Abstract
The growing antimicrobial resistance to last-line antimicrobials among Gram-positive pathogens remains a major healthcare emergency worldwide. Therefore, the search for new small molecules targeting multidrug-resistant pathogens remains of great importance. In this paper, we report the synthesis and in vitro antimicrobial activity characterisation of novel thiazole derivatives using representative Gram-negative and Gram-positive strains, including tedizolid/linezolid-resistant S. aureus, as well as emerging fungal pathogens. The 4-substituted thiazoles 3h, and 3j with naphthoquinone-fused thiazole derivative 7 with excellent activity against methicillin and tedizolid/linezolid-resistant S. aureus. Moreover, compounds 3h, 3j and 7 showed favourable activity against vancomycin-resistant E. faecium. Compounds 9f and 14f showed broad-spectrum antifungal activity against drug-resistant Candida strains, while ester 8f showed good activity against Candida auris which was greater than fluconazole. Collectively, these data demonstrate that N-2,5-dimethylphenylthioureido acid derivatives could be further explored as novel scaffolds for the development of antimicrobial candidates targeting Gram-positive bacteria and drug-resistant pathogenic fungi.
1. Introduction
Infections caused by Gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) remain among one of the most common infectious agents worldwide. Infections caused by MRSA and VRE are responsible for increased mortality rates among hospitalised patients with chronic illness [1,2]. Numerous virulence factors are harboured by MRSA and VRE as well as biofilm production often leading to infections in surgically implanted catheters, especially in patients receiving cancer chemotherapy, hematopoietic stem-cell transplantation, and solid-organ transplantation. The profound antimicrobial resistance among MRSA and VRSA shortens the available treatment options, resulting in the systemic manifestation of the pathogen and death. Therefore, it is important to develop and investigate novel compounds with antimicrobial activity directed to MRSA and VRE.
The ability of pathogens to form biofilms on indwelling catheters has previously been associated with greater morbidity [3,4]. Moreover, the formation of interkingdom biofilms, consisting of bacterial pathogens and Candida species worsens the clinical prognosis. Despite being the most common fungal pathogen in clinical settings responsible for great morbidity worldwide, Candida spp. can form synergistic relationships with S. aureus leading to bacterial protection from antimicrobial treatment. Moreover, there is evidence that S. aureus provides a niche for C. albicans to evade antifungal drugs and thus survive the treatment [5,6]. Moreover, the recent emergence of the highly resistant C. auris with an instinctive resistance to azoles remains an increasing hardcore threat. Therefore, it is critical to apply novel concepts and develop multifunctional compounds with the ability to evade pre-existing antimicrobial activity in Gram-positive pathogens and clinically important fungi [7,8,9].
The development of new antimicrobials is focused on aspects of enhancing antimicrobial properties as well as evading antimicrobial resistance of bacterial and fungal pathogens or restoring the susceptibility of the pathogens to clinically approved antimicrobials [10,11,12,13,14,15]. In addition, compounds should remain minimally toxic to the host and show good pharmacological properties. However, the main aspect of developing effective drugs is their structural characteristics and the rate of activity. Lipophilicity is a significant physicochemical parameter that influences the membrane’s transport and the binding’s ability to act [16,17,18,19]. The study of lipophilicity-related parameters of thiazole derivatives showed these compounds to be promising drug candidates [20].
Thiazole derivatives are a family of heterocyclic compounds with large-scale biological properties [21] and are well-known in medicinal chemistry as promising drug candidates [22,23,24]. Synthesis of variously substituted thiazole ring led to novel compounds with numerous interesting pharmacological properties, including antibacterial [25,26,27,28,29], antifungal [15,20,23,30,31], antiviral [32,33,34,35], anthelmintic [36], antihypertensive, antihistaminic [37,38] and analgesic [39,40,41] effect. For instance, a naphthyl-substituted thiazole was established to inhibit the allosteric cysteine in the p10 subunit of caspase-5, thus acting as a cell’s protective compound [42]. Furthermore, benzimidazole-thiazole hybrid [43] was shown to be a privileged scaffold with a potent anti-inflammatory effect [40], and benzene sulphonamide thiazoles revealed its potent inhibitory properties against DPP-4 [27].
The 2,5-dimethylphenyl scaffold is a common structural feature in many antimicrobial compounds, particularly in the class of compounds known as phenylpropanoids [44]. These compounds have been found to have antimicrobial activity against a wide range of microorganisms, including bacteria, fungi and viruses [45,46,47]. They have been the subject of extensive research for the development of new antimicrobial agents, particularly in the fight against antibiotic-resistant infections [48]. Some examples of drugs that have been developed from the 2,5-dimethylphenyl scaffold include antifungal echinocandins and antibacterial agents such as linezolid. Therefore, novel compounds bearing 2,5-dimethylphenyl substituents may pose antimicrobial activity against Gram-positive and Gram-negative pathogens with novel or emerging resistance mechanisms. To explore aminothiazole derivatives as novel candidates targeting clinically important and multidrug-resistant WHO priority pathogens, we generated a series of aminothiazole derivatives bearing N-2,5-dimethylphenyl and β-alanine and characterised their antimicrobial activity using pathogens with defined resistance mechanisms. In addition to that, we characterised anticancer activity using A549 and Caco-2 cell culture models. A SAR investigation of aminothiazole derivatives revealed the influence of modification to a carboxylic acid moiety on the antimicrobial activity of the synthesized compounds [49]. In this paper, we describe the synthesis and in vitro characterisation of antimicrobial and anticancer properties of a series of novel thiazole derivatives bearing 4-quinolone, quinoxaline, naphthoquinone, hydrazone, benzimidazole, and benzenesulphonamide moieties. It is known that thiazoles can be synthesized from α-bromoketone and thiourea via Hantzsch thiazole synthesis in high yields.
2. Results and Discussion
2.1. Synthesis
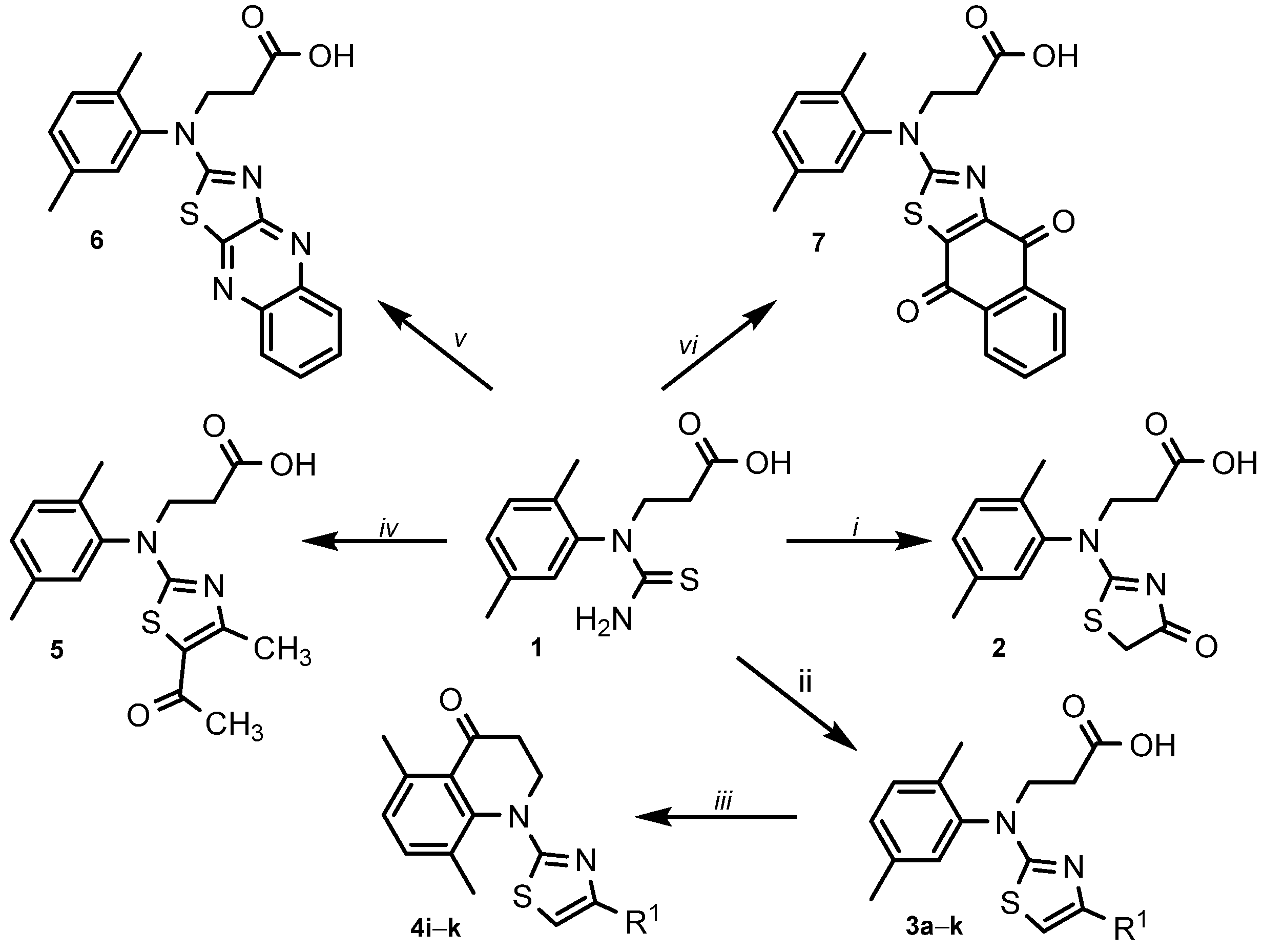
This research work is a continuation of projects on the synthesis and biological evaluation of various thiazole derivatives. 3-(1-(2,5-Dimethylphenyl)thioureido)propanoic acid (1) as the starting compound was synthesized by multistep reactions according to the method described in [50]. It is known that thiazoles can be synthesised from α-haloketone and a thioamide via Hantzsch thiazole synthesis in high yields [51]. To synthesize thiazolone 2 (Scheme 1), thioureido acid 1 was reacted with monochloroacetic acid in an aqueous 10% potassium carbonate solution at room temperature followed by the acidification with acetic acid to pH 6. In this reaction, the conventional reaction conditions at reflux were not suitable due to the formation of a large number of impurities. A singlet at 3.91 ppm of CH2 group protons in the 1H NMR spectrum and resonance lines at 183.3 ppm (C=O) and 187.0 ppm (C=N) in the 13C NMR spectrum of compound 2 confirmed the formation of the thiazolone ring. 2-Amino-1,3-thiazole derivative 3a was obtained from thioureido acid 1 and a chloroacetaldehyde 50% aqueous solution. The reaction was performed by refluxing acetone for 12 h. Product 3a was obtained in the form of water-soluble hydrochloride salt [52]. The convenience of the method is the crystallisation of the product from the reaction mixture already during the process. The data of the 1H and 13C NMR spectra of synthesized compounds 1, 2 3a are included in the Supplementary Material (Figures S1–S6).
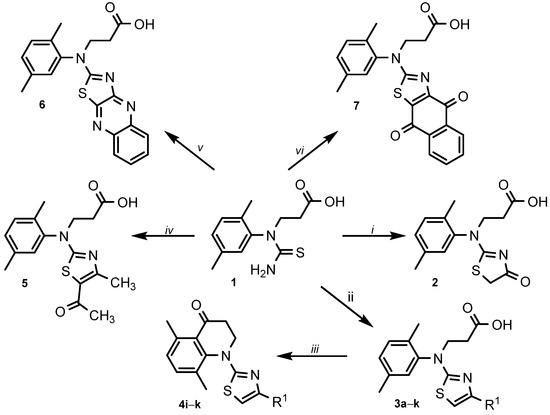
Scheme 1.
Synthesis of thiazole derivatives 2–7. R1 = (a) H; (b) CH3; (c) C6H5; (d) 4-F-C6H4; (e) 4-CN-C6H4; (f) 4-Cl-C6H4; (g) 4-NO2-C6H4; (h) 3,4-diCl-C6H4; (i) 4-Br-C6H4; (j) naphthalen-2-yl; (k) chromenon-3-yl. Reagents and conditions: (i) ClCH2COOH, 10% K2CO3, r.t. 24 h, AcOH to pH 6; (ii) a 50% ClCH2CHO, acetone, reflux, 12 h; b ClCH2COCH3, water, rt, 24 h, AcONa, heated to boil; c–k α-bromoacetophenone, acetone, reflux, 2–5 h, (iii) PPA, 120 °C, 2–3 h, crushed ice; (iv) 3-chloropentane-2,4-dione, acetone, reflux, 2 h; (v) 2,3-dichloroquinoxaline or (vi) 2,3-dichloro-1,4-naphthoquinone, AcOH, AcONa, rt, 24 h, 70–80 °C, 10 h, water.
Next, the 4-methyl substituted thiazole 3b was synthesized as shown in Scheme 1. Previously, in [53], 4-methyl-2-aminothiazole was prepared in acetone, but for 3b, the interaction of thioureido acid 1 with chloroacetone proceeded better in water than in acetone. The reaction at room temperature for 24 h and the following addition of sodium acetate to the reaction mixture gave the desired 4-methyl-1,3-thiazole derivative 3b, the structure of which was easily confirmed by the NMR spectral data (Supplementary Material, Figures S7 and S8).
According to the publication [54], 4-substituted thiazoles and especially those with chromen-3-yl and naphthalen-2-yl moieties demonstrate convincing antibacterial properties. Based on this, we have prepared a library of 4-substituted thiazoles 3c–k. The well-known Hantzsch thiazole synthesis method was applied and the generation of the target products 3c–k was performed by condensation of thioureido acid 1 with a series of α-bromoacetophenones without the use of a base. The isolated hydrobromide salts were transformed to free-based by dissolving them in 10% aqueous sodium carbonate and acidifying the solutions with acetic acid to pH 6. The structures of the obtained compounds 3c–k were confirmed by the data of the 1H and 13C NMR spectra (Supplementary Material, Figures S9–S26).
The activity of broad-spectrum quinolone-based antibiotics against both Gram-positive and Gram-negative bacteria, including mycobacteria and anaerobes, promotes the synthesis and development of new quinolone-type compounds which are relevant to the development of knowledge on this topic [55]. Heating of N-aryl-β-alanines with strong dehydrating agents such as Eaton’s reagent [56], polyphosphoric acid [57,58] or phosphorus pentoxide [59] causes the formation of compounds containing 2,3-dihydroquinolin-4(1H)-one moiety. To obtain quinolone-type compounds 4i–k, thiazoles 3i–k were treated with polyphosphoric acid at 120 °C. The intramolecular cyclisation occurred over 2–3 h and 2,3-dihydroquinolin-4(1H)-ones 4i–k were obtained in 71–88% yield. The data of the 1H and 13C NMR spectra of synthesized compounds 4i–k are included in the Supplementary Material (Figures S27–S32).
Compounds bearing a 5-acetyl-4-methylthiazole structure were found to exhibit high cytotoxicity against the MCF-7 cell line [60] as well as demonstrate up-and-coming antimicrobial properties [61,62]. For that purpose, we have included the synthesis of a compound containing this fragment into the goals of our study, and 5-acetyl-4-methylthiazole 5 was prepared by the same technique as for 3c–k. The product was separated in 75% yield and its structure was confirmed by the methods of IR, NMR spectroscopy and the data of elemental analysis (NMR data are included in the Supplementary Material, Figures S33 and S34).
The previous study [63] on the synthesis and the assessment of biological properties of thiazole derivatives revealed naphthoquinone-fused derivatives to demonstrate good antimicrobial properties against Gram-positive and Gram-negative bacteria strains. The efforts to synthesize quinoxaline- and naphthoquinone-fused thiazoles 6 and 7 by the interaction of thioureido acid 1 with 2,3-dichloroquinoxaline or 2,3-dichloro-1,4-naphthoquinone, respectively, were successful. The stirring of the reaction mixture in glacial acetic acid with the presence of sodium acetate in the mixture at room temperature for 24 h and the additional stirring at the higher temperature of 70–80 °C for 10 h afforded thiazoles 6 and 7 (NMR data are included in the Supplementary Material, Figures S35–S38). An even higher temperature of the reaction mixture strongly reduces the yield of the products due to the formation 1-(2,5-dimethylphenyl)-2-thioxotetrahydropyrimidin-4(1H)-one caused by the intramolecular cyclisation of thioureido acid 1 [64]. Noteworthy, Matsuoka et al. state that in some cases the reactions of 2,3-dichloro-1,4-naphthoquinone with thioamides, thiourea and dithiooxamide led to the formation of dibenzo[b,i]thianthrene-5,7,12,14-tetraone as the main product of the reaction [65,66,67]. The same by-product was identified in the synthesis route of 3-[(2,5-dimethylphenyl)(4,9-dioxo-4,9-dihydronaphtho [2,3-d][1,3]thiazol-2-yl)amino]propanoic acid (7).
