiol. 2018, 58, 76–80. [Google Scholar] [CrossRef]
- Cote, C.K.; Buhr, T.; Bernhards, C.B.; Bohmke, M.D.; Calm, A.M.; Esteban-Trexler, J.S.; Hunter, M.; Katoski, S.E.; Kennihan, N.; Klimko, C.P.; et al. A Standard Method To Inactivate Bacillus anthracis Spores to Sterility via Gamma Irradiation. Appl. Environ. Microbiol. 2018, 84, e00106-18. [Google Scholar] [CrossRef]
- He, L.; Chen, Z.; Wang, S.; Wu, M.; Setlow, P.; Li, Y.Q. Germination, Outgrowth, and Vegetative-Growth Kinetics of Dry-Heat-Treated Individual Spores of Bacillus Species. Appl. Environ. Microbiol. 2018, 84, e02618-17. [Google Scholar] [CrossRef]
- Shirey, B.T.; Schubert, W.; Benardini, J. An Overview of Surface Heat Microbial Reduction as a Viable Microbial Reduction Modality for Spacecraft Surfaces. In Proceedings of the 47th International Conference on Environmental Systems (ICES), Charleston, SC, USA, 16–20 July 2017. [Google Scholar]
- Joslyn, L. Sterilization by Heat in Disinfection, Sterlization, and Preservation; Block, S.S., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Sandle, T.S. Sterility, Sterilisation and Sterility Assurance for Pharmaceuticals; Elsevier: Amsterdam, The Netherlands, 2013; pp. 93–109. [Google Scholar]
- Soni, A.; Parlane, N.A.; Khan, F.; Derraik, J.G.B.; Wild, C.E.K.; Anderson, Y.C.; Brightwell, G. Efficacy of Dry Heat Treatment against Clostridioides difficile Spores and Mycobacterium tuberculosis on Filtering Facepiece Respirators. Pathogens 2022, 11, 871. [Google Scholar] [CrossRef]
- Huesca-Espitia, L.C.; Suvira, M.; Rosenbeck, K.; Korza, G.; Setlow, B.; Li, W.; Wang, S.; Li, Y.Q.; Setlow, P. Effects of steam autoclave treatment on Geobacillus stearothermophilus spores. J. Appl. Microbiol. 2016, 121, 1300–1311. [Google Scholar] [CrossRef]
- Wen, J.; Smelt, J.; Vischer, N.O.E.; de Vos, A.L.; Setlow, P.; Brul, S. Heat Activation and Inactivation of Bacterial Spores: Is There an Overlap? Appl. Environ. Microbiol. 2022, 88, e0232421. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, D.; Dahlberg, T.; Wiklund, K.; Andersson, P.O.; Henriksson, S.; Andersson, M. Mode of Action of Disinfection Chemicals on the Bacterial Spore Structure and Their Raman Spectra. Anal. Chem. 2021, 93, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Kenters, N.; Huijskens, E.G.W.; de Wit, S.C.J.; Sanders, I.; van Rosmalen, J.; Kuijper, E.J.; Voss, A. Effectiveness of various cleaning and disinfectant products on Clostridium difficile spores of PCR ribotypes 010, 014 and 027. Antimicrob. Resist. Infect. Control 2017, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Uwamahoro, M.C.; Massicotte, R.; Hurtubise, Y.; Gagne-Bourque, F.; Mafu, A.A.; Yahia, L. Evaluating the Sporicidal Activity of Disinfectants against Clostridium difficile and Bacillus amyloliquefaciens Spores by Using the Improved Methods Based on ASTM E2197-11. Front. Public Health 2018, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Stier, P.; Kulozik, U. Effect of Sporulation Conditions Following Submerged Cultivation on the Resistance of Bacillus atrophaeus Spores against Inactivation by H2O2. Molecules 2020, 25, 2985. [Google Scholar] [CrossRef]
- Liao, X.; Muhammad, A.I.; Chen, S.; Hu, Y.; Ye, X.; Liu, D.; Ding, T. Bacterial spore inactivation induced by cold plasma. Crit. Rev. Food Sci. Nutr. 2019, 59, 2562–2572. [Google Scholar] [CrossRef]