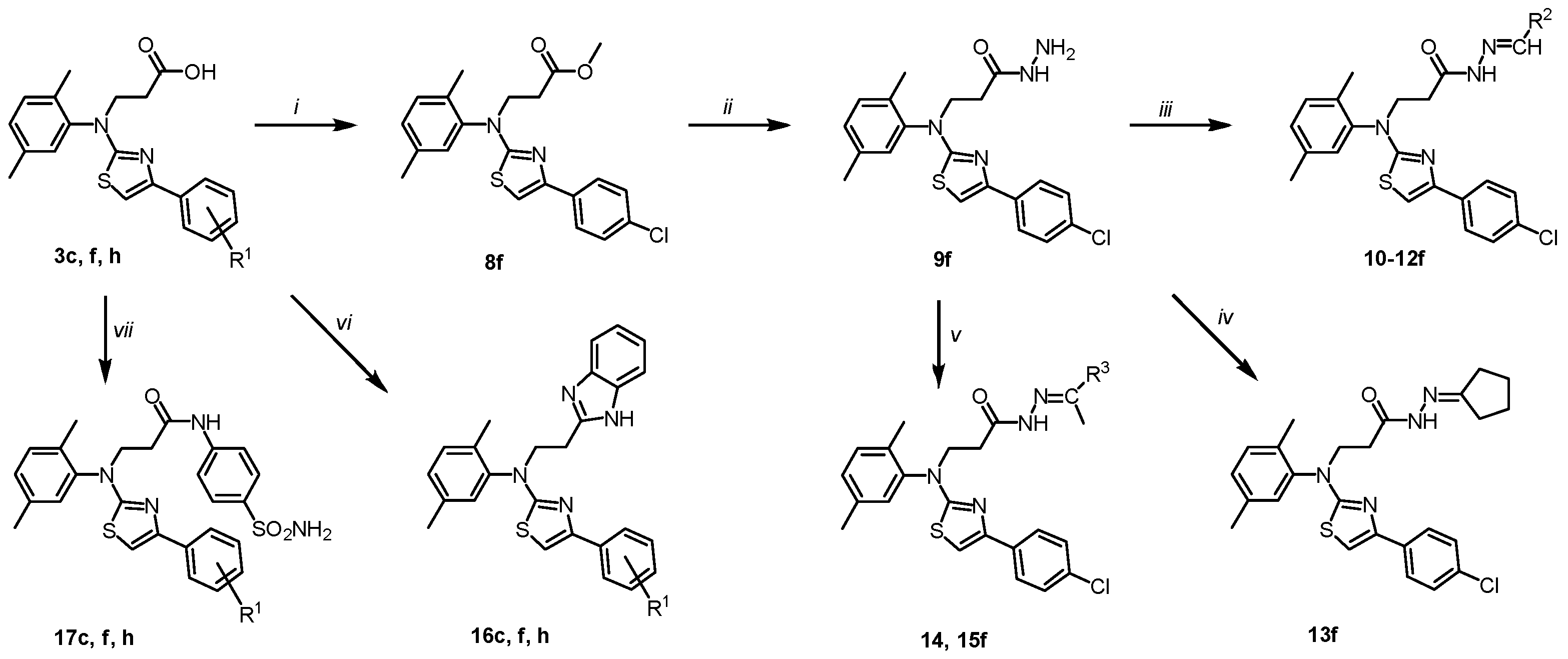
The carboxyl functional group can be an important constituent of a pharmacophore [68]. Its functionalisation can greatly expand the library of biological properties of compounds [69,70]. To expand the library of thiazole derivatives and to evaluate the influence of substituents on their biological properties, some transformations of the carboxyl group were performed (Scheme 2). Firstly, acid 3f was esterified with methanol to obtain ester 8f. No additional catalyst was required for this reaction with hydrobromide analogue. Afterwards, ester 8f was refluxed in 1,4-dioxane with hydrazine monohydrate and the resulting acid hydrazide 9f was then condensed with aldehydes and ketones. The structure of 9f was confirmed by the NMR techniques and microanalysis data. The presence of the CONHNH2 group protons were indicated by the singlets at 9.11 (NH) and 4.07 (NH2) ppm in the 1H NMR spectrum of 9f, whereas the spectral line of the carbon of the C=O group resonated at the characteristic area of the 13C NMR spectrum, i.e., at 169.4 ppm (Supplementary Material, Figures S39 and S42).
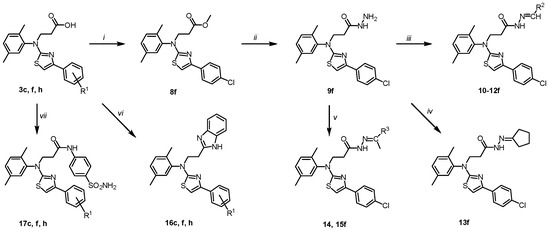
Scheme 2.
Chemical transformations of carboxylic acids 3c, f, h and hydrazide 9f. (3, 16, 17c) R1 = H; (3, 16, 17f) R1 = 4-Cl; (3, 16, 17h) R1 = 2,4-diCl; (10f) R2 = 5-nitrothiophen-2-yl; (11f) R2 = 5-nitrofuran-2-yl; (12f) R2 = indol-3-yl; (14f) R3 = Et; (15f) Hex. Reagents and conditions: (i) MeOH, a few drops of conc. H2SO4, reflux, 4 h; 5% Na2CO3 (ii) N2H4·H2O, 1,4-dioxane, reflux, 5 h; (iii) corresponding aldehyde, 1,4-dioxane, a few drops of glacial acetic acid, reflux, 2–12 h; (iv) cyclopentanone, 1,4-dioxane, a few drops of conc. acetic acid, reflux, 3 h; (v) corresponding ketone, 1,4-dioxane, a few drops of conc. acetic acid, reflux, 4 (14) or 8 (15) h; (vi) o-phenylenediamine, 15% HCl, reflux 72 h, water, 10% K2CO3; (vii) sulphanilamide, TEA, DMF, rt, 0.5 h, HBTU, DMF, argon, r.t. 72 h, 10% K2CO3.
As previously reported in [71], 4-aryl substituted thiazoles bearing hydrazonyl fragments appeared to be an eligible scaffold for the future development of antifungal and antibacterial agents targeting highly resistant pathogenic microorganisms. Further, a series of various hydrazones were synthesized and investigated for their bioactivity. To obtain hydrazones 10–15f, hydrazide 9f was condensed with heterocyclic and aliphatic ketones. The products were isolated in a 75–85% yield. The NMR spectra of the synthesized hydrazones 10–15f showed that in the DMSO-d6 solution, they exist as a mixture of E/Z conformers due to the presence of a CO-NH fragment in the molecule and the restricted rotation around it; in the studies [57,72], the Z-form predominate are described NMR data are included in the Supplementary Material, Figures S43–S54).
One of the methods for the synthesis of a benzimidazole heterosystem involves to condensation of carboxylic acids with 1,2-diaminobenzenes. The target compounds 16c, f, h were synthesized by Phillip’s method (heating of both reagents in 4 M hydrochloric acid). The reflux for 72 h gave benzimidazoles 16c, f, h in good yields (NMR data are in the Supplementary Material, Figures S45–S60).
Finally, amides 17c, f, h were prepared by direct coupling of acids 3 with the sulphanilamide using HBTU as the coupling reagent and triethylamine as the base (Scheme 2).
The reactions were performed in dimethylformamide at room temperature in an inert argon atmosphere. The products were isolated by the dilution of the reaction mixture with an aqueous 10% potassium carbonate and were characterised using NMR and IR spectroscopy and elemental analysis (NMR data are included in the Supplementary Material, Figures S61–S66).
It is worth noting that the analysis of NMR spectra revealed that the CH2N protons are often observed as two broad singlets. The characteristic splitting of resonances was observed for all compounds except 4i–k. The literature in [64] suggests that such splitting of the proton resonances of the compounds possessing methyl group in the 2nd position of the benzene ring was caused by the restricted rotation of benzene and other N-substituents around the C(1′)-N(1) bond and the possibility of diastereomers.
2.2. Antibacterial Activity of Compounds 1–17 against Multidrug-Resistant Pathogens
In order to understand the structure-dependent antibacterial activity of novel thiazole derivatives 1–17, we first used a representative collection of multidrug-resistant Gram-positive and Gram-negative bacterial pathogens with genetically defined antimicrobial resistance profiles [73]. The bacterial strains were selected to represent WHO ESKAPE group pathogens with emerging and challenging antimicrobial resistance mechanisms. To do so, the compounds were screened by using clinically approved minimal inhibitory concentration (MIC) determination by using the Clinical Laboratory Standard Institute (CLSI) recommendations.
Novel thiazole derivatives 1–17 exhibited structure-dependent antibacterial and antifungal activity. Interestingly, compounds 1–17 failed to show antimicrobial activity against multidrug-resistant Gram-negative pathogens, such as K. pneumoniae, P. aeruginosa, A. baumannii and E. coli (MIC > 64 µg/mL), suggesting the possible existence of Gram-positive bacteria-derived targets of compounds 1–17 (Table 1). On the other hand, only 4-substituted thiazoles 3h, 3j and ring-fused 7 showed favourable activity against Gram-positive bacteria (S. aureus and E. faecium) harbouring multidrug-resistance profiles (Table 2). Compound 3h bearing 3,4-diCl-C6H3 moiety showed antimicrobial activity against S. aureus TCH 1516 (MIC 8 µg/mL) harbouring mecA gene conferring resistance to β-lactam antibiotics [1]. Moreover, 3h showed one-fold lover antimicrobial activity against E. faecium AR-0783 (MIC 16 µg/mL) strain-harbouring vanB gene conferring resistance to vancomycin. The chlorines on the 3,4-diCl-C6H4 substituent could pose a synergistic activity by targeting multiple cellular targets on bacterial cells, in comparison to mono chlorine derivatives, thus exerting more potent antimicrobial activity. Moreover, dichloro substitutions could potentially greatly enhance lipophilicity, thus increasing compound penetration to bacterial cells. Finally, chlorine atoms are electron-windrowing groups and diCl substitution can greatly increase the electrophilicity of the compounds, thus greatly increasing reactivity and stronger interaction with microbial targets. Therefore, the chlorines at 3,4-positions of the phenyl ring are important for antimicrobial activity against Gram-positive multidrug-resistant pathogens [53]. The introduction of the 4-Cl-C6H4 substitution (3f) or the replacement of 3,4-diCl-C6H4 with 4-fluoro (3d) or 4-bromo (3i) phenyl substituents results in a complete loss of antimicrobial activity (MIC >64 µg/mL) against S. aureus and E. faecium suggesting the high importance of 3,4-diCl-C6H4 for the antimicrobial activity. Interestingly, the introduction of naphthalen-2-yl substitution (3j) greatly enhances the antimicrobial activity against S. aureus and E. faecium (MIC 2 µg/mL).

Table 1.
In vitro antibacterial activity of compounds 1–17 against the representative, multidrug-resistant Gram-positive and Gram-negative bacterial strains harbouring genetically defined resistance mechanisms.

Table 2.
In vitro antibacterial activity of the most promising thiazole derivatives 3h, 3j and 7 against tedizolid/linezolid resistant S. aureus strains.
The replacement of the thiazole ring with the ring-fused structure strongly enhanced the antibacterial activity against S. aureus TCH 1516 strain (MIC 1 µg/mL), while the antibacterial activity against E. faecium AR-0783 increased two-fold (MIC 8 µg/mL) if compared to 3h (Table 1). The antibacterial activity of compound 7 against S. aureus was similar to daptomycin (MIC 1 µg/mL) and twice stronger than vancomycin (MIC 2 µg/mL) and excellent if compared to ampicillin (MIC > 64 µg/mL).
The emerging resistance to oxazolidinones (linezolid and tedizolid) which are last-line antimicrobial pharmaceuticals used to treat infections caused by Staphylococcus remains one of the major healthcare threats worldwide. Therefore, we further evaluated if the most promising thiazole derivatives 3h, 3j and 7 will be active against linezolid/tedizolid-resistant S. aureus strains (Table 2). The compounds 3h, 3j and 7 showed promising activity against linezolid/tedizolid-resistant S. aureus strains with MIC ranging from 1 to 32 µg/mL (Table 2). Compound 3j showed the highest activity against tested strains (MIC 1–2 µg/mL), and its antimicrobial activity was greater than linezolid (8–32 µg/mL).
Collectively, these results demonstrated that substituted thiazoles exert in vitro antibacterial activity against Gram-positive bacterial pathogens harbouring emerging antibacterial resistance profiles. Moreover, 3,4-diCl-C6H3 and naphthalen-2-yl substitutions, as well as naphthothiazoledione moiety, are important for the potent in vitro antibacterial activity against S. aureus with challenging resistance mechanisms.
2.3. Antifungal Activity of Thiazoles 1–17 against Drug-Resistant Candida Species
Antifungal drugs of the azole group are one of the most widely used for the treatment of invasive and systemic fungal infections [74]. Candida species are responsible for the majority of fungal infections, as rapidly rising resistance to azole antifungals among Candida species, in particular C. albicans is responsible for increased morbidity and mortality worldwide [75]. We, therefore, evaluated novel thiazoles 1–17 for their in vitro antifungal activity against multidrug-resistant Candida species by exposing the fungal strains with clinically relevant concentrations of each compound or control antifungal drug (Table 3).

Table 3.
In vitro antifungal activity of compounds 1–17 against the representative, multidrug-resistant fungal pathogens.
Interestingly, only thiazoles 8f, 9f and 14f showed in vitro antifungal activity against Candida strains, expressing the multidrug-resistance phenotype to azoles (Table 3). The transformation of thiazole 3f (MIC > 64 µg/mL) to ester 8f was observed to result in striking changes in antifungal activity. (Table 3). The derivative 8f showed antifungal activity against all tested strains (1–8 µg/mL) including Candida auris AR-0383 strains to harbour instinctive resistance to clinically approved antifungal drugs. The ester 8f transformation to hydrazide 9f greatly affected the antifungal activity. The hydrazide 9f was no longer active against C. auris (MIC > 64 µg/mL) and had decreased activity against C. albicans (MIC 8 µg/mL) in comparison to ester 8f (MIC 1 µg/mL). Interestingly, despite the loss of activity against C. auris, hydrazide 9f showed increased activity against C. parapsilosis AR-0335 (MIC 4 µg/mL). Numerous hydrazones were previously reported to show antifungal activity [76,77,78]; therefore, we further transformed hydrazide 9f to the corresponding hydrazones 10–15f and evaluated their structure-dependent antifungal activity in vitro. Hydrazone 14f bearing the butan-2-ylidene substitution exhibited antifungal activity against the majority of tested fungi (MIC 8–32 µg/mL) with the exception of C. auris (MIC > 64 µg/mL). The substitution of this moiety with a longer octan-2-ylidene carbon chain (15f) results in a complete loss of antifungal activity against all tested strains.
Thus, these results demonstrate novel thiazole derivatives 9f, 14f and, especially, 8f with a moderately functionalised carboxyl group exert the most promising antifungal activity against highly multidrug-resistant fungal pathogens. On the other hand, the functionalization of the carboxyl group is important for the broad-spectrum antifungal activity since bulky substitutions result in decreased antifungal activity spectrum or potency in these novel compounds.
2.4. Anticancer Activity of Compounds 1–17
The thiazole scaffold of often explored by medicinal chemists as an attractive pharmacophore for the development of novel antineoplastic drug candidates [79,80,81]. Therefore, we used two well-described pulmonary (A549) [82] and corectal adenocarcinoma (Caco-2) [83] models to evaluate the anticancer activity of compounds 1–17. We exposed the cells to 100 µM of each compound or cisplatin (CP) that was used as a clinically approved drug for the neoplastic disease treatment. The treatment was initiated for 24 h and after that, the cellular viability was measured by using MTT assay.
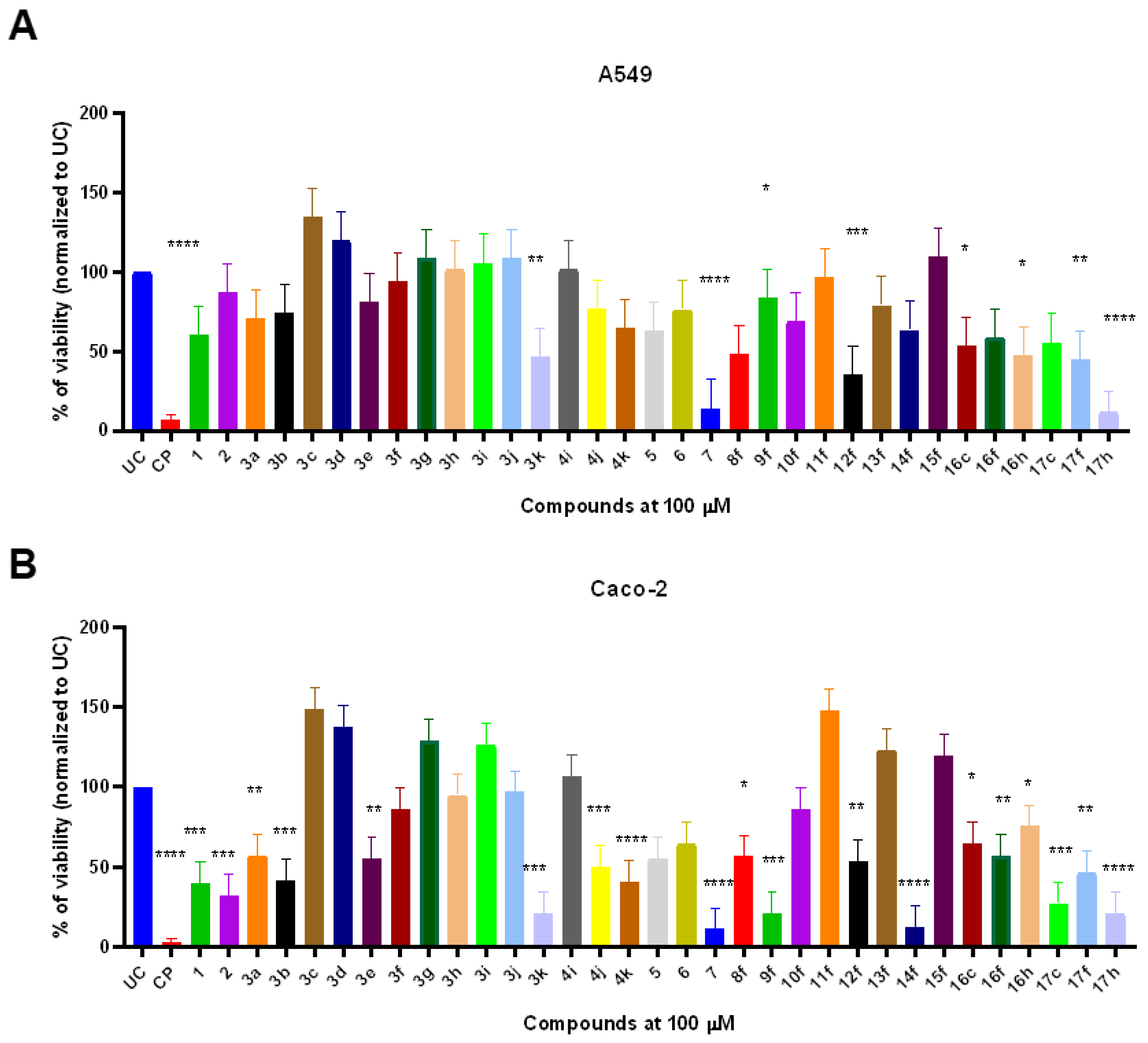
The novel thiazoles 1–17 demonstrated structure-dependent anticancer activity on A549 and Caco-2 cells. Interestingly, Caco-2 corectal adenocarcinoma cells showed significantly higher susceptibility to the treatment compounds in comparison to A549 cells (p < 0.05) suggesting the possible existence of selective novel thiazole-response involved targets in Caco-2. The starting compound 1 showed no significant anticancer activity against A549 human pulmonary adenocarcinoma cells. On the other hand, compound 1 significantly decreased the viability of Caco-2 cells (39.8%) in comparison to untreated control (UC) (p < 0.001) (Figure 1B). Thiazolone 2 resulted in slightly enhanced anticancer activity against Caco-2 (31.9%) while 2-amino-1,3-thiazole derivative 3a showed decreased (56.9%) anticancer activity in comparison to UC (p = 0.0019). The addition of a 4-methyl group on the 1,3-thiazole ring (3b) enhanced the anticancer activity against Caco-2 cells (p = 0.004). Interestingly, compounds 2–3b showed no anticancer activity against A549 cells (Figure 1A).
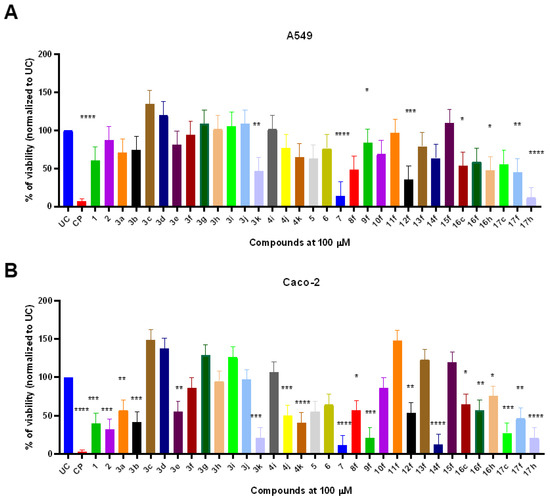
Figure 1.
The in vitro anticancer activity of compounds 1–17 on A549 (A) and Caco-2 (B) adenocarcinoma cell line. Cells were exposed with fixed 100 µM of compounds 1–17 or cisplatin (CP) that served as a cytotoxicity control for 24 h. After the treatment, the remaining viability was determined by using MTT assay. Data represent the mean ± SD of triplicate experiments (n = 3). Statistical differences were determined using the Kruskal–Wallis test. * p < 0.05; ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Amongst 4-substituted thiazoles, only 3e bearing 4-CN-C6H4 and 3k with chromenon-3-yl substitutions showed anticancer activity against A549 and Caco-2 cells. Thiazole 3e (R: 4-CN-C6H4) was able to significantly reduce the Caco-2 viability (54.9%) (p = 0.0011) while having no significant effect against A549 cells. Interestingly, the incorporation of chromenon-3-yl moiety (3k) significantly enhances the anticancer activity against Caco-2 (p = 0.001) as well as A549 cells (p = 0.0084) (Figure 1A,B).
Quinolone-type compound 4i bearing 4-Br-C6H4 substituent failed to significantly decrease the Caco-2 and A549 viability (106.1 and 101.4%, respectively). The incorporation of naphthalen-2-yl substitution (4j) significantly enhanced the anticancer activity against Caco-2 cells (p = 0.002) while chromenon-3-yl substitution (4k) resulted in significant 40.2% post-treatment viability of Caco-2 cells (p < 0.001) (Figure 1B).
5-Acetyl-4-methylthazole 5 did not significantly reduce the viability of A549 or Caco-2, although the compound was able to decrease the viability to 63 and 55%, respectively. Thiazole fusion with quinoxaline (compound 6) did not result in significant viability reduction, although fusion with naphthoquinone lead to compound 7 with significantly high anticancer activity. Compound 7 exhibited broad-spectrum anticancer activity and was able to reduce A549 and Caco-2 viability to 14.0 and 13.1% compared to UC (p < 0.001) (Figure 1A,B).
The carboxyl group transformation to ester 8f in compound 3f slightly enhanced the anticancer activity if compared to parent acid 3f and showed similar anticancer efficacy against A549 and Caco-2 by reducing the viability to 48.1 and 56.4%, respectively. Interestingly subsequent ester 8f transformation to hydrazide 9f greatly enhanced the anticancer activity against Caco-2 cells (20.6%) while ameliorating compound-mediated cytotoxic activity against A549 cells (Figure 1A,B).
On the other hand, hydrazones 10–15f showed highly structure-dependent activity. Hydrazones 10f and 11f bearing the 5-nitrothien-2-yl and 5-nitrofuran-2-yl substitutions showed no anticancer activity against both tested cancer cell lines. Compound 12f containing indol-3-yl substitution demonstrated greater anticancer activity against A549 (35.0%) than Caco-2 (53.1%). The incorporation of cyclopentyl substitution resulted in compound 13f with a complete loss of anticancer activity against both cell lines, while the incorporation of ethyl radical (14f) significantly enhanced the anticancer activity against Caco-2 cells, but not A549. On the other hand, the incorporation of the hexyl chain (15f) resulted in a complete loss of anticancer activity against Caco-2, suggesting the importance of ethyl radical in novel thiazole structures (Figure 1A,B).
Benzimidazoles 16c,f,h demonstrated similar anticancer activity in both A549 and Caco-2 cell lines by decreasing viability to approximately 47–60%. Amide 17c containing 4-phenyl substituted thiazole showed potent anticancer activity against Caco-2 cells (27.2%) and little activity against A549 cells (55.4%). Compound 17f bearing 4-Cl-C6H4 substituent at 4th position of the thiazole cycle showed similar activity in both cell lines (44.3 and 46.0%, respectively, while the incorporation of 2,4-diCl-C6H3 into the same position resulted in strong anticancer activity against both cell lines (Figure 1A,B).
These results demonstrate that novel thiazole derivatives possess strong anticancer activity against different adenocarcinoma cell lines. The structure–activity relation studies showed that naphthoquinone-fusion and 2,4-diCl-C6H3 substitution are required for broad-spectrum anticancer activity.
3. Materials and Methods
3.1. Synthesis
All reagents and used solvents were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used as received without any purification. Reaction progress and compound purity were monitored by TLC using aluminium plates precoated with Silica gel with F254 nm (Merck KGaA, Darmstadt, Germany). Melting points were determined in an open capillary with a B-540 melting point apparatus (Büchi Corporation, New Castle, DE, USA) and were uncorrected. A Perkin–Elmer Spectrum BX FT–IR spectrometer (Perkin–Elmer Inc., Waltham, MA, USA) was used to record the IR spectra (ν, cm−1) and the pellets were prepared using KBr. The NMR spectra were recorded on Bruker Ascend 400 (1H 400 MHz, 13C 101 MHz) and Bruker Ascend (1H 700 MHz, 13C 176 MHz) spectrometers (Bruker BioSpin AG, Fällanden, Switzerland) using DMSO-d6 and CDCl3 as solvents and TMS as an internal reference. The spectra data are presented as follows: chemical shift, multiplicity, integration, coupling constant [Hz] and allocation. The Elemental Analyzer CE-440 was used for elemental analyses (C, H, N) in good agreement (±0.3%) with the calculated values.
3-[Carbamothioyl(2,5-dimethylphenyl)amino]propanoic acid (1). The thioureido acid 1 was synthesized according to the method described in the literature [64]. Yield 85%, m.p. 134–135 °C (from water).
IR (KBr): ν 1167 (C=S), 1746 (C=O), 3342 (NH2), 3445 (OH) cm−1.
1H NMR (400 MHz, CDCl3): δ 2.13 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.49–2.56 (m, 2H, CH2CO), 3.76 (br. s,, 1H, NCH2), 4.44 (br. s, 1H, NCH2), 5.95 (br. s, 1H, NH2), 6.96 (s, 1H, HAr), 7.10 (s, 1H, HAr), 7.21 (s, 1H, HAr), 7.50 (br. s, 1H, NH2), ppm.
13C NMR (101 MHz, CDCl3): δ 16.9 (CH3), 21.6 (CH3), 32.9 (CH2CO), 50.3 (NCH2), 128.4, 130.3, 132.1, 132.3, 138.1, 138.9 (CAr), 176.9 (COOH), 181.6 (C=O) ppm.
Anal. Calcd for C12H16N2O2S: C, 57.12%; H, 6.39%; N, 11.10%; Found: C, 57.33%; H, 6.10%; N, 10.84%.
3-[(2,5-Dimethylphenyl)(4-oxo-4,5-dihydro-1,3-thiazol-2-yl)amino]propanoic acid (2). A mixture of thioureido acid 1 (1.26 g, 5 mmol), 40 mL of aqueous 10% potassium carbonate and chloroacetic acid (0.57 g, 6 mmol) was stirred at room temperature for 24 h. Then, the mixture was acidified with acetic acid to pH 6. Obtained the precipitate was filtered off, washed with water, and crystallized. Yield 0.88 g, 60%; m.p. 159–160 °C (from 2-propanol and water mixture 1:1).
IR (KBr): ν 1581 (C=N), 1674, 1725 (2C=O), 3290 (OH) cm−1.
1H NMR (700 MHz, DMSO-d 6): δ 2.16 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.57 (t, 2H, J = 7.5 Hz, CH2CO), 3.72–3.81 (m, 1H, NCH2), 3.91 (s, 2H, SCH2), 4.24–4.39 (m, 1H, NCH2), 7.16 (s,1H, HAr), 7.22 (d, J = 7.8 Hz, 1H, HAr), 7.29 (d, J = 7.7 Hz, 1H, HAr), 11.07 (br. s, 1H, OH) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.5 (CH3), 20.3 (CH3), 32.3 (CH2CO), 40.5 (SCH2), 49.5 (NCH2), 129.2, 130.6, 131.4, 132.7, 136.9, 138.7 (CAr), 172.2 (COOH), 183.2 (C=O), 187.0 (C=N) ppm.
Anal. Calcd for C14H16N2O3S: C, 57.52%; H, 5.52%; N, 9.58%; Found: C, 57.27%; H, 4.28%; N, 9.39%.
3-[(2,5-Dimethylphenyl)(1,3-thiazol-2-yl)amino]propanoic acid (3a). A mixture of the thioureido acid 1 (0.5 g, 2 mmol), aqueous 50% chloroacetaldehyde solution (0.79 g, 10 mmol) and acetone (20 mL) was refluxed for 12 h. After that, the formed precipitate was filtered, washed with acetone and dried. Purification was performed by dissolving the crystals in aqueous 10% sodium carbonate (75 mL), filtering, and acidifying the filtrate with acetic acid to pH 6 (the procedure was repeated 2 times). Yield 0.35 g, 64%, m.p. 112–113 °C.
IR (KBr): ν 3076 (OH), 1717 (CO), 1514 (CN) cm−1.
1H NMR (700 MHz, DMSO-d 6): δ 2.10 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.62 (t, J = 7.3 Hz, 2H, CH2CO), 3.98 (br. s, 2H, NCH2), 6.66, 7.16 (2d, 2H, J = 3.6 Hz, SCH, NCH), 7.11 (s, 1H, HAr), 7.13–7.16 (m, 1H, HAr), 7.26 (d, 1H, J = 7.8 Hz, HAr), 12.29 (s, 1H, OH) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.7 (CH3), 20.4 (CH3), 32.4 (CH2CO), 48.1 (NCH2), 108.0, 129.1, 129.2, 131.6, 133.0, 137.2, 139.3, 143.0 (CAr + SCH + NCH), 170.1 (C=N), 172.7 (COOH) ppm.
Anal. Calcd for C14H16N2O2S: C, 60.85%; H, 5.48%; N, 10.14%; Found: C, 60.67%; H, 5.62%; N, 9.87%.
3-[(2,5-Dimethylphenyl)(4-methyl-1,3-thiazol-2-yl)amino]propanoic acid (3b). A mixture of thioureido acid 1 (1.26 g, 5 mmol), chloroacetone (0.64 g, 7 mmol) and water (20 mL) was stirred at room temperature for 24 h. Then, sodium acetate (0.82 g, 10 mmol) was added and the reaction mixture was brought to reflux. After cooling, the formed precipitate was filtered off and washed with water. Obtained organic salt was transformed to free-base by dissolving the crystals in 10% aqueous sodium carbonate (75 mL), filtering, and acidifying the filtrate with acetic acid to pH 6 (the purification procedure was repeated 2 times). Yield 0.88 g, 67%, m.p. 155–156 °C.
IR (KBr): ν 1527 (C=N), 1706 (C=O), 3392 (OH) cm−1.
1H NMR (700 MHz, DMSO-d 6): δ 2.09 (s, 3H, CH3), 2.15 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.60 (t, 2H, J = 7.3 Hz, CH2CO), 3.96 (br. s, 2H, NCH2), 6.20 (d, 1H, J = 1.1 Hz, SCH), 7.09 (d, 1H, J = 1.2 Hz, HAr), 7.12 (dd, 1H, J = 7.9, 1.3 Hz, HAr), 7.25 (d, 1H, J = 7.8 Hz, HAr), 12.35 (s, 1H, OH) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.8 (CH3), 17.6 (CH3), 20.4 (CH3), 32.4 (CH2CO), 47.9 (NCH2), 102.1 (SCH), 129.2, 129.4, 131.6, 133.1, 137.1, 142.8, 148.5 (CAr + NC), 169.3 (C=N), 172.8 (COOH) ppm.
Anal. Calcd for C15H18N2O2S: C, 62.04%; H, 6.25%; N, 9.65%; Found: C, 62.02%; H, 6.28%; N, 9.40%.
General procedure for the synthesis of compounds3c–k. A mixture of the thioureido acid 1 (0.5 g, 2 mmol), the appropriate α-bromoacetophenone (2.2 mmol) and acetone (20 mL) was refluxed for 2–5 h and cooled down. The precipitate was filtered off, washed with acetone and dried. Obtained organic salts were transformed to free-base by dissolving the crystals in 10% aqueous sodium carbonate (75 mL), filtering and acidifying the filtrate with acetic acid to pH 6 (the purification procedure was repeated 2 times).
3-[(2,5-Dimethylphenyl)(4-phenyl-1,3-thiazol-2-yl)amino]propanoic acid (3c)
Yield 0.47 g, 67%, m.p. 104–105 °C.
IR (KBr): ν 1537 (C=N), 1710 (C=O), 3390 (OH) cm−1.
1H NMR (700 MHz, DMSO-d6): δ 2.14 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.63 (t, 2H, J = 7.3 Hz, CH2CO), 4.07 (br. s, 2H, NCH2), 7.08 (s, 1H, SCH), 7.11–7.17 (m, 2H, HAr), 7.25–7.30 (m, 2H, HAr), 7.39 (t, 2H, J = 7.7 Hz, HAr) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 33.1 (CH2CO), 48.5 (NCH2), 102.6 (SCH), 125.7, 127.4, 128.5, 129.2, 129.3, 131.5, 133.1, 134.8, 137.2, 142.7, 150.5 (CAr + NC), 169.3, (C=N), 173.1 (COOH) ppm.
Anal. Calcd for C20H20N2O2S: C, 68.16%; H, 5.72%; N, 7.95%; Found: C, 68.08%; H, 5.66%; N, 7.73%.
3-{(2,5-Dimethylphenyl)[4-(4-fluorophenyl)-1,3-thiazol-2-yl]amino}propanoic acid (3d)
Yield 0.56 g, 75%, m.p. 91–92 °C.
IR (KBr): ν 1223 (C-F), 1540 (C=N), 1711 (C=O), 3384 (OH) cm−1.
1H NMR (700 MHz, DMSO-d6): δ 2.14 (s, 3H, CH3), 2.30 (s, 3H, 2CH3), 2.68 (t, 2H, J = 7.2 Hz, CH2CO), 4.07 (br. s, 2H, NCH2), 7.07 (s, 1H, SCH), 7.12–7.17 (m, 2H, HAr), 7.22 (t, 2H, J = 8.8 Hz, HAr), 7.28 (d, 2H, J = 8.2 Hz, HAr), 7.85–7.95 (m, 2H, HAr) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 32.6 (CH2CO), 48.2 (NCH2), 102.5 (SCH), 115.3, 115.4, 127.7, 129.3, 129.4, 131.3, 131.6, 133.0, 137.2, 142.7, 149.4, 160.9, 162.3 (CAr + NC), 169.4 (C=N), 172.8 (COOH) ppm.