- Obileke, K.; Onyeaka, H.; Miri, T.; Nwabor, O.F.; Hart, A.; Al-Sharify, Z.T.; Al-Najjar, S.; Anumudu, C. Recent advances in radio frequency, pulsed light, and cold plasma technologies for food safety. J. Food Process. Eng. 2022, 45, e14138. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Ayub, Z.; Muhammad Aadil, R.; Abid, M.; Zhang, J.; Liqing, Z. Recent Advances in Plasma Technology: Influence of Atmospheric Cold Plasma on Spore Inactivation. Food Rev. Int. 2021, 38, 789–811. [Google Scholar] [CrossRef]
- Buszewski, B.; Wrona, O.; Mayya, R.P.; Zakharenko, A.M.; Kalenik, T.K.; Golokhvast, K.S.; Piekoszewski, W.; Rafinska, K. The potential application of supercritical CO(2) in microbial inactivation of food raw materials and products. Crit. Rev. Food Sci. Nutr. 2022, 62, 6535–6548. [Google Scholar] [CrossRef]
- Hart, A.; Anumudu, C.; Onyeaka, H.; Miri, T. Application of supercritical fluid carbon dioxide in improving food shelf-life and safety by inactivating spores: A review. J. Food Sci. Technol. 2022, 59, 417–428. [Google Scholar] [CrossRef]
- Wang, W.; Rao, L.; Wu, X.; Wang, Y.; Zhao, L.; Liao, X. Supercritical Carbon Dioxide Applications in Food Processing. Food Eng. Rev. 2020, 13, 570–591. [Google Scholar] [CrossRef]
- Delbruck, A.I.; Tritten, Y.; Nanni, P.; Heydenreich, R.; Mathys, A. Moderate High-Pressure Superdormancy in Bacillus Spores: Properties of Superdormant Spores and Proteins Potentially Influencing Moderate High-Pressure Germination. Appl. Environ. Microbiol. 2022, 88, e0240621. [Google Scholar] [CrossRef]
- Lay, C.L.; Dridi, L.; Bergeron, M.G.; Ouellette, M.; Fliss, I.L. Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. J. Med. Microbiol. 2016, 65, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mathys, A. Superdormant Spores as a Hurdle for Gentle Germination-Inactivation Based Spore Control Strategies. Front. Microbiol. 2018, 9, 3163. [Google Scholar] [CrossRef] [PubMed]
- Tetz, G.; Tetz, V. Introducing the sporobiota and sporobiome. Gut Pathog. 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Filippidou, S.; Junier, T.; Wunderlin, T.; Lo, C.C.; Li, P.E.; Chain, P.S.; Junier, P. Under-detection of endospore-forming Firmicutes in metagenomic data. Comput. Struct. Biotechnol. J. 2015, 13, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Stevens, D.L.; Aldape, M.J.; Bryant, A.E. Life-threatening clostridial infections. Anaerobe 2012, 18, 254–259. [Google Scholar] [CrossRef]
- Milton, A.A.P.; Sanjukta, R.; Gogoi, A.P.; Momin, K.M.; Priya, G.B.; Das, S.; Ghatak, S.; Sen, A.; Kandpal, B.K. Prevalence, molecular typing and antibiotic resistance of Clostridium perfringens in free range ducks in Northeast India. Anaerobe 2020, 64, 102242. [Google Scholar] [CrossRef]
- Kiersnowska, Z.M.; Lemiech-Mirowska, E.; Michalkiewicz, M.; Marczak, M. Hand hygiene as the basic method of reducing Clostridium difficile infections (CDI) in a hospital environment. Ann. Agric. Environ. Med. 2021, 28, 535–540. [Google Scholar] [CrossRef]
- Sasahara, T.; Hayashi, S.; Hosoda, K.; Morisawa, Y.; Hirai, Y. Comparison of hand hygiene procedures for removing Bacillus cereus spores. Biocontrol Sci. 2014, 19, 129–134. [Google Scholar] [CrossRef]
- Turner, N.A.; Anderson, D.J. Hospital Infection Control: Clostridioides difficile. Clin. Colon Rectal Surg. 2020, 33, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Pilo, P.; Frey, J. Pathogenicity, population genetics and dissemination of Bacillus anthracis. Infect. Genet. Evol. 2018, 64, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Anthrax. 2017. Available online: https://www.cdc.gov/anthrax/index.html (accessed on 1 November 2022).
- Moayeri, M.; Leppla, S.H.; Vrentas, C.; Pomerantsev, A.P.; Liu, S. Anthrax Pathogenesis. Annu. Rev. Microbiol. 2015, 69, 185–208. [Google Scholar] [CrossRef] [PubMed]
- Carlson, C.J.; Getz, W.M.; Kausrud, K.L.; Cizauskas, C.A.; Blackburn, J.K.; Bustos Carrillo, F.A.; Colwell, R.; Easterday, W.R.; Ganz, H.H.; Kamath, P.L.; et al. Spores and soil from six sides: Interdisciplinarity and the environmental biology of anthrax (Bacillus anthracis). Biol. Rev. 2018, 93, 1813–1831. [Google Scholar] [CrossRef]
- Warda, A.K.; Siezen, R.J.; Boekhorst, J.; Wells-Bennik, M.H.; de Jong, A.; Kuipers, O.P.; Nierop Groot, M.N.; Abee, T. Linking Bacillus cereus Genotypes and Carbohydrate Utilization Capacity. PLoS ONE 2016, 11, e0156796. [Google Scholar] [CrossRef]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on Clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef]
- Schaumann, R.; Dallacker-Losensky, K.; Rosenkranz, C.; Genzel, G.H.; Stingu, C.S.; Schellenberger, W.; Schulz-Stubner, S.; Rodloff, A.C.; Eschrich, K. Discrimination of Human Pathogen Clostridium Species Especially of the Heterogeneous C. sporogenes and C. botulinum by MALDI-TOF Mass Spectrometry. Curr. Microbiol. 2018, 75, 1506–1515. [Google Scholar] [CrossRef]
- Connan, C.; Popoff, M.R. Two-component systems and toxinogenesis regulation in Clostridium botulinum. Res. Microbiol. 2015, 166, 332–343. [Google Scholar] [CrossRef]
- Tiwari, A.; Nagalli, S. Clostridium botulinum. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Antonucci, L.; Locci, C.; Schettini, L.; Clemente, M.G.; Antonucci, R. Infant botulism: An underestimated threat. Infect. Dis. 2021, 53, 647–660. [Google Scholar] [CrossRef]
- Edmunds, S.; Vugia, D.J.; Rosen, H.E.; Wong, K.K.; Dykes, J.K.; Griffin, P.M.; Chatham-Stephens, K. Inadequate Refrigeration of Some Commercial Foods Is a Continued Cause of Foodborne Botulism in the United States, 1994–2021. Foodborne Pathog. Dis. 2022, 19, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, K.; Shimizu, T. Regulation of Toxin Production in Clostridium perfringens. Toxins 2016, 8, 207. [Google Scholar] [CrossRef]
- Kiu, R.; Hall, L.J. An update on the human and animal enteric pathogen Clostridium perfringens. Emerg. Microbes Infect. 2018, 7, 141. [Google Scholar] [CrossRef]
- Li, J.; Paredes-Sabja, D.; Sarker, M.R.; McClane, B.A. Clostridium perfringens Sporulation and Sporulation-Associated Toxin Production. Microbiol. Spectr. 2016, 4, 3. [Google Scholar] [CrossRef]
- George, E.K.; De Jesus, O.; Vivekanandan, R. Clostridium tetani. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2020. [Google Scholar]
- Yen, L.M.; Thwaites, C.L. Tetanus. Lancet 2019, 393, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Kuehne, S.A.; Rood, J.I.; Lyras, D. Clostridial Genetics: Genetic Manipulation of the Pathogenic Clostridia. Microbiol. Spectr. 2019, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Bruggemann, H.; Brzuszkiewicz, E.; Chapeton-Montes, D.; Plourde, L.; Speck, D.; Popoff, M.R. Genomics of Clostridium tetani. Res. Microbiol. 2015, 166, 326–331. [Google Scholar] [CrossRef]
- Hall, A.J.; Curns, A.T.; McDonald, L.C.; Parashar, U.D.; Lopman, B.A. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin. Infect. Dis. 2012, 55, 216–223. [Google Scholar] [CrossRef]
- Burke, K.E.; Lamont, J.T. Clostridium difficile infection: A worldwide disease. Gut Liver 2014, 8, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Auchtung, J.M. Control of Clostridium difficile Infection by Defined Microbial Communities. Microbiol. Spectr. 2017, 5, 267–289. [Google Scholar] [CrossRef]