Anal. Calcd for C20H19FN2O2S: C, 64.85%; H, 5.17%; N, 7.56%; Found: C, 64.87%; H, 5.20%; N, 7.18%.
3-{[4-(4-Cyanophenyl)-1,3-thiazol-2-yl](2,5-dimethylphenyl)amino}propanoic acid (3e)
Yield 0.60 g, 80%, m.p. 132–133 °C.
IR (KBr): ν 1536 (C=N), 1737 (C=O), 2223 (C≡N), 3261 (OH) cm−1.
1H NMR (700 MHz, DMSO-d6): δ 2.15 (2s, 3H, CH3), 2.30 (2s, 3H, CH3), 2.65–2.75 (m, 2H, CH2CO), 3.86–4.28 (m, 2H, NCH2), 7.12–7.21 (m, 2H, HAr + SCH), 7.25–7.32 (m, 1H, HAr), 7.41 (d, 1H, J = 8.0 Hz, HAr), 7.85 (dd, 2H, J = 8.0, 4.9 Hz, HAr), 8.05 (m, 2H, J = 8.3 Hz, HAr) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.6 (CH3), 20.4 (CH3), 32.2 (CH2CO), 47.4 (NCH2), 91.8 (SCH), 109.5, 119.0, 126.3, 129.5, 131.7, 132.61, 132.63, 133.0, 137.3, 138.8, 142.5, 148.7 (CAr + C≡N + NC), 169.6 (N=C), 172.7 (COOH) ppm.
Anal. Calcd for C21H19N3O2S: C, 66.82%; H, 5.07%; N, 11.13%; Found: C, 66.63%; H, 5.04%; N, 11.06%.
3-{[4-(4-Chlorophenyl)-1,3-thiazol-2-yl](2,5-dimethylphenyl)amino}propanoic acid (3f)
Yield 0.64 g, 83%, m.p. 94–95 °C.
IR (KBr): ν 835 (C-Cl), 1536 (C=N), 1710 (C=O), 3391 (OH) cm−1.
1H NMR (700 MHz, DMSO-d6): δ 2.14, 2.16 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.57–2.75 (m, 2H, CH2CO), 4.10 (br. s, 2H, NCH2), 7.18 (d, 2H, J = 7.5 Hz, HAr + SCH), 7.30 (d, 1H, J = 7.6 Hz, HAr), 7.47, 7.54 (2d, 2H, J = 8.4 Hz, HAr), 7.80–8.01 (m, 2H, HAr) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 32.4 (CH2CO), 48.1 (NCH2), 103.7 (SCH), 127.4, 128.4, 128.6, 129.3, 129.4, 129.7, 131.7, 131.9, 133.0, 133.5, 137.3, 142.6, 149.1 (CAr + NC), 169.5 (C=N), 172.7 (COOH) ppm.
Anal. Calcd for C20H19ClN2O2S: C, 62.09%; H, 4.95%; N, 7.24%; Found: C, 62.27%; H, 4.91%; N, 7.03%.
3-{(2,5-Dimethylphenyl)[4-(4-nitrophenyl)-1,3-thiazol-2-yl]amino}propanoic acid (3g)
Yield 0.63 g, 79%, m.p. 136–137 °C.
IR (KBr): ν 1334, 1509 (NO2), 1540 (C=N), 1712 (C=O); 3430 (OH) cm−1.
1H NMR (700 MHz, DMSO-d6): δ 2.13 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.46–2.61 (m, 2H, CH2CO), 3.85–4.22 (m, 2H, NCH2), 7.08–7.18 (m, 2H, HAr + SCH), 7.26 (d, 1H, J = 8.2 Hz, HAr), 7.45 (s, 1H, HAr), 8.10 (d, 2H, J = 8.8 Hz, HAr), 8.24 (d, 2H, J = 8.9 Hz, HAr) ppm. 13C NMR (176 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 34.1 (CH2CO), 49.2 (NCH2), 107.3 (SCH), 124.0, 126.4, 129.3, 129.4, 131.6, 133.0, 137.2, 140.8, 142.5, 146.1, 148.5 (CAr + NC), 169.7 (C=N), 173.8 (COOH) ppm.
Anal. Calcd for C20H19N3O4S: C, 60.44%; H, 4.82%; N, 10.57%; Found: C, 60.50%; H, 4.64%; N, 10.35%.
3-{[4-(3,4-Dichlorophenyl)-1,3-thiazol-2-yl](2,5-dimethylphenyl)amino}propanoic acid (3h)
Yield 0.71 g, 84%, m.p. 114–115 °C.
IR (KBr): ν 1535 (C=N), 1710 (C=O), 3320 (OH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.11, 2.27 (2s, 6H, 2CH3), 2.46–2.61 (m, 2H, CH2CO), 3.85–4.22 (m, 2H, NCH2), 7.04–7.19 (m, 2H, HAr + SCH), 7.20–7.38 (m, 2H, HAr), 7.61 (d, 1H, J = 8.4 Hz, HAr), 7.83 (dd, 1H, J = 8.2, 1.4 Hz, HAr), 8.06 (s, 1H, HAr) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.8, 20.4 (2CH3), 33.8 (CH2CO), 48.9 (NCH2), 103.8 (SCH), 125.8, 127.1, 129.3, 129.4, 129.6, 130.8, 131.4, 131.6, 133.0, 135.3, 137.3, 142.5, 147.9 (CAr, N–C), 169.5 (C=N), 171.7 (COOH) ppm.
Anal. Calcd for C20H18Cl2N2O2S: C, 57.01%; H, 4.31% N, 6.65%; Found: C, 57.25%; H, 4.42%; N, 6.73%.
3-{[4-(4-Bromophenyl)-1,3-thiazol-2-yl](2,5-dimethylphenyl)amino}propanoic acid (3i)
Yield 0.64 g, 76%, m.p. 127–128 °C.
IR (KBr): ν 1539 (C=N), 1710 (C=O), 3310 (OH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.12 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.50–2.62 (m, 2H, CH2CO), 4.02 (br. s, 2H, NCH2), 7.06–7.30 (m, 4H, HAr + SCH), 7.56 (d, 2H, J = 8.5 Hz, HAr), 7.81 (d, 2H, J = 8.6 Hz, HAr) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 33.7 (CH2CO), 49.0 (NCH2), 103.4 (SCH), 120.4, 127.7, 129.3, 129.4, 131.5, 131.6, 133.1, 134.0, 137.2, 142.7, 149.3 (CAr, N–C), 169.5 (C=N); 173.7 (COOH) ppm.
Anal. Calcd for C20H19BrN2O2S: C, 55.69%; H, 4.44%; N, 6.49%; Found: C, 55.93%; H, 4.46%; N, 6.28%.
3-{(2,5-Dimethylphenyl)[4-(naphthalen-2-yl)thiazol-2-yl]amino}propanoic acid (3j)
Yield 0.68 g, 84%, m.p. 121–122 °C.
IR (KBr): ν 1536 (C=N), 1707 (C=O), 3363 (OH) cm−1.
1H NMR (700 MHz, DMSO-d6): δ 2.16 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.48–2.54 (m, 2H, CH2CO), 4.09 (br. s, 2H, NCH2), 7.06–7.18 (m, 2H, HAr + SCH), 7.21 (s, 1H, HAr), 7.27 (d, 1H, J = 7.7 Hz, HAr), 7.36–7.61 (m, 2H, HAr), 7.88, 7.95 (2d, 2H, J = 7.7 Hz, HAr), 7.90 (d, 1H, J = 8.6 Hz, HAr), 8.00 (d, 1H, J = 8.5 Hz, HAr), ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 34.6 (CH2CO), 49.3 (NCH2), 103.1 (SCH), 124.1, 124.2, 125.8, 126.3, 127.5, 128.0, 128.1, 129.2, 129.4, 131.5, 132.3, 132.4, 133.1, 133.2, 137.1, 142.7, 150.5 (CAr, N–C), 170.0 (C=N), 174.3 (COOH) ppm.
Anal. Calcd for C24H22N2O2S: C, 71.62%; H, 5.51%; N, 6.96%; Found: C, 71.79%; H, 5.57%; N, 6.91%.
3-{(2,5-Dimethylphenyl)[4-(2-oxo-2H-chromen-3-yl)thiazol-2-yl]amino}propanoic acid (3k)
Yield 0.72 g, 86%, m.p. 164–165 °C.
IR (KBr): ν 1540 (C=N), 1711, 1736 (2C=O), 3510 (OH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.15 (s, 3H, CH3), 2.31 (s, 3H, CH3), 2.70 (t, 2H, J = 7.0 Hz, CH2CO), 3.95–4.35 (m, 2H, NCH2), 7.13–7.23 (m, 2H, HAr + SCH), 7.30 (d, 1H, J = 7.9 Hz, HAr), 7.39 (t, 1H, J = 7.5 Hz, HAr), 7.44 (d, 1H, J = 8.3 Hz, HAr), 7.57 (s, 1H, HAr), 7.62 (t, 1H, J = 7.8 Hz, HAr), 7.90 (d, 1H, J = 7.7 Hz, HAr), 8.67 (s, 1H, HAr), 12.30 (s, 1H, OH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.7 (CH3), 20.4 (CH3), 32.4 (CH2CO), 47.7 (NCH2), 110.0, 115.9, 119.3, 120.5, 124.8, 128.9, 129.4, 129.6, 131.6, 131.7, 133.1, 137.3, 138.5, 142.4, 144.0, 152.3 (CAr, N–C, SCH), 158.8 (O–C=O), 168.8 (C=N), 172.8 (COOH) ppm.
Anal. Calcd for C23H20N2O4S: C, 65.70%; H, 4.79%; N, 6.66%; Found: C, 65.89%; H, 4.89%; N, 6.75%.
General procedure for the synthesis of compounds4i–k. A mixture of the corresponding compound 3i–k (2 mmol) and polyphosphoric acid (15 g) was stirred at 120 °C for 2–3 h; then, the reaction mixture was cooled down and crushed ice (150 g) was added. The precipitate was filtered off, washed with water and dried. Purification was performed by recrystallization from 2-propanol.
1-[4-(4-Bromophenyl)-1,3-thiazol-2-yl]-5,8-dimethyl-2,3-dihydroquinolin-4(1H)-one (4i)
Yield 0.73 g, 88%, m.p. 145–146 °C.
IR (KBr): ν 1507 (C=N), 1673 (C=O) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.21 (s, 3H, CH3), 2.53 (s, 3H, CH3), 2.79 (t, 2H, J = 6.1 Hz, CH2CO), 4.28 (t, 2H, J = 6.0 Hz, NCH2), 7.18 (d, 1H, J = 7.9 Hz, HAr), 7.42–7.49 (m, 2H, HAr + SCH), 7.57, 7.78 (2d, 4H, J = 8.5 Hz, HAr) ppm.
13C NMR (101 MHz, DMSO-d6): δ 18.1 (CH3), 22.4 (CH3), 39.3 (CH2CO), 49.8 (NCH2), 106.8, 121.3, 127.3, 128.2, 130.7, 131.9, 132.0, 133.9, 136.1, 139.1, 146.7, 149.9 (CAr, SCN, N-C), 168.5 (C=N), 196.7 (C=O) ppm.
Anal. Calcd for C20H17BrN2OS: C, 58.12%; H, 4.15%; N, 6.78%; Found: C, 58.32%; H, 4.23%; N, 6.80%.
5,8-Dimethyl-1-[4-(naphthalen-2-yl)-1,3-thiazol-2-yl]-2,3-dihydroquinolin-4(1H)-one (4j)
Yield 0.62 g, 81%, m.p. 141–142 °C.
IR (KBr): ν 1547 (C=N), 1740 (C=O) cm−1.
1H NMR (400 MHz, (CDCl3): δ 2.34 (s, 3H, CH3), 2.66 (s, 3H, CH3), 2.91 (t, 2H, J = 6.1 Hz, CH2CO), 4.48 (t, 2H, J = 6.0 Hz, NCH2), 6.96 (s, 1H, SCH), 7.12, 7.36 (2d, 2H, J = 7.8 Hz, HAr), 7.39–7.56 (m, 2H, HAr), 7.74–7.98 (m, 4H, HAr), 8.38 (s, 1H, HAr) ppm.
13C NMR (101 MHz, (CDCl3): δ 18.2 (CH3), 22.9 (CH3), 39.4 (CH2CO), 49.9 (NCH2), 104.6 (SCH), 124.1, 125.2, 126.1, 126.4, 127.2, 127.8, 128.4, 128.5, 130.7, 132.0, 132.1, 133.2, 133.8, 136.1, 140.3, 147.0, 152.1 (CAr, N–C), 169.0 (C=N), 196.9 (C=O) ppm.
Anal. Calcd for C24H20N2OS: C, 74.97%; H, 5.24%; N, 7.29%; Found: C, 75.01%; H, 5.20%; N, 7.35%.
5,8-Dimethyl-1-[4-(2-oxo-2H-1-benzopyran-3-yl)-1,3-thiazol-2-yl]-2,3-dihydroquinolin-4(1H)-one (4k)
Yield 0.57 g, 71%, m.p. 205–206 °C.
IR (KBr): ν 1518 (C=N), 1681, 1716 (2C=O) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.23 (s, 3H, CH3), 2.54 (s, 3H, CH3), 2.81 (t, 2H, J = 6.2 Hz, CH2CO,), 4.37 (t, 2H, J = 6.1 Hz, NCH2), 7.21 (d, 1H, J = 7.9 Hz, HAr), 7.34–7.54 (m, 3H, HAr), 7.56–7.65 (m, 1H, HAr), 7.79 (s, 1H, SCH), 7.86 (d, 1H, J = 6.9 Hz, HAr), 8.63 (s, 1H, HChrom) ppm.
13C NMR (101 MHz, DMSO-d6): δ 17.5 (CH3), 22.0 (CH3), 38.9 (CH2CO), 49.2 (NCH2), 112.0, 115.9, 119.2, 120.2, 124.7, 126.9, 128.9, 130.5, 131.3, 131.8, 135.8, 138.8, 139.1, 144.1, 146.2, 152.4 (CAr, SCN, N-C), 158.7 (O-C=O), 167.6 (C=N), 196.2 (CH2C=O) ppm.
Anal. Calcd for C23H18N2O3S: C, 68.64%; H, 4.51%; N, 6.96%; Found: C, 68.49%; H, 4.53%; N, 6.99%.
3-[(5-Acetyl-4-methyl-1,3-thiazol-2-yl)(2,5-dimethylphenyl)amino]propanoic acid (5). A mixture of the thioureido acid 1 (0.5 g, 2 mmol), 3-chloropentane-2,4-dione (0.29 g, 2.2 mmol) and acetone (20 mL) was refluxed for 2 h and cooled down. The formed precipitate was filtered off, washed with acetone and dried. Purification was performed by dissolving the crystals in 10% aqueous sodium carbonate, filtering and acidifying the filtrate with acetic acid to pH 6 (the procedure was repeated 2 times).
Yield 0.50 g, 75%, m.p. 172–173 °C.
IR (KBr): ν 1521 (C=N), 1656, 1717 (2C=O), 3631 (OH) cm−1.
1H NMR (700 MHz, DMSO-d6): δ 2.11 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.50 (s, 3H, CH3), 2.57–2.65 (m, 2H, CH2CO), 3.79–3.93 (m, 1H, NCH2), 4.13–4.32 (m, 1H, NCH2), 7.13 (s, 1H, HAr), 7.20 (d, 1H, J = 7.8 Hz, HAr), 7.30 (d, 1H, J = 7.8 Hz, HAr),12.36 (s, 1H, OH) ppm.
13C NMR (176 MHz, DMSO-d6): δ 16.5 (CH3), 18.6 (CH3), 20.4 (CH3), 29.5 (CH3), 32.1 (CH2CO), 47.5 (NCH2), 122.8, 128.8, 130.0, 131.8, 132.5, 137.5, 141.5, 157.5 (CAr + SC + NC), 170.5 (C=N), 172.3 (COOH), 188.6 (CH3C=O) ppm.
Anal. Calcd for C17H20N2O3S: C, 61.42%; H, 6.06%; N, 8.43%; Found: C, 61.19%; H, 6.20%; N, 8.53%.
General procedure for the preparation of compounds 6 and 7. A mixture of thioureido acid 1 (1.26 g, 5 mmol), 2,3-dichloroquinoxaline (6) (1 g, 5 mmol) or 2,3-dichloro-1,4-naphthoquinone (1.18 g, 5 mmol), sodium acetate (1.64 g, 20 mmol) and glacial acetic acid (20 mL) was stirred at room temperature for 24 h. Then, the temperature was raised to 70–80 °C and the reaction was continued for another 10 h. After the completion of the rection, the mixture was diluted with water (30 mL), the precipitate was filtered off, washed with water and dried. Purification was performed by dissolving the crystals in 10% aqueous sodium carbonate, filtering and acidifying the filtrate with acetic acid to pH 6 (the procedure was repeated 2 times).
3-[(2,5-Dimethylphenyl)([1,3]thiazolo [4,5-b]quinoxalin-2-yl)amino]propanoic acid (6)
Yield 1.30 g, 70%, m.p. 132–133 °C.
IR (KBr): ν 1520, 1562, 1591 (3C=N), 1724 (C=O), 2922 (OH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.20 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.50–2.61 (m, 2H, CH2CO), 3.91–4.00 (m, 1H, NCH2), 4.34–4.54 (m, 1H, NCH2), 7.06–7.37 (m, 3H, HAr), 7.58, 7.67 (2t, 2H, J = 7.3 Hz, HAr), 7.86 (t, 2H, J = 6.9 Hz, HAr)ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.6, 20.4 (2CH3), 34.2 (CH2CO), 49.6 (NCH2), 126.9, 127.9, 129.0, 129.4, 130.7, 131.8, 132.8, 137.6, 137.9, 140.0, 140.6, 154.2, 158.7, 169.5 (CAr + 3C=N), 175.3 (COOH) ppm.
Anal. Calcd for C20H18N4O2S: C, 63.47%; H, 4.79%; N, 14.80%; Found: C, 63.63%; H, 4.69%; N, 14.24%.
3-[(2,5-Dimethylphenyl)(4,9-dioxo-4,9-dihydronaphtho [2,3-d][1,3]thiazol-2-yl)amino]propanoic acid (7)
Yield 1.32 g, 65%, m.p. 194–195 °C.
IR (KBr): ν 1530 (C=N), 1627, 1642, 1720 (3C=O), 3550 (OH) cm−1.
1H NMR (400 MHz, CDCl3): δ 2.21 (s, 3H, CH3), 2.35 (s, 3H, CH3), 2.75–3.01 (m, 2H, CH2CO), 3.93–4.14 (m, 1H, NCH2), 4.30–4.92 (m, 1H, NCH2), 7.04, 7.06 (2s, 1H, HAr), 7.20, 7.27 (2d, 2H, J = 7.8 Hz, HAr), 7.42, 7.61 (2t, 2H, J = 7.4 Hz, HAr), 7.99 (d, 1H, J = 7.5 Hz, HAr), 8.04 (d, 1H, J = 6.3 Hz, HAr) ppm.
13C NMR (101 MHz, CDCl3): δ 17.1, 21.0 (2CH3), 32.5 (CH2CO), 48.4 (NCH2), 122.30, 126.5, 127.4, 128.6, 129.7, 130.7, 130.8, 131.1, 132.3, 132.5, 132.6, 132.7, 133.5, 135.2, 138.5, 141.1, 162.0, 170.7 (CAr), 176.2 (COOH), 177.3, 181.1 (2C=O) ppm.
Anal. Calcd for C22H18N2O4S: C, 65.01%; H, 4.46%; N, 6.89%; Found: C, 64.88%; H, 4.57%; N, 6.74%.
Methyl 3-{[4-(4-chlorophenyl)-1,3-thiazol-2-yl](2,5-dimethylphenyl)amino}propanoate (8f). A mixture of acid 3f (1.94 g, 5 mmol), methanol (50 mL) and H2SO4 (0.5 mL) was refluxed for 4 h. Then, the mixture was cooled down and the solvent was evaporated. Sodium bicarbonate solution (5%) was used to neutralize the residues, which was then extracted with diethyl ether (3 × 100 mL). Afterwards, the ether was evaporated to give the title compound 8f (brown resin, 1.84 g, 92%), Rf = 0.57 (ethyl acetate: hexane (1:10)).
IR (KBr): ν 1174 (C-O), 1733 (C=O) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.13 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.78 (t, J = 6.9 Hz, CH2CO), 3.53 (s, 3H, OCH3), 4.12 (br. s, 2H, NCH2), 7.07–7.22 (m, 3H, HAr), 7.28 (d, 1H, J = 7.7 Hz, HAr), 7.45 (d, 2H, J = 8.3 Hz, HAr), 7.88 (d, 2H, J = 8.3 Hz, HAr) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.7 (CH3), 20.4 (CH3), 32.3 (CH2CO), 48.0 (NCH2), 51.4 (OCH3), 103.7 (SCH), 127.3, 128.5, 129.2, 129.5, 131.6, 131.9, 133.0, 133.5, 137.2, 142.4, 149.2 (CAr),169.4 (C=N), 171.6 (C=O) ppm.
Anal. Calcd for C21H21ClN2O2S: C, 62.91%; H, 5.28%; N, 6.99%. Found: C, 62.99%; H, 5.14%; N, 7.07%.
3-{[4-(4-Chlorophenyl)-1,3-thiazol-2-yl](2,5-dimethylphenyl)amino}propanehydrazide (9f). A mixture of ester 8f (1.20 g, 1 mmol), hydrazine monohydrate (0,45 g, 9 mmol) and 1,4-dioxane (20 mL) was refluxed in for 5 h. Then, the reaction mixture was cooled down, the formed precipitate was filtered off, washed with 2-propanol and dried. Purification was performed by recrystallization from 2-propanol.
Yield 1.02 g, 85%, m.p. 136–137 °C.
IR (KBr): ν 1538 (C=N), 1662 (C=O), 3046 (NH), 3248 (NH2) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.13 (s, 3H, CH3), 2.30 (s, 3H, CH3), 2.51–2.55 (m, 2H, CH2CO), 4.07 (br. s, 2H, NH2), 4.24 (br. s, 2H, NCH2), 7.08–7.24 (m, 3H, HAr), 7.28 (d, 1H, J = 7.7 Hz, HAr), 7.45 (d, 2H, J = 8.6 Hz, HAr), 7.90 (d, 2H, J = 8.5 Hz, HAr), 9.11 (s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 31.9 (CH2CO), 48.7 (NCH2), 103.5 (SCH), 127.4, 128.5, 129.3, 129.4, 131.6, 131.8, 133.0, 133.6, 137.2, 142.7, 149.3 (CAr, NC), 169.35 (C=N), 169.4 (C=O) ppm.
Anal. Calcd for C20H21ClN4OS: C, 59.92%, H, 5.28%; N, 13.97%; Found: C, 59.75%; H, 5.36%; N, 13.91%.
General procedure for the synthesis of hydrazones10–12f. A mixture of compound 8f (0.40 g, 1 mmol), corresponding carbaldehyde (1.1 mmol), glacial acetic acid (2 drops) and 1,4-dioxane (20 mL) was heated at refluxed for 2–12 h. Then, the reaction mixture was cooled down, the formed precipitate was filtered off, washed with 2-propanol and dried. Purification was performed by recrystallization from 2-propanol.
(Z/E)-3-{[4-(4-Chlorophenyl)thiazol-2-yl](2,5-dimethylphenyl)amino}-N’-[(5-nitrothiophen-2-yl)methylene]propanehydrazide (10f)
Yield 0.43 g, 79%, m.p. 102–103 °C.
IR (KBr): ν 1534 (C=N), 1679 (C=O), 3121 (NH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.15, 2.16 (2s, 3H, CH3), 2.28, 2.29 (2s, 3H, CH3), 2.75 (t, 0.8H, J = 7.0 Hz, CH2CO), 3.08 (t, 1.2H, J = 7.0 Hz, CH2CO), 4.18 (br. s, 2H, NCH2), 6.96–7.35 (m, 6H, HAr), 7.41, 7.44 (2d, 2H, J = 8.6 Hz, HAr), 7.74, 7.77 (2d, 1H, J = 3.9 Hz, HAr), 7.86–7.90 (m, 1H, HAr), 7.91 (s, 0.6H, N=CH), 8.12 (s, 0.4H, N=CH), 11.78 (s, 0.6H, NH), 11.88 (s, 0.4H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 20.5 (CH3), 30.5 (CH2CO), 33.1 (CH2CO), 47.7 (NCH2), 48.2 (NCH2), 103.5 (SCH), 103.6 (SCH), 114.4, 114.6, 115.1, 127.3, 127.4, 128.5, 129.3, 129.4, 130.9, 131.6, 131.8, 133.0, 133.1, 133.5, 133.6, 134.0, 137.2, 142., 142.7, 149.2, 149.3, 151.6, 151.7 (CAr, N-Cthiaz), 167.4 (N-C=N), 169,4 (C=N), 169,5 (C=N), 172,9 (C=O) ppm.
Anal. Calcd for C20H21ClN4OS: C, 55.60%; H, 4.11%; N, 12.97%; Found: C, 55.63%; H, 3.97%; N, 13.06%.
(Z/E)-3-((4-(4-Chlorophenyl)thiazol-2-yl)(2,5-dimethylphenyl)amino)-N’-((5-nitrofuran-2-yl)methylene)propanehydrazide (11f)
Yield 0.45 g, 85%, m.p. 78–79 °C.
IR (KBr): ν 1535 (C=N), 1679 (C=O), 3108 (NH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.15, 2.16 (2s, 3H, CH3), 2.28, 2.30 (2s, 3H, CH3), 2.73 (t, 0.8H, J = 7.0 Hz, CH2CO), 3.08 (t, 1.2H, J = 7.0 Hz, CH2CO), 4.15 (br. s, 2H, NCH2), 7.07–7.23 (m, 3H, HAr + SCH), 7.28 (d, 1H, J = 7.7 Hz, HAr), 7.32–7.44 (m, 2H, HAr), 7.46, 7.50 (2d, 1H, J = 4.2 Hz, HAr), 8.12, 8.41 (2s, 1H, N=CH), 11.79 (s, 0.6H, NH), 11.83 (s, 0.4H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.7 (CH3), 16.8 (CH3), 20.4 (CH3), 30.7 (CH2CO), 33.1 (CH2CO), 47.9 (NCH2), 48.3 (NCH2), 103.5 (SCH), 103.6 (SCH), 127.3, 127.4, 128.4, 128.5, 128.8, 129.4, 129.5, 130.4, 130.5, 131.7, 131.8, 132.9, 133.0, 133.5, 133.6, 136.1, 137.2, 139.7, 142.4, 142.6, 146.8, 146.9, 149.3, 150.3, 150.64 (CAr, N-Cthiaz), 167.3 (N-C=N), 169.4 (C=N), 169.5 (C=N), 172.7 (C=O) ppm.
Anal. Calcd for C25H22ClN5O4S: C, 57.31%; H, 4.23%; N, 13.37%; Found: C, 57.11%; H, 4.15%; N, 13.33%.
(Z/E)-N′-((1H-Indol-3-yl)methylene)-3-((4-(4-chlorophenyl)thiazol-2-yl)(2,5-dimethylphenyl)amino)propanehydrazide (12f)
Yield 0.40 g, 75%, m.p. 72–73 °C.
IR (KBr): ν 1534, 1612 (C=N), 1660 (C=O), 3108, 3178 (2NH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.16, 2.21, (2s, 3H, CH3), 2.27 (s, 3H, CH3), 2.69 (t, 0.8H, J = 7.2 Hz, CH2CO), 3.13 (s, 1.2H, CH2CO), 4.28 (br. s, 2H, NCH2), 6.92 (t, 1H, J = 7.5 Hz, HAr), 7.10–7.45 (m, 8H, HAr), 7.74, 7.76 (2d, 1H, J = 2.5 Hz, HAr), 7.89, 7.92 (2d, 2.7H, J = 8.5 Hz, HAr), 8.16, 8.32 (2s, 1H, N=CH), 8.21 (d, 0.3H, J = 7.8 Hz, HAr), 11.06 (s, 0.6H, NHind), 11.13 (s, 0.4H, NHind), 11.50 (s, 0.6H, NH), 11.53 (s, 0.4H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.80 (CH3), 16.83 (CH3), 20.4 (CH3), 20.5 (CH3), 30.6 (CH2CO), 33.0 (CH2CO), 47.9 (NCH2), 48.7 (NCH2), 103.4 (SCH), 103.5 (SCH), 111.5, 111.6, 111.7, 111.8, 120.3, 121.3, 121.9, 122.5, 122.6, 124.0, 124.3, 127.3, 127.4, 128.5, 128.6, 129.3. 129.4, 129.5, 130.0, 130.2, 131.6, 131.8, 131.9, 133.0, 133.1, 133.6, 133.7, 137.0, 137.1, 137.2, 140.3, 142.6, 142.7, 143.3, 149.3 (CAr, N-Cthiaz), 165.7 (N-C=N), 169.5 (C=N), 169.6 (C=N), 171.5 (C=O) ppm.
Anal. Calcd for C29H26ClN5OS: C, 65.96%; H, 4.96%; N, 13.26%; Found: C, 66.17%; H, 4.91%; N, 13.02%.
3-{[4-(4-Chlorophenyl)thiazol-2-yl](2,5-dimethylphenyl)amino}-N’-cyclopentylidenepropanehydrazide (13f)
A mixture of compound 8f (0.40 g, 1 mmol), cyclopentanone (0.092 g, 1.1 mmol), a few drops of glacial acetic acid and 1,4-dioxane (20 mL) was refluxed for 3 h. Then, the reaction mixture was cooled, and the precipitate was filtered, washed with 2-propanol and dried. Purification was performed by recrystallization from 1,4-dioxane.
Yield 0.34 g (73 %), m.p. 117–118 °C;
IR (KBr): ν (cm−1) 1540 (C=N), 1662 (C=O), 3187 (NH) cm−1;
1H NMR (400 MHz, DMSO-d6): δ 1.55–1.79 (m, 4H, CH2), 2.14 (s, 3H, CH3), 2.17–2.26 (m, 4H, CH2), 2.29 (s, 3H, CH3), 2.68 (t, 0.9H, J = 7.3 Hz, CH2CO), 2.97 (t, 1.1H, J = 7.3 Hz, CH2CO), 4.13 (br. s, 2H, NCH2), 7.13–7.18 (m, 3H, HAr + SCH), 7.25–7.30 (m, 1H, HAr), 7.41–7.46 (m, 2H, HAr), 7.88, 7.90 (2d, 2H, J = 6.0 Hz, HAr), 9.93 (s, 0.45H, NH), 9.97 (s, 0.55H, NH);
13C NMR (101 MHz, DMSO-d6): δ 16.8 (CH3), 20.4 (CH3), 24.2, 24.3, 24.4, 24.5, 28.0, 28.3, 32.3,32.8 (4CH2), 30.8 (CH2CO), 32.9 (CH2CO), 48.1 (NCH2), 48.5 (NCH2), 103.38 (SCH), 103.43 (SCH), 127.3, 127.4, 128.5, 129.29, 129.33, 131.6 131.8, 132.96, 133.02, 133.58, 133.62, 137.09, 137.14, 142.6, 142.7, 149.21, 149.24, (CAr, N-Cthiaz), 162.2 (N-C=N), 165.7, 166.4 (N-C=N), 169.38 (C=N), 169.43 (C=N), 172.3 (C=O);
Anal. Calcd for C25H27ClN4OS: C, 65.96%; H, 4.96%; N, 13.26%; Found: C, 66.17%; H, 4.91%; N, 13.02%.
General procedure for the synthesis of compounds14fand15f. A mixture of compound 8f (0.40 g, 1 mmol), the corresponding ketone (1.1 mmol), 1,4-dioxane (20 mL) and glacial acetic acid (2 drops) was refluxed for 4–8 h. Then, the reaction mixture was cooled down, the obtained precipitate was filtered off, washed with 2-propanol and dried. Purification was performed by recrystallization from 1,4-dioxane.
(Z/E)-N′-(Butan-2-ylidene)-3-{[4-(4-chlorophenyl)thiazol-2-yl](2,5-dimethylphenyl)amino}propanehydrazide (14f)
Yield 0.35 g, 77%, m.p. 86–87 °C.
IR (KBr): ν 1514, 1537 (2C=N), 1669 (C=O), 3187 (NH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 1.69 (m, 5H, CH3 and CH2), 2.14 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.50 (s, 3H, CH3), 2.68 (t, 1H, J = 7.3 Hz, CH2CO), 2.98 (t, 1H, J = 7.3 Hz, CH2CO), 4.12 (br. s, 2H, NCH2), 7.13–7.18 (m, 3H, HAr + SCH), 7.28 (d, 1H, J = 7.6 Hz, HAr), 7.44 (d, 2H, J = 8.3 Hz, HAr), 7.89 (dd, 2H, J = 8.0, 6.1 Hz, HAr), 10.03 (s, 0.5H, NH), 10.08 (s, 0.5H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.8 (CH3), 17.0 (CH3), 17.5 (CH3), 20.4 (CH3), 24.9 (CH2), 25.1 (CH2), 30.9 (CH2CO), 32.4 (CH2CO), 48.1 (NCH2), 48.5 (NCH2), 103.4 (SCH), 103.5 (SCH), 127.3, 127.4, 128.5, 129.3, 131.6, 131.8, 132.9, 133.0, 133.58, 133.62, 137.1, 137.2, 142.6, 142.7 149.2, 150.1, 154.7, (CAr, N-Cthiaz), 166.5 (N-C=N), 169.38 (C=N), 169.44 (C=N), 172.4 (C=O) ppm.
Anal. Calcd for C24H27ClN4OS: C, 63.35%; H, 5.98%; N, 12.31%; Found: C, 63.41%; 5.92%; N, 12.09%.
(Z/E)-3-{[4-(4-Chlorophenyl)thiazol-2-yl](2,5-dimethylphenyl)amino}-N’-(octan-2-ylidene)propanehydrazide (15f)
Yield 0.38 g, 75%, m.p. 70–71 °C.