- Bhatnagar, J.; Deleon-Carnes, M.; Kellar, K.L.; Bandyopadhyay, K.; Antoniadou, Z.A.; Shieh, W.J.; Paddock, C.D.; Zaki, S.R. Rapid, simultaneous detection of Clostridium sordellii and Clostridium perfringens in archived tissues by a novel PCR-based microsphere assay: Diagnostic implications for pregnancy-associated toxic shock syndrome cases. Infect. Dis. Obstet. Gynecol. 2012, 2012, 972845. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Bhatnagar, J.; Reagan, S.; Zane, S.B.; D’Angeli, M.A.; Fischer, M.; Killgore, G.; Kwan-Gett, T.S.; Blossom, D.B.; Shieh, W.J.; et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet. Gynecol. 2007, 110, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Andre, S.; Vallaeys, T.; Planchon, S. Spore-forming bacteria responsible for food spoilage. Res. Microbiol. 2017, 168, 379–387. [Google Scholar] [CrossRef]
- McHugh, A.J.; Feehily, C.; Hill, C.; Cotter, P.D. Detection and Enumeration of Spore-Forming Bacteria in Powdered Dairy Products. Front. Microbiol. 2017, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Wells-Bennik, M.H.; Eijlander, R.T.; den Besten, H.M.; Berendsen, E.M.; Warda, A.K.; Krawczyk, A.O.; Nierop Groot, M.N.; Xiao, Y.; Zwietering, M.H.; Kuipers, O.P.; et al. Bacterial Spores in Food: Survival, Emergence, and Outgrowth. Annu. Rev. Food Sci. Technol. 2016, 7, 457–482. [Google Scholar] [CrossRef]
- Doll, E.V.; Scherer, S.; Wenning, M. Spoilage of Microfiltered and Pasteurized Extended Shelf Life Milk Is Mainly Induced by Psychrotolerant Spore-Forming Bacteria that often Originate from Recontamination. Front. Microbiol. 2017, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Anumudu, C.; Hart, A.; Miri, T.; Onyeaka, H. Recent Advances in the Application of the Antimicrobial Peptide Nisin in the Inactivation of Spore-Forming Bacteria in Foods. Molecules 2021, 26, 5552. [Google Scholar] [CrossRef]
- Gut, I.M.; Blanke, S.R.; van der Donk, W.A. Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem. Biol. 2011, 6, 744–752. [Google Scholar] [CrossRef]
- Omardien, S.; Drijfhout, J.W.; Zaat, S.A.; Brul, S. Cationic Amphipathic Antimicrobial Peptides Perturb the Inner Membrane of Germinated Spores Thus Inhibiting Their Outgrowth. Front. Microbiol. 2018, 9, 2277. [Google Scholar] [CrossRef]
- Hofstetter, S.; Gebhardt, D.; Ho, L.; Ganzle, M.; McMullen, L.M. Effects of nisin and reutericyclin on resistance of endospores of Clostridium spp. to heat and high pressure. Food Microbiol. 2013, 34, 46–51. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, C.; O’Connor, P.M.; O’Sullivan, O.; Rea, M.C.; Hill, C.; Ross, R.P. Impact of nisin on Clostridioides difficile and microbiota composition in a faecal fermentation model of the human colon. J. Appl. Microbiol. 2022, 132, 1397–1408. [Google Scholar] [CrossRef]
- Udompijitkul, P.; Paredes-Sabja, D.; Sarker, M.R. Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood-borne isolates. J. Food Sci. 2012, 77, M51–M56. [Google Scholar] [CrossRef] [PubMed]
- Portinha, I.M.; Douillard, F.P.; Korkeala, H.; Lindström, M. Sporulation Strategies and Potential Role of the Exosporium in Survival and Persistence of Clostridium botulinum. In Int. J. Mol. Sci. 2022, 23, 754. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, J.; Lu, X.; Lu, Z.; Bie, X.; Zhao, H.; Zhang, C.; Lu, F. Purification, Characterization, and Mode of Action of Plantaricin GZ1-27, a Novel Bacteriocin against Bacillus cereus. J. Agric. Food Chem. 2018, 66, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Miao, L.; Ma, H.; Bai, F.; Lin, Y.; Sun, M.; Li, J. Purification, characterization and action mechanism of plantaricin JY22, a novel bacteriocin against Bacillus cereus produced by Lactobacillus plantarum JY22 from golden carp intestine. Food Sci. Biotechnol. 2018, 27, 695–703. [Google Scholar] [CrossRef]