IR (KBr): ν 1538 (C=N), 1669 (C=O), 3178 (NH) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 0.72–0.83 (m, 3H, CH3), 1.10–1.45 (m, 8H, 4CH2), 1.78, 1.81, 1.87 (3s, 3H, CH3), 2.01–2.25 (m, 5H, CH2 and CH3), 2.29 (2s, 3H, CH3), 2.69 (t, 0.8H, J = 7.3 Hz, CH2CO), 3.00 (t, 1.2H, J = 7.3 Hz, CH2CO), 4.12 (br. s, 2H, NCH2), 7.01–7.22 (m, 3H, HAr + SCH), 7.23–7.31 (m, 1H, HAr), 7.36–7.51 (m, 2H, HAr), 7.89 (t, 2H, J = 8.0 Hz, HAr), 10.00 (s, 0.3H, NH), 10.08 (s, 0.1H, NH), 10.10 (s, 0.5H, NH), 10.22 (s, 0.1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 13.88 (CH3), 13.92 (CH3), 15.9 (CH3), 16.0 (CH3), 16.75 (CH3), 16.79 (CH3), 20.4 (CH3), 20.4 (CH3), 22.0 (CH2), 25.0 (CH2), 25.3 (CH2), 26.0 (CH2), 28.2 (CH2), 28.4 (CH2), 28.7 (CH2), 30.0 (CH2CO), 31.05 (CH2CO), 31.09 (CH2CO), 32.5 (CH2CO), 38.3 (CH2CO), 48.2 (NCH2), 48.5 (NCH2), 103.3 (SCH), 103.4 (SCH), 127.3, 127.4, 128.5, 129.27, 129.31, 131.5, 131.6, 131.7, 131.8, 132.95, 133.02, 133.6, 133.6, 137.1, 137.12, 142.6, 142.7, 149.2, 152.6, 157.5 (CAr, N-Cthiaz), 166.5 (N-C=N), 169.3 (C=N), 169.4 (C=N), 172.5 (C=O), 172.6 (C=O) ppm.
Anal. Calcd for C24H27ClN4OS: C, 65.80%; H, 6.90%; N, 10.96%; Found: C, 65.94%; H, 6.86%; N, 11.15%.
General procedure for the synthesis of compounds 16c, f, h. A mixture of the corresponding compound 3c, f, h (2 mmol), o-phenylenediamine (0.32 g, 3 mmol) and 15% hydrochloric acid (30 mL) was refluxed for 72 h. Then, the reaction mixture was cooled down, diluted with water (150 mL), and the obtained precipitate was filtered off, washed with water and dried. Then, the crystals were dissolved in 2-propanol and diluted with an aqueous 10% potassium carbonate solution. The formed precipitate was filtered off, washed with water and dried. Purification was performed by recrystallization from a mixture of toluene with hexane (1:1).
N-[2-(1H-Benzo[d]imidazol-2-yl)ethyl]-N-(2,5-dimethylphenyl)-4-phenylthiazol-2-amine (16c)
Yield 0.55 g (65 %), m.p. 95–97 °C;
IR (KBr): ν 1538, 1621 (C=N), 3055 (NH) cm−1;
1H NMR (400 MHz, CDCl3): δ 2.12 (s, 3H, CH3), 2.34 (s, 3H, CH3), 3.40–3.59 (m, 2H, CH2), 4.53, 4.67 (2br. s, 2H, NCH2), 6.72 (s, 1H, HAr), 7.01 (s, 1H, SCH), 7.14–7.47 (m, 7H, HAr), 7.54 (t, 2H, J = 7.5 Hz, HAr), 8.00 (d, 2H, J = 7.7 Hz, HAr), 12.39 (br. s, 1H, NH) ppm;
13C NMR (101 MHz, CDCl3): δ 17.1 (CH3), 21.0 (CH3), 29.2 (CH2), 49.8 (NCH2), 102.5 (SCH), 122.1, 126.0, 128.3, 129.1, 129.6, 130.27, 132.3, 133.6, 134.6, 138.3, 141.8, 150.7, 153.2 (CAr, Cbenzimid.), 172.4 (N-C=N) ppm;
Anal. Calcd for C26H24N4S: C, 73.55%; H, 5.70%; N, 13.20%; Found: C, 73.71%; H, 5.93%; N, 13.37%.
N-[2-(1H-Benzo[d]imidazol-2-yl)ethyl]-4-(4-chlorophenyl)-N-(2,5-dimethylphenyl)thiazol-2-amine (16f)
Yield 0.59 g (64 %), m.p. 85–86 °C;
IR (KBr): ν 1537, 1620 (2C=N), 3051 (NH) cm−1;
1H NMR (400 MHz, CDCl3): δ 2.04 (s, 3H, CH3), 2.11 (s, 3H, CH3), 3.36 (s, 2H, CH2), 4.16, 4.45 (2br. s, 2H, NCH2), 6.79 (s, 1H, 1H, HAr), 7.08–7.27 (m, 5H, HAr + SCH), 7.39, 7.52 (2d, 2H, J = 6.1 Hz, HAr), 7.45 (d, 2H, J = 8.1 Hz, HAr), 7.91 (d, 2H, J = 7.8 Hz, HAr), 12.30 (s, 1H, NH) ppm;
13C NMR (101 MHz, CDCl3) δ 16.7 (CH3), 20.1 (CH3), 27.2 (CH2), 50.8 (NCH2), 103.6 (SCH), 110.8, 118.2, 120.9, 121.6, 127.4, 128.5, 129.3, 129.3, 131.5, 131.8, 132.8, 133.6, 134.2, 137.1, 142.8, 143.3, 149.4, 152.4 (CAr, Cbenzimid.), 169.3 (N-C=N) ppm;
Anal. Calcd for C26H23ClN4S: C, 68.03%; H, 5.05%; N, 12.21%; Found: C, 68.22%; H, 5.29%; N, 12.39%.
N-[2-(1H-Benzo[d]imidazol-2-yl)ethyl]-4-(3,4-dichlorophenyl)-N-(2,5-dimethylphenyl)thiazol-2-amine (16h)
Yield 0.61 g (62 %), m.p. 100–101 °C;
IR (KBr): ν 1533 (2C=N), 1621, 3051 (NH) cm−1;
1H NMR (400 MHz, DMSO-d6): δ 2.04 (s, 3H, CH3), 2.10 (s, 3H, CH3), 3.30–3.34 (m, 2H, CH2), 4.13, 4.50 (br. s, 2H, NCH2), 6.78 (s, 1H, 1H, HAr), 7.05–7.15 (m, 3H, HAr + SCH), 7.25 (d, 1H, J = 7.8 Hz, HAr), 7.32 (s, 1H, HAr), 7.39 (d, 1H, J = 6.7 Hz, HAr), 7.52 (d, 1H, J = 7.9 Hz, HAr), 7.65 (d, 1H, J = 8.4 Hz, HAr), 7.87 (dd, 1H, J = 8.4, 1.8 Hz, HAr), 8.13 (d, 1H, J = 1.8 Hz, HAr), 12.30 (s, 1H, NH) ppm;
13C NMR (101 MHz, DMSO-d6): δ 16.6 (CH3), 20.1 (CH3), 27.2 (CH2), 50.8 (NCH2), 105.0 (SCH), 110.7, 118.2, 120.9, 121.6, 125.8, 127.2, 129.3, 129.4, 129.6, 130.7, 131.4, 131.6, 132.8, 134.2, 135.3, 137.1, 142.7, 143.3, 148.0, 152.4 (CAr, Cbenzimid.), 169.4 (N-C=N) ppm;
Anal. Calcd for C26H22Cl2N4S: C, 63.29%; H, 4.49%; N, 11.35%; Found: C, 63.10%; H, 4.55%; N, 11.47%.
General procedure for the synthesis of compounds 17c, f, h. A mixture of the corresponding compound 3c, f, h (1 mmol), sulfanilamide (0.19 g, 1.1 mmol), triethylamine (0.30 g, 3 mmol) and DMF (5 mL) was stirred at room temperature for 0.5 h. HBTU (0.57 g, 1.5 mmol) was dissolved in DMF (3 mL) at room temperature in an inert atmosphere and then added to the reaction mixture over 15 min. The reaction mixture was stirred at room temperature for 72 h, then diluted with an aqueous 10% potassium carbonate (50 mL) solution. The obtained precipitate was filtered off, washed with water and dried. Purification was performed by column chromatography using hexane: ethyl acetate (1:2) as eluent.
3-[(2,5-Dimethylphenyl)(4-phenylthiazol-2-yl)amino]-N-(4-sulfamoylphenyl)propanamide (17c)
Yield 0.43 g, 85%, Rf = 0.62 (hexane: ethyl acetate (1:2)), m.p. 78–79 °C.
IR (KBr): ν (cm−1) 1538 (C=N), 1681 (C=O), 3253 (NH), 3600 (NH2) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.15 (s, 3H, CH3), 2.22 (s, 3H, CH3), 2.71–2.93 (m, 3H, COCH2), 4.11 (br. s, 2H, NCH2), 7.11, 7.15 (2s, 3H, HAr, SCH), 7.24 (s, 2H, NH2), 7.25, 7.29 (2d, 2H, J = 6.9 Hz, HAr), 7.38, 7.89 (t, 4H, J = 7.5 Hz, HAr), 7.70, 7.74 (2d, 4H, J = 8.9 Hz, HAr), 10.35 (s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.5 (CH3), 16.6 (CH3), 20.2 (CH3), 34.9 (CH2CO), 48.1 (NCH2), 102.6 (SCH), 118.4, 125.5, 126.4, 127.3, 128.3, 128.6, 129.0, 129.1, 131.4, 132.8, 134.5, 134.6, 136.9, 138.0, 141.8, 142.6, 150.3 (CAr, N-Cthiaz), 169.0 (N-C=N), 169.1 (N-C=N), 169.6 (C=O) ppm.
Anal. Calcd for C26H22Cl2N4S: C, 61.64%; H, 5.17%; N, 11.06%; Found: C, 61.37%; H, 5.05%; N, 10.98%.
3-[(4-(4-Chlorophenyl)thiazol-2-yl)(2,5-dimethylphenyl)amino]-N-(4-sulfamoylphenyl)propanamide (17f)
Yield 0.44 g, 81%, Rf = 0.63 (hexane: ethyl acetate (1:2)), m.p. 126–127 °C.
IR (KBr): ν 1533 (C=N), 1667 (C=O), 3248 (NH), 3600 (NH2) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.14 (s, 3H, CH3), 2.22 (s, 3H, CH3), 2.74–2.89 (m, 2H, COCH2), 4.19 (br. s, 2H, NCH2), 7.14 (s, 2H, HAr), 7.18 (s, 1H, SCH), 7.25–7.29 (m, H, HAr), 7.44, 7.90 (2d, 4H, J = 8.4 Hz, HAr), 7.61, 7.66 (2d, 4H, J = 8.6 Hz, HAr), 8.76 (br. s, 2H, NH2) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.7 (CH3), 20.4 (CH3), 34.9 (CH2CO), 48.4 (NCH2), 103.6 (SCH), 118.3, 126.2, 127.4, 128.5, 129.3, 129.4, 131.6, 131.8, 133.0, 133.6, 137.2, 140.7, 141.8, 142.7, 149.3 (CAr, N-Cthiaz), 169.5 (N-C=N), 169.6 (C=O) ppm.
Anal. Calcd for C26H25ClN4O3S2: C, 57.72%; H, 4.66%; N, 10.35%; Found: C, 57.86%; H, 4.79%; N, 10.51%.
3-((4-(3,4-Dichlorophenyl)thiazol-2-yl)(2,5-dimethylphenyl)amino)-N-(4-sulfamoylphenyl)propanamide (17h)
Yield 0.45 g, 78%, Rf = 0.62 (hexane: ethyl acetate (1:2)), m.p. 98–99 °C.
IR (KBr): ν 1532 (C=N), 1682 (C=O), 3253 (NH), 3653 (NH2) cm−1.
1H NMR (400 MHz, DMSO-d6): δ 2.14 (s, 3H, CH3), 2.22 (s, 3H, CH3), 2.84 (t, 2H, J = 6.9 Hz, COCH2), 4.21 (br. s, 2H, NCH2), 7.14, 7.15 (2s, 2H, HAr, SCH), 7.24 (s, 2H, NH2), 7.28 (d, 1H, J = 8.2 Hz, HAr), 7.33 (s, 1H, HAr), 7.64 (d, 1H, J = 8.4 Hz, HAr), 7.70, 7.74 (2d, 4H, J = 8.9 Hz, HAr), 7.87 (dd, 1H, J = 8.4, 1.5 Hz, HAr), 8.12 (s, 1H, HAr), 10.35 (s, 1H, NH) ppm.
13C NMR (101 MHz, DMSO-d6): δ 16.7 (CH3), 20.4 (CH3), 35.0 (CH2CO), 48.3 (NCH2), 105.0 (SCH), 118.6, 125.7, 126.6, 127.2, 129.2, 129.5, 129.6, 130.7, 131.4, 131.6, 132.9, 135.3, 137.2, 138.2, 141.9, 142.6, 147.9 (CAr, N-Cthiaz), 169.6 (N-C=N), 169.8 (C=O) ppm.
Anal. Calcd for C26H24Cl2N4O3S2: C, 54.26%; H, 4.20%; N, 9.74%; Found: C, 54.08%; H, 4.12%; N, 9.83%.
3.2. Bacterial Strains and Culture Conditions
The multidrug-resistant and genetically defined isolates were obtained from the AR isolate bank at the Centre for Disease Control (CDC, United States). S. aureus TCH 1516 (USA300) was obtained from the American Type Culture Collection. Prior to the study, all strains were maintained in commercial cryopreservation systems at −80 °C. Bacterial strains were subcultured on Columbia Sheep Blood agar or Tryptic-Soy agar (Becton Dickenson, Franklin Lakes, NJ, USA). Fungal strains were cultured on Saburoud-Dextrose agar. Unless otherwise specified, all antimicrobial susceptibility studies with bacterial pathogens were performed in Cation-Adjusted Mueller–Hinton broth (CAMBH) for liquid cultures (Liofilchem, Italy). Antifungal studies were conducted using RPMI/MOPS broth.
3.3. Minimal Inhibitory Concentration Determination
3.3.1. Antibacterial Activity Characterization
The minimal inhibitory concentrations (MICs) of compounds 1–17 as well as various antibiotics were determined according to the recommendations of the Clinical and Laboratory Standards Institute (CLSI). The MICs for the compounds and comparator antibiotics were determined according to the testing standard broth microdilution methods described in CLSI document M07-A8 against the libraries of Gram-positive and Gram-negative pathogens. Compounds and antibiotics that were used as a control were dissolved in dimethyl sulfoxide (DMSO) to achieve a final concentration of 15–30 mg/mL. A series of compound dilutions were prepared in deep, polypropylene 96-well microplates to achieve 2× of concentrations (0.5–64 µg/mL) and were then transferred to the assay single-use, flat bottom plates. The prepared microplates were stored at −80 °C until the day of the experiment.
A standardized bacterial inoculum was prepared using the colony suspension method. The inoculum suspension was diluted in sterile CAMBH to achieve final concentrations of approximately 5 × 105 CFU/mL (range, 2 × 105 to 8 × 105 CFU/mL) in each well. The inoculum was transferred to the assay plates to achieve 1× assay concentration. Inoculated microdilution plates were incubated at 35 °C for 16 to 20 h in an ambient-air incubator within 15 min of the addition of the inoculum.
3.3.2. Antifungal Activity Characterization
The MIC of compounds 1–17 as well as clinically approved antifungal drugs was determined by CLSI recommendations, described in document M27-A3 [84,85]. Briefly, before the experiments, multidrug-resistant Candida spp., strains were sub-cultured on Saburoud-Dextrose agar for 24 h at 35 °C. The colonies were suspended in sterile saline to reach approximately 5 × 106 CFU/mL. Then, the fungal suspension is diluted in RPMI/MOPS broth to reach 5 × 105 CFU/mL and microplates containing test compounds, prepared as described above are inoculated using a multichannel pipette. Inoculated microdilution plates were incubated at 35 °C for 16 to 20 h in an ambient-air incubator within 15 min of the addition of the inoculum.
3.4. Cell Lines and Culture Conditions
The non-small-cell human lung carcinoma A549 cells were obtained from American Type Culture Collection (Rockville, MD, USA). Caco-2 human corectal adenocarcinoma cells were obtained from Dr. Iliyan IIiev’s laboratory (Institute for Research in IBD, Weill Cornell Medicine of Cornell University). Cells were maintained in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12) (Gibco, Waltham, MA, USA) supplemented with 10% fetal bovine serum (10% FBS) (Gibco, Waltham, MA, USA) and 100 U/mL penicillin and 100 μg/mL streptomycin (P/S). Cells were cultured at 37 °C humidified atmosphere containing 5% of CO2. Cells were fed every 2–3 days and subculture upon reaching 70–80% confluence.
3.5. In Vitro Cytotoxic Activity Determination
The viability of A549 and Caco-2 cells after the treatment with compounds or cisplatin that served as cytotoxicity control was evaluated by using commercial MTT (3-[4,5-methylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay (ThermoFisher Scientific, United States). Briefly, cells were plated in the flat-bottomed 96-well microplates (1 × 104 cells/well) and incubated overnight to facilitate the attachment. The test compounds were dissolved in hybridoma-grade DMSO (Sigma-Aldrich, St. Louis, MO, USA) and then further serially diluted in cell culture media containing 0.25 % DMSO to achieve 100 µM of each compound.
Subsequently, the media from the cells was aspirated and the compounds were added to the microplates. The cells were incubated at 37 °C, 5% CO2 for 24 h. After incubation, a 10 μL of Vybrant® MTT Cell Proliferation Reagent (ThermoFisher Scientific) was added, and cells were further incubated for 4 h. After incubation, the media was aspirated, and the resulting formazan was solubilized by the addition of 100 μL of DMSO. The absorbance was then measured at 570 nm by using a microplate reader (Multiscan, ThermoFisher Scientific). The following formula was used to calculate the % of A549 viability: ([AE-AB]/[AC-AB]) × 100%. AE, AC, and AB were defined as the absorbance of experimental samples, untreated samples, and blank controls, respectively. The experiments were performed in triplicates.
3.6. Statistical Analysis
The results are expressed as mean ± standard deviation (SD). Statistical analyses were performed with Prism (GraphPad Software, San Diego, CA, USA), using Kruskal–Wallis test and two-way ANOVA. p < 0.05 was accepted as significant.
4. Conclusions
In the present study, a series of 2-aminothiazole derivatives containing N-2,5-dimethyl phenyl and β-alanine moieties in the molecules were synthesized and evaluated for their in vitro antimicrobial activity using a panel of multidrug-resistant bacterial and fungal pathogens with emerging and genetically defined resistance mechanisms. In addition, we characterized the cytotoxic and anticancer properties using A549 and Caco-2 pulmonary and corectal adenocarcinoma models.
The results revealed that thiazoles with the 4-aryl substituted showed profound and selective antimicrobial activity against Gram-positive pathogens. Compound 3j possessed the most potent activity against tested strains multidrug-resistant S. aureus and E. faecium (MIC 1–2 µg/mL). Strikingly, compounds 3h, 3j and 7 showed antibacterial activity against especially clinically challenging tedizolid/linezolid-resistant S. aureus strains.
On the other hand, the modified carboxyl group in thiazoles 8f, 9f and 14f provided promising antifungal properties against drug-resistant Candida strains, including C. glabrata, C. parapsilosis and C. hemulonii. Moreover, ester 8f was found to have broader antifungal activity on multiple Candida species (1–8 µg/mL) including the emerging fungal pathogen C. auris.
During the anticancer activity study, a structure-dependent anticancer activity against A549 and Caco-2 cells was observed. Structure–activity relation analysis revealed naphthoquinone-fused thiazole 7 structure and 2,4-diCl-C6H3 moiety 3j to provide broad-spectrum anticancer against both A549 and Caco-2 cells.
Compounds based on 2-aminothiazole derivatives containing N-2,5-dimethyl phenyl and β-alanine moieties could be further explored as novel scaffolds for the development of novel and highly active compounds targeting multidrug-resistant pathogens, including tedizolid/linezolid-resistant S. aureus as well as vancomycin-resistant Enterococcus. Furthermore, a series of novel compounds based on 8f could be further explored as novel antifungals targeting challenging and multidrug-resistant Candida auris. Further studies are needed to better understand the safety, pharmacological properties as well as cellular targets of 2-aminothiazole derivatives 1–17.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12020220/s1, Figure S1: 1H-NMR of compound 1, Figure S2: 13C-NMR of compound 1, Figure S3: 1H-NMR of compound 2, Figure S4: 13C-NMR of compound 2, Figure S5: 1H-NMR of compound 3a, Figure S6: 13C-NMR of compound 3a, Figure S7: 1H-NMR of compound 3b, Figure S8: 13C-NMR of compound 3b, Figure S9: 1H-NMR of compound 3c, Figure S10: 13C-NMR of compound 3c, Figure S11: 1H-NMR of compound 3d, Figure S12: 13C-NMR of compound 3d, Figure S13: 1H-NMR of compound 3e, Figure S14: 13C-NMR of compound 3e, Figure S15: 1H-NMR of compound 3f, Figure S16: 13C-NMR of compound 3f, Figure S17: 1H-NMR of compound 3g, Figure S18: 13C-NMR of compound 3g, Figure S19: 1H-NMR of compound 3h, Figure S20: 13C-NMR of compound 3h, Figure S21: 1H-NMR of compound 3i, Figure S22: 13C-NMR of compound 3i, Figure S23: 1H-NMR of compound 3j, Figure S24: 13C-NMR of compound 3j, Figure S25: 1H-NMR of compound 3k, Figure S26: 13C-NMR of compound 3k, Figure S27: 1H-NMR of compound 4i, Figure S28: 13C-NMR of compound 4i, Figure S29: 1H-NMR of compound 4j, Figure S30: 13C-NMR of compound 4j, Figure S31: 1H-NMR of compound 4k, Figure S32: 13C-NMR of compound 4k, Figure S33: 1H-NMR of compound 5, Figure S34: 13C-NMR of compound 5, Figure S35: 1H-NMR of compound 6, Figure S36: 13C-NMR of compound 6, Figure S37: 1H-NMR of compound 7, Figure S38: 13C-NMR of compound 7, Figure S39: 1H-NMR of compound 8f, Figure S40: 13C-NMR of compound 8f, Figure S41: 1H-NMR of compound 9f, Figure S42: 13C-NMR of compound 9f, Figure S43: 1H-NMR of compound 10f, Figure S44: 13C-NMR of compound 10f, Figure S45: 1H-NMR of compound 11f, Figure S46: 13C-NMR of compound 11f, Figure S47: 1H-NMR of compound 12f, Figure S48: 13C-NMR of compound 12f, Figure S49: 1H-NMR of compound 13f, Figure S50: 13C-NMR of compound 13f, Figure S51: 1H-NMR of compound 14f, Figure S52: 13C-NMR of compound 14f, Figure S53: 1H-NMR of compound 15f, Figure S54: 13C-NMR of compound 15f, Figure S55: 1H-NMR of compound 16c, Figure S56: 13C-NMR of compound 16c, Figure S57: 1H-NMR of compound 16f, Figure S58: 13C-NMR of compound 16f, Figure S59: 1H-NMR of compound 16h, Figure S60: 13C-NMR of compound 16h, Figure S61: 1H-NMR of compound 17c, Figure S62: 13C-NMR of compound 17c, Figure S63: 1H-NMR of compound 17f, Figure S64: 13C-NMR of compound 17f, Figure S65: 1H-NMR of compound 17h, Figure S66: 13C-NMR of compound 17h. References [86,87] are cited in the supplementary materials.
Author Contributions
Conceptualization, V.M.; Data curation, B.G., B.S.-B., K.A., A.K., G.S., V.P., R.P., E.N. and A.G.; Formal analysis, P.K., R.V., E.N. and A.G.; Funding acquisition, V.M.; Investigation, P.K., B.G., R.V., B.S.-B., K.A., A.K. and G.S.; Methodology, B.G., R.V., B.S.-B., A.K., G.S. and K.A.; Project administration, B.S.-B. and V.M.; Resources, P.K. and K.A.; Software, P.K., K.A. and A.G.; Supervision, V.M.; Validation, P.K., V.P. and R.P.; Visualization, P.K., K.A. and R.V.; Writing—original draft, R.V., K.A., A.K., G.S. and P.K.; Writing—review and editing, B.G., R.V., V.P., R.P. and V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Petraitis, V.; Petraitiene, R.; Kavaliauskas, P.; Naing, E.; Garcia, A.; Sutherland, C.; Kau, A.Y.; Goldner, N.; Bulow, C.; Nicolau, D.P.; et al. Pharmacokinetics, Tissue Distribution, and Efficacy of VIO-001 (Meropenem/Piperacillin/Tazobactam) for Treatment of Methicillin-Resistant Staphylococcus Aureus Bacteremia in Immunocompetent Rabbits with Chronic Indwelling Vascular Catheters. Antimicrob. Agents Chemother. 2021, 65, e0116821. [Google Scholar] [CrossRef] [PubMed]
- Holubar, M.; Meng, L.; Deresinski, S. Bacteremia Due to Methicillin-Resistant Staphylococcus Aureus. Infect. Dis. Clin. N. Am. 2016, 30, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Hashem, Y.A.; Amin, H.M.; Essam, T.M.; Yassin, A.S.; Aziz, R.K. Biofilm Formation in Enterococci: Genotype-Phenotype Correlations and Inhibition by Vancomycin. Sci. Rep. 2017, 7, 5733–5745. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Sherman, E.; Colmer-Hamood, J.A.; Nelius, T.; Myntti, M.; Hamood, A.N. Urinary Catheters Coated with a Novel Biofilm Preventative Agent Inhibit Biofilm Development by Diverse Bacterial Uropathogens. Antibiotics 2022, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Kean, R.; Rajendran, R.; Haggarty, J.; Townsend, E.M.; Short, B.; Burgess, K.E.; Lang, S.; Millington, O.; Mackay, W.G.; Williams, C.; et al. Candida Albicans Mycofilms Support Staphylococcus Aureus Colonization and Enhances Miconazole Resistance in Dual-Species Interactions. Front. Microbiol. 2017, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Kong, E.F.; Tsui, C.; Kucharíková, S.; Andes, D.; Van Dijck, P.; Jabra-Rizk, M.A. Commensal Protection of Staphylococcus Aureus against Antimicrobials by Candida Albicans Biofilm Matrix. MBio 2016, 7, e01365-16. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida Auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimicrob. Agents Chemother. 2022, 66, e0005322. [Google Scholar] [CrossRef]
- Thatchanamoorthy, N.; Rukumani Devi, V.; Chandramathi, S.; Tay, S.T. Candida Auris: A Mini Review on Epidemiology in Healthcare Facilities in Asia. J. Fungi 2022, 8, 1126. [Google Scholar] [CrossRef]
- Rybak, J.M.; Cuomo, C.A.; David Rogers, P. The Molecular and Genetic Basis of Antifungal Resistance in the Emerging Fungal Pathogen Candida Auris. Curr. Opin. Microbiol. 2022, 70, 102208–102216. [Google Scholar] [CrossRef]
- Short, F.L.; Lee, V.; Mamun, R.; Malmberg, R.; Li, L.; Espinosa, M.I.; Venkatesan, K.; Paulsen, I.T. Benzalkonium Chloride Antagonises Aminoglycoside Antibiotics and Promotes Evolution of Resistance. EBioMedicine 2021, 73, 103653–103659. [Google Scholar] [CrossRef]
- Liu, G.; Stokes, J.M. A Brief Guide to Machine Learning for Antibiotic Discovery. Curr. Opin. Microbiol. 2022, 69, 102190–102197. [Google Scholar] [CrossRef] [PubMed]
- Evren, A.E.; Dawbaa, S.; Nuha, D.; Yavuz, Ş.A.; Gül, Ü.D.; Yurttaş, L. Design and Synthesis of New 4-Methylthiazole Derivatives: In Vitro and in Silico Studies of Antimicrobial Activity. J. Mol. Struct. 2021, 1241, 130692–130706. [Google Scholar] [CrossRef]
- Moreira, J.; Durães, F.; Freitas-Silva, J.; Szemerédi, N.; Resende, D.I.S.P.; Pinto, E.; da Costa, P.M.; Pinto, M.; Spengler, G.; Cidade, H.; et al. New Diarylpentanoids and Chalcones as Potential Antimicrobial Adjuvants. Bioorg. Med. Chem. Lett. 2022, 67, 128743–128752. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, S.; Park, J.H.; Kim, H.S.; Seo, C.D.; Ajish, C.; Kim, E.Y.; Lim, H.S.; Shin, S.Y. Cationic, Amphipathic Small Molecules Based on a Triazine-Piperazine-Triazine Scaffold as a New Class of Antimicrobial Agents. Eur. J. Med. Chem. 2022, 243, 114747–114763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Wang, S.; Wu, H. Synthesis and Biological Evaluation of Novel Pyrimidine Amine Derivatives Bearing Bicyclic Monoterpene Moieties. Molecules 2022, 27, 8104. [Google Scholar] [CrossRef] [PubMed]
- Hosny, Y.; Abutaleb, N.S.; Omara, M.; Alhashimi, M.; Elsebaei, M.M.; Elzahabi, H.S.; Seleem, M.N.; Mayhoub, A.S. Modifying the Lipophilic Part of Phenylthiazole Antibiotics to Control Their Drug-Likeness. Eur. J. Med. Chem. 2020, 185, 111830–111847. [Google Scholar] [CrossRef]
- AboulMagd, A.M.; Abdelwahab, N.S.; Abdelrahman, M.M.; Abdel-Rahman, H.M.; Farid, N.F. Lipophilicity Study of Different Cephalosporins: Computational Prediction of Minimum Inhibitory Concentration Using Salting-out Chromatography. J. Pharm. Biomed. Anal. 2021, 206, 114358–114368. [Google Scholar] [CrossRef]
- Sadowski, E.; Bercot, B.; Chauffour, A.; Gomez, C.; Varon, E.; Mainardis, M.; Sougakoff, W.; Mayer, C.; Sachon, E.; Anquetin, G.; et al. Lipophilic Quinolone Derivatives: Synthesis and in Vitro Antibacterial Evaluation. Bioorg. Med. Chem. Lett. 2022, 55, 128450–128456. [Google Scholar] [CrossRef]
- Mohanty, P.; Behera, S.; Behura, R.; Shubhadarshinee, L.; Mohapatra, P.; Barick, A.K.; Jali, B.R. Antibacterial Activity of Thiazole and Its Derivatives: A Review. Biointerface Res. Appl. Chem. 2022, 12, 2171–2195. [Google Scholar] [CrossRef]
- Constantinescu, T.; Lungu, C.N.; Lung, I. Lipophilicity as a Central Component of Drug-like Properties of Chalchones and Flavonoid Derivatives. Molecules 2019, 24, 1505. [Google Scholar] [CrossRef]
- Pivovarova, E.; Climova, A.; Świątkowski, M.; Staszewski, M.; Walczyński, K.; Dzięgielewski, M.; Bauer, M.; Kamysz, W.; Krześlak, A.; Jóźwiak, P.; et al. Synthesis and Biological Evaluation of Thiazole-Based Derivatives with Potential against Breast Cancer and Antimicrobial Agents. Int. J. Mol. Sci. 2022, 23, 9844. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.F.; Alam, A.; Alshammari, A.A.; Alhazza, M.B.; Alzimam, I.M.; Alam, M.A.; Mustafa, G.; Ansari, M.S.; Alotaibi, A.M.; Alotaibi, A.A.; et al. Thiazole: A Versatile Standalone Moiety Contributing to the Development of Various Drugs and Biologically Active Agents. Molecules 2022, 27, 3994. [Google Scholar] [CrossRef] [PubMed]
- Kamat, V.; Santosh, R.; Poojary, B.; Nayak, S.P.; Kumar, B.K.; Sankaranarayanan, M.; Faheem; Khanapure, S.; Barretto, D.A.; Vootla, S.K. Pyridine- And Thiazole-Based Hydrazides with Promising Anti-Inflammatory and Antimicrobial Activities along with Their in Silico Studies. ACS Omega 2020, 5, 25228–25239. [Google Scholar] [CrossRef]
- Othman, I.M.M.; Alamshany, Z.M.; Tashkandi, N.Y.; Gad-Elkareem, M.A.M.; Abd El-Karim, S.S.; Nossier, E.S. Synthesis and Biological Evaluation of New Derivatives of Thieno-Thiazole and Dihydrothiazolo-Thiazole Scaffolds Integrated with a Pyrazoline Nucleus as Anticancer and Multi-Targeting Kinase Inhibitors. RSC Adv. 2022, 12, 561–577. [Google Scholar] [CrossRef]
- Yurttaş, L.; Özkay, Y.; Karaca Gençer, H.; Acar, U. Synthesis of Some New Thiazole Derivatives and Their Biological Activity Evaluation. J. Chem. 2015, 2015, 464379. [Google Scholar] [CrossRef]
- Elsebaei, M.M.; Mohammad, H.; Abouf, M.; Abutaleb, N.S.; Hegazy, Y.A.; Ghiaty, A.; Chen, L.; Zhang, J.; Malwal, S.R.; Oldfield, E.; et al. Alkynyl-Containing Phenylthiazoles: Systemically Active Antibacterial Agents Effective against Methicillin-Resistant Staphylococcus Aureus (MRSA). Eur. J. Med. Chem. 2018, 148, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.D.; Liu, P.; Yang, Y.; Gao, F. Sulfonamide-1,3,5-Triazine-Thiazoles: Discovery of a Novel Class of Antidiabetic Agents: Via Inhibition of DPP-4. RSC Adv. 2016, 6, 83438–83447. [Google Scholar] [CrossRef]
- Santosh, R.; Selvam, M.K.; Kanekar, S.U.; Nagaraja, G.K.; Kumar, M. Design, Synthesis, DNA Binding, and Docking Studies of Thiazoles and Thiazole-Containing Triazoles as Antibacterials. ChemistrySelect 2018, 3, 3892–3898. [Google Scholar] [CrossRef]
- Cheng, K.; Xue, J.Y.; Zhu, H.L. Design, Synthesis and Antibacterial Activity Studies of Thiazole Derivatives as Potent EcKAS III Inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 4235–4238. [Google Scholar] [CrossRef]
- Parašotas, I.; Anusevičius, K.; Jonuškiene, I.; Mickevičius, V. Synthesis and Antibacterial Activity of N-Carboxyethyl-N-(4-Hydroxyphenyl)- 2-Aminothiazoles and Dihydrothiazolones. Chemija 2014, 25, 107–114. [Google Scholar]
- Prusiner, S.B. Molecular Biology of Prion Diseases. Science 1991, 252, 1515–1522. [Google Scholar] [CrossRef] [PubMed]