- Yue, T.; Pei, J.; Yuan, Y. Purification and characterization of anti-Alicyclobacillus bacteriocin produced by Lactobacillus rhamnosus. J. Food Prot. 2013, 76, 1575–1581. [Google Scholar] [CrossRef]
- Draper, L.A.; Cotter, P.D.; Hill, C.; Ross, R.P. Lantibiotic resistance. Microbiol. Mol. Biol. Rev. MMBR 2015, 79, 171–191. [Google Scholar] [CrossRef]
- Mazzotta, A.S.; Crandall, A.D.; Montville, T.J. Nisin Resistance in Clostridium botulinum Spores and Vegetative Cells. Appl. Environ. Microbiol. 1997, 63, 2654–2659. [Google Scholar] [CrossRef]
- Oman, T.J.; van der Donk, W.A. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem. Biol. 2009, 4, 865–874. [Google Scholar] [CrossRef]
- Ros-Chumillas, M.; Esteban, M.D.; Huertas, J.P.; Palop, A. Effect of Nisin and Thermal Treatments on the Heat Resistance of Clostridium sporogenes Spores. J. Food Prot. 2015, 78, 2019–2023. [Google Scholar] [CrossRef]
- Mazzotta, A.S.; Montville, T.J. Characterization of fatty acid composition, spore germination, and thermal resistance in a nisin-resistant mutant of Clostridium botulinum 169B and in the wild-type strain. Appl. Environ. Microbiol. 1999, 65, 659–664. [Google Scholar] [CrossRef]
- Huertas, J.P.; Esteban, M.D.; Antolinos, V.; Palop, A. Combined effect of natural antimicrobials and thermal treatments on Alicyclobacillus acidoterrestris spores. Food Control 2014, 35, 73–78. [Google Scholar] [CrossRef]
- Grande, M.J.; Lucas, R.; Abriouel, H.; Omar, N.B.; Maqueda, M.; Martinez-Bueno, M.; Martinez-Canamero, M.; Valdivia, E.; Galvez, A. Control of Alicyclobacillus acidoterrestris in fruit juices by enterocin AS-48. Int. J. Food Microbiol. 2005, 104, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Martínez Viedma, P.; Abriouel, H.; Ben Omar, N.; Lucas López, R.; Gálvez, A. Effect of enterocin EJ97 against Geobacillus stearothermophilus vegetative cells and endospores in canned foods and beverages. Eur. Food Res. Technol. 2009, 230, 513–519. [Google Scholar] [CrossRef]
- Pei, J.; Yue, T.; Yuan, Y. Control of Alicyclobacillus acidoterrestris in fruit juices by a newly discovered bacteriocin. World J. Microbiol. Biotechnol. 2014, 30, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Carmen Martínez-Cuesta, M.; Bengoechea, J.; Bustos, I.; Rodríguez, B.; Requena, T.; Peláez, C. Control of late blowing in cheese by adding lacticin 3147-producing Lactococcus lactis IFPL 3593 to the starter. Int. Dairy J. 2010, 20, 18–24. [Google Scholar] [CrossRef]
- Gonzalez, L.; Zarate, V. Inhibitory activity of Lactobacillus plantarum TF711 against Clostridium sporogenes when used as adjunct culture in cheese manufacture. J. Dairy Res. 2015, 82, 236–241. [Google Scholar] [CrossRef]
- Pei, J.; Jin, W.; Wang, J.; Huang, Y.; Li, X.; Zhang, H.; Zhang, Y.; Ramadan, A.; Abd El-Aty, A.M. Purification and Characterization of Plantaricin YKX and Assessment of Its Inhibitory Activity Against Alicyclobacillus spp. Front. Microbiol. 2021, 12, 783266. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Manns, D.C.; Guron, G.K.; Churey, J.J.; Worobo, R.W. Large-Scale Purification, Characterization, and Spore Outgrowth Inhibitory Effect of Thurincin H, a Bacteriocin Produced by Bacillus thuringiensis SF361. Probiotics Antimicrob Proteins 2014, 6, 105–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).