- Al-Humaidi, J.Y.; Badrey, M.G.; Aly, A.A.; Nayl, A.E.A.A.; Zayed, M.E.M.; Jefri, O.A.; Gomha, S.M. Evaluation of the Binding Relationship of the RdRp Enzyme to Novel Thiazole/Acid Hydrazone Hybrids Obtainable through Green Synthetic Procedure. Polymers 2022, 14, 3160. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.A.; Bawazeer, T.M.; Asghar, B.; Alharbi, A.; Aljohani, M.M.; Khalifa, M.E.; El-Metwaly, N. Synthesis and Characterization of New Thiazole-Based Co(II) and Cu(II) Complexes; Therapeutic Function of Thiazole towards COVID-19 in Comparing to Current Antivirals in Treatment Protocol. J. Mol. Struct. 2021, 1244, 130961–130973. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, E.; Dincel, E.D.; Naesens, L.; Ulusoy Güzeldemirci, N. Design and Synthesis of Novel Imidazo[2,1-b]Thiazole Derivatives as Potent Antiviral and Antimycobacterial Agents. Bioorg. Chem. 2020, 95, 103496–103505. [Google Scholar] [CrossRef]
- Pacca, C.C.; Marques, R.E.; Espindola, J.W.P.; Filho, G.B.O.O.; Leite, A.C.L.; Teixeira, M.M.; Nogueira, M.L. Thiosemicarbazones and Phthalyl-Thiazoles Compounds Exert Antiviral Activity against Yellow Fever Virus and Saint Louis Encephalitis Virus. Biomed. Pharmacother. 2017, 87, 381–387. [Google Scholar] [CrossRef]
- Abdel-Sattar, N.E.A.; El-Naggar, A.M.; Abdel-Mottaleb, M.S.A. Novel Thiazole Derivatives of Medicinal Potential: Synthesis and Modeling. J. Chem. 2017, 2017, 4102796. [Google Scholar] [CrossRef]
- Raghunatha, P.; Inamdar, M.N.; Asdaq, S.M.B.; Almuqbil, M.; Alzahrani, A.R.; Alaqel, S.I.; Kamal, M.; Alsubaie, F.H.; Alsanie, W.F.; Alamri, A.S.; et al. New Thiazole Acetic Acid Derivatives: A Study to Screen Cardiovascular Activity Using Isolated Rat Hearts and Blood Vessels. Molecules 2022, 27, 6138. [Google Scholar] [CrossRef]
- Bagheri, M.; Shekarchi, M.; Jorjani, M.; Ghahremani, M.H.; Vosooghi, M.; Shafiee, A. Synthesis and Antihypertensive Activity of 1-(2-Thiazolyl)-3,5-Disubstituted -2-Pyrazolines. Arch. Pharm. 2004, 337, 25–34. [Google Scholar] [CrossRef]
- Pember, S.O.; Mejia, G.L.; Price, T.J.; Pasteris, R.J. Piperidinyl Thiazole Isoxazolines: A New Series of Highly Potent, Slowly Reversible FAAH Inhibitors with Analgesic Properties. Bioorg. Med. Chem. Lett. 2016, 26, 2965–2973. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, N.P. Synthesis, Anti-Inflammatory and Analgesic Evaluation of Thiazole/Oxazole Substituted Benzothiazole Derivatives. Bioorg. Chem. 2021, 107, 104608. [Google Scholar] [CrossRef]
- Kalkhambkar, R.G.; Kulkarni, G.M.; Shivkumar, H.; Rao, R.N. Synthesis of Novel Triheterocyclic Thiazoles as Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem. 2007, 42, 1272–1276. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wells, J.A. Identification of Specific Tethered Inhibitors for Caspase-5. Chem. Biol. Drug Des. 2012, 79, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Maghraby, M.T.E.; Abou-Ghadir, O.M.F.; Abdel-Moty, S.G.; Ali, A.Y.; Salem, O.I.A. Novel Class of Benzimidazole-Thiazole Hybrids: The Privileged Scaffolds of Potent Anti-Inflammatory Activity with Dual Inhibition of Cyclooxygenase and 15-Lipoxygenase Enzymes. Bioorganic Med. Chem. 2020, 28, 115403–115422. [Google Scholar] [CrossRef] [PubMed]
- Neelam; Khatkar, A.; Sharma, K.K. Phenylpropanoids and Its Derivatives: Biological Activities and Its Role in Food, Pharmaceutical and Cosmetic Industries. Crit. Rev. Food Sci. Nutr. 2020, 60, 2655–2675. [Google Scholar] [CrossRef]
- Sahu, S.; Sahu, T.; Kalyani, G.; Gidwani, B. Synthesis and Evaluation of Antimicrobial Activity of 1, 3, 4-Thiadiazole Analogues for Potential Scaffold. J. Pharmacopuncture 2021, 24, 32–40. [Google Scholar] [CrossRef]
- Coluccia, A.; Bufano, M.; La Regina, G.; Puxeddu, M.; Toto, A.; Paone, A.; Bouzidi, A.; Musto, G.; Badolati, N.; Orlando, V.; et al. Anticancer Activity of (S)-5-Chloro-3-((3,5-Dimethylphenyl) Sulfonyl)-N-(1-Oxo-1-((Pyridin-4-Ylmethyl)Amino)Propan-2-Yl)-1H-Indole-2-Carboxamide (RS4690), a New Dishevelled 1 Inhibitor. Cancers 2022, 14, 1358. [Google Scholar] [CrossRef]
- Moore, B.P.; Chung, D.H.; Matharu, D.S.; Golden, J.E.; Maddox, C.; Rasmussen, L.; Noah, J.W.; Sosa, M.I.; Ananthan, S.; Tower, N.A.; et al. (S)-N-(2,5-Dimethylphenyl)-1-(Quinoline-8-Ylsulfonyl)Pyrrolidine-2-Carboxamide as a Small Molecule Inhibitor Probe for the Study of Respiratory Syncytial Virus Infection. J. Med. Chem. 2012, 55, 8582–8587. [Google Scholar] [CrossRef]
- Abbasi, M.A.; Irshad, M.; Aziz-Ur-Rehman; Siddiqui, S.Z.; Nazir, M.; Ali Shah, S.A.; Shahid, M. Synthesis of Promising Antibacterial and Antifungal Agents: 2-[[(4-Chlorophenyl)Sulfonyl](2,3-Dihydro-1,4-Benzodioxin-6-Yl)Amino]-N(Un/Substituted-Phenyl)Acetamides. Pak. J. Pharm. Sci. 2020, 33, 2161–2170. [Google Scholar] [CrossRef]
- Minickaitė, R.; Grybaitė, B.; Vaickelionienė, R.; Kavaliauskas, P.; Petraitis, V.; Petraitienė, R.; Tumosienė, I.; Jonuškienė, I.; Mickevičius, V. Synthesis of Novel Aminothiazole Derivatives as Promising Antiviral, Antioxidant and Antibacterial Candidates. Int. J. Mol. Sci. 2022, 23, 7688. [Google Scholar] [CrossRef]
- Anusevicius, K.; Vaickelioniene, R.; Mickevicius, V. Unexpected Cyclization of N-Aryl-N-Carboxy-Ethyl-β-Alanines to 5,6-Dihydrouracils. Chem. Heterocycl. Compd. 2012, 48, 1105–1107. [Google Scholar] [CrossRef]
- Bouherrou, H.; Saidoun, A.; Abderrahmani, A.; Abdellaziz, L.; Rachedi, Y.; Dumas, F.; Demenceau, A. Synthesis and Biological Evaluation of New Substituted Hantzsch Thiazole Derivatives from Environmentally Benign One-Pot Synthesis Using Silica Supported Tungstosilisic Acid as Reusable Catalyst. Molecules 2017, 22, 757. [Google Scholar] [CrossRef] [PubMed]
- Anusevičius, K.; Jonuškiene, I.; Mickevičius, V. Synthesis and Antimicrobial Activity of N-(4-Chlorophenyl)-β-Alanine Derivatives with an Azole Moiety. Mon. Chem. 2013, 144, 1883–1891. [Google Scholar] [CrossRef]
- Vaickelioniene, R.; Mickeviciene, K.; Anusevicius, K.; Siugzdaite, J.; Kantminiene, K.; Mickevicius, V. Synthesis and Antibacterial Activity of Novel N-Carboxyalkyl-N-Phenyl-2-Aminothia(Oxa)Zole Derivatives. Heterocycles 2015, 91, 747–763. [Google Scholar] [CrossRef]
- Vaickelioniene, R.; Mickevičus, V.; Vaickelionis, G.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Novikov, V. Synthesis and Antibacterial and Antifungal Activity of N-(4-Fluorophenyl)-N-Carboxyethylaminothiazole Derivatives. Arkivoc 2015, 2015, 303–318. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Grybaite, B.; Jonuškiene, I.; Vaickelioniene, R.; Mickevičius, V. Synthesis, Transformation and Antibacterial Activity of New N,N-Disubstituted 2-Aminothiazole Derivatives. Chemija 2017, 28, 64–73. [Google Scholar]
- Parašotas, I.; Urbonavičiute, E.; Anusevičius, K.; Tumosiene, I.; Jonuškiene, I.; Kantminiene, K.; Vaickelioniene, R.; Mickevičius, V. Synthesis and Biological Evaluation of Novel DI- and Trisubstituted Thiazole Derivatives. Heterocycles 2017, 94, 1074–1097. [Google Scholar] [CrossRef]
- Parašotas, I.; Anusevičius, K.; Vaickelioniene, R.; Jonuškiene, I.; Stasevych, M.; Zvarych, V.; Olena, K.P.; Novikov, V.; Belyakov, S.; Mickevičius, V. Synthesis and Evaluation of the Antibacterial, Antioxidant Activities of Novel Functionalized Thiazole and Bis(Thiazol-5-Yl)Methane Derivatives. Arkivoc 2018, 2018, 240–256. [Google Scholar] [CrossRef]
- Kappe, T.; Karem, A.S.; Stadlbauer, W. Synthesis of Benzo-Halogenated 4-Hydroxy-2(1 H)-Quinolones. J. Heterocycl. Chem. 1988, 25, 857–862. [Google Scholar] [CrossRef]
- Zaki, Y.H.; Al-Gendey, M.S.; Abdelhamid, A.O. A Facile Synthesis, and Antimicrobial and Anticancer Activities of Some Pyridines, Thioamides, Thiazole, Urea, Quinazoline, β-Naphthyl Carbamate, and Pyrano[2,3-d]Thiazole Derivatives. Chem. Cent. J. 2018, 12, 70–84. [Google Scholar] [CrossRef]
- Omar, A.M.; Ihmaid, S.; Habib, E.-S.E.; Althagfan, S.S.; Ahmed, S.; Abulkhair, H.S.; Ahmed, H.E.A. The Rational Design, Synthesis, and Antimicrobial Investigation of 2-Amino-4-Methylthiazole Analogues Inhibitors of GlcN-6-P Synthase. Bioorg. Chem. 2020, 99, 103781–103793. [Google Scholar] [CrossRef] [PubMed]
- Muluk, M.B.; Dhumal, S.T.; Rehman, N.N.M.A.; Dixit, P.P.; Kharat, K.R.; Haval, K.P. Synthesis, Anticancer and Antimicrobial Evaluation of New (E)-N′-Benzylidene-2-(2-ethylpyridin-4-yl)-4-methylthiazole-5-carbohydrazides. ChemistrySelect 2019, 4, 8993–8997. [Google Scholar] [CrossRef]
- Mickevičius, V.; Voskiene, A.; Jonuškiene, I.; Kolosej, R.; Šiugždaite, J.; Venskutonis, P.R.; Kazernavičiute, R.; Braziene, Z.; Jakiene, E. Synthesis and Biological Activity of 3-[Phenyl(1,3-Thiazol-2-Yl)-Amino] Propanoic Acids and Their Derivatives. Molecules 2013, 18, 15000–15018. [Google Scholar] [CrossRef]
- Vaickelioniene, R.; Mickevicius, V.; Mikulskiene, G. Molecules Synthesis and Cyclizations of N-(2,3-,3,4- and 3,5-Dimethylphenyl)-β-Alanines. Molecules 2005, 10, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, M.; Iwamoto, A.; Kitao, T. Reaction of 2,3-dichloro-1,4-naphthoquinone with Dithiooxamide. Synthesis of Dibenzo[b,i]Thianthrene-5,7,12,14-tetrone. J. Heterocycl. Chem. 1991, 28, 1445–1447. [Google Scholar] [CrossRef]
- Matsuoka, M.; Iwamoto, A.; Furukawa, N.; Kitao, T. Syntheses of Polycyclic-1,4-dithiines and Related Heterocycles. J. Heterocycl. Chem. 1992, 29, 439–443. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Fan, W.-Q. A Reexamination of the Reactions of 2,3-dichloro-1,4-naphthoquinone with Thioamides. J. Heterocycl. Chem. 1993, 30, 1679–1681. [Google Scholar] [CrossRef]
- Ballatore, C.; Huryn, D.M.; Smith, A.B. Carboxylic Acid (Bio)Isosteres in Drug Design. ChemMedChem 2013, 8, 385–395. [Google Scholar] [CrossRef]
- Grybaitė, B.; Vaickelionienė, R.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Novikov, V.; Mickevičius, V. Synthesis, Transformation of 3-[(4-Arylthiazol-2-Yl)-(p-Tolyl)Amino]Propanoic Acids, Bis(Thiazol-5-Yl)Phenyl-, Bis(Thiazol-5-Yl)Methane Derivatives, and Their Antimicrobial Activity. Heterocycles 2018, 96, 86–105. [Google Scholar] [CrossRef]
- Grybaitė, B.; Vaickelionienė, R.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Kantminienė, K.; Novikov, V.; Mickevičius, V. Synthesis and Antimicrobial Activity of Novel Thiazoles with Reactive Functional Groups. ChemistrySelect 2019, 4, 6965–6970. [Google Scholar] [CrossRef]
- Malūkaitė, D.; Grybaitė, B.; Vaickelionienė, R.; Vaickelionis, G.; Sapijanskaitė-Banevič, B.; Kavaliauskas, P.; Mickevičius, V. Synthesis of Novel Thiazole Derivatives Bearing β-Amino Acid and Aromatic Moieties as Promising Scaffolds for the Development of New Antibacterial and Antifungal Candidates Targeting Multidrug-Resistant Pathogens. Molecules 2022, 27, 74. [Google Scholar] [CrossRef]
- Anusevičius, K.; Mickevičius, V.; Mikulskiene, G. Synthesis and Structure of N-(4-Bromophenyl)-N-Carboxyethyl-β-Alanine Derivatives. Chemija 2010, 21, 127–134. [Google Scholar]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.; Wilson, D.; Drew, R.; Perfect, J. Azole Antifungals: 35 Years of Invasive Fungal Infection Management. Expert Rev. Anti. Infect. Ther. 2015, 13, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216, S445–S451. [Google Scholar] [CrossRef] [PubMed]
- Nastasă, C.; Tiperciuc, B.; Duma, M.; Benedec, D.; Oniga, O. New Hydrazones Bearing Thiazole Scaffold: Synthesis, Characterization, Antimicrobial, and Antioxidant Investigation. Molecules 2015, 20, 17325–17338. [Google Scholar] [CrossRef] [PubMed]
- Zha, G.-F.; Leng, J.; Darshini, N.; Shubhavathi, T.; Vivek, H.K.; Asiri, A.M.; Marwani, H.M.; Rakesh, K.P.; Mallesha, N.; Qin, H.-L. Synthesis, SAR and Molecular Docking Studies of Benzo[d]Thiazole-Hydrazones as Potential Antibacterial and Antifungal Agents. Bioorg. Med. Chem. Lett. 2017, 27, 3148–3155. [Google Scholar] [CrossRef]
- Kauthale, S.; Tekale, S.; Damale, M.; Sangshetti, J.; Pawar, R. Synthesis, Antioxidant, Antifungal, Molecular Docking and ADMET Studies of Some Thiazolyl Hydrazones. Bioorg. Med. Chem. Lett. 2017, 27, 3891–3896. [Google Scholar] [CrossRef]
- Sahil; Kaur, K.; Jaitak, V. Thiazole and Related Heterocyclic Systems as Anticancer Agents: A Review on Synthetic Strategies, Mechanisms of Action and SAR Studies. Curr. Med. Chem. 2022, 29, 4958–5009. [Google Scholar] [CrossRef]
- Pawar, S.; Kumar, K.; Gupta, M.K.; Rawal, R.K. Synthetic and Medicinal Perspective of Fused-Thiazoles as Anticancer Agents. Anticancer. Agents Med. Chem. 2020, 21, 1379–1402. [Google Scholar] [CrossRef]
- Sharma, P.C.; Bansal, K.K.; Sharma, A.; Sharma, D.; Deep, A. Thiazole-Containing Compounds as Therapeutic Targets for Cancer Therapy. Eur. J. Med. Chem. 2020, 188, 112016–112063. [Google Scholar] [CrossRef] [PubMed]
- Swain, R.J.; Kemp, S.J.; Goldstraw, P.; Tetley, T.D.; Stevens, M.M. Assessment of Cell Line Models of Primary Human Cells by Raman Spectral Phenotyping. Biophys. J. 2010, 98, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chow, E.C.Y.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 Cell Monolayer: Usefulness and Limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Espinel-Ingroff, A.; Bustamante, B.; Canton, E.; Diekema, D.J.; Fothergill, A.; Fuller, J.; Gonzalez, G.M.; Guarro, J.; Lass-Flörl, C.; et al. Multicenter Study of Anidulafungin and Micafungin MIC Distributions and Epidemiological Cutoff Values for Eight Candida Species and the CLSI M27-A3 Broth Microdilution Method. Antimicrob. Agents Chemother. 2014, 58, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Peano, A.; Beccati, M.; Chiavassa, E.; Pasquetti, M. Evaluation of the Antifungal Susceptibility of Malassezia Pachydermatis to Clotrimazole, Miconazole and Thiabendazole Using a Modified CLSI M27-A3 Microdilution Method. Vet. Dermatol. 2012, 23, 131-e29. [Google Scholar] [CrossRef]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; De Amicis, C.V.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).