Stability of Important Veterinary Antibiotics Amoxicillin, Sulfadiazine, and Trimethoprim in Practice-Relevant Model Solutions
Abstract
:1. Introduction
2. Results
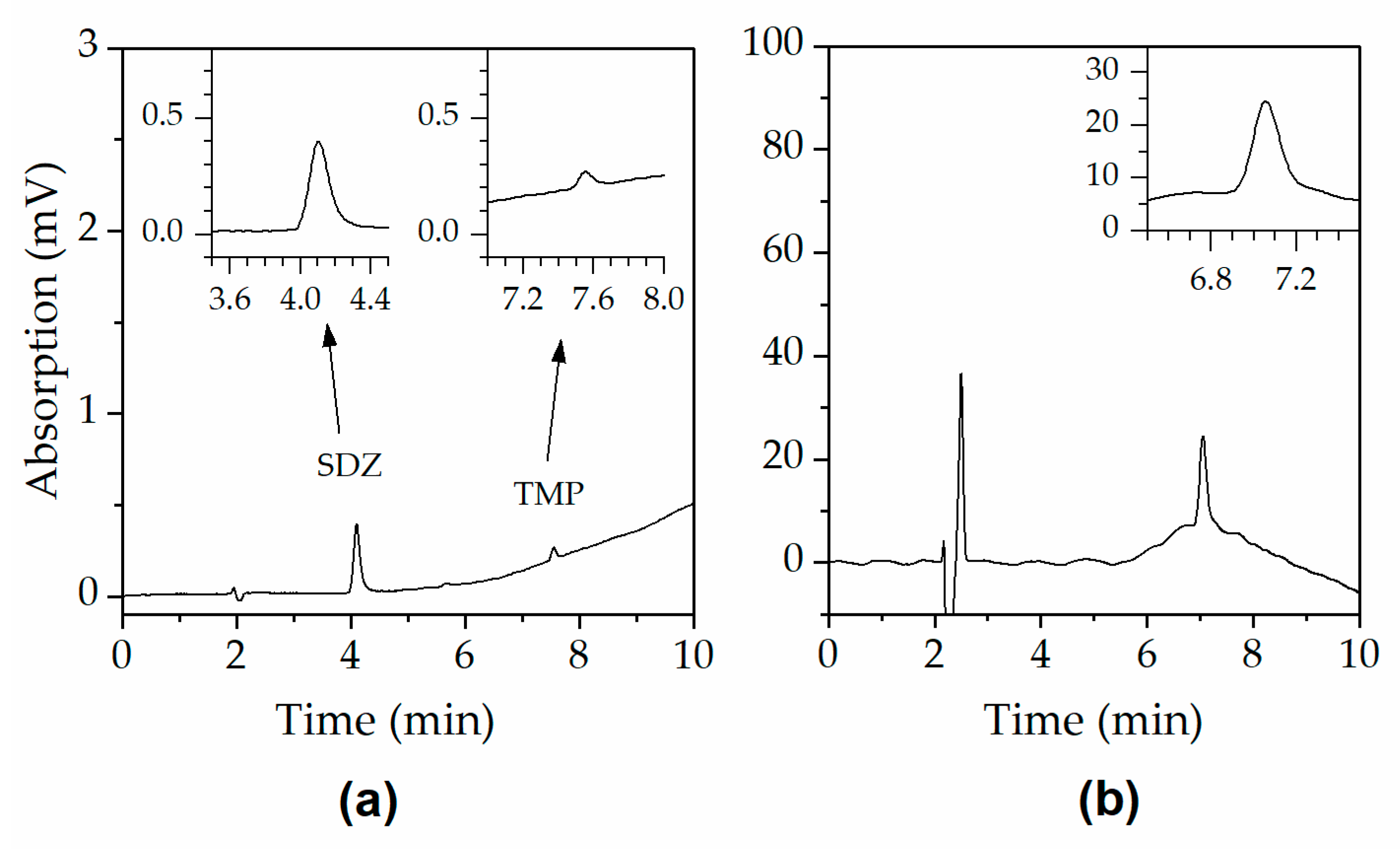
2.1. Stability of Sulfadiazine (SDZ) and Trimethoprim (TMP) Solutions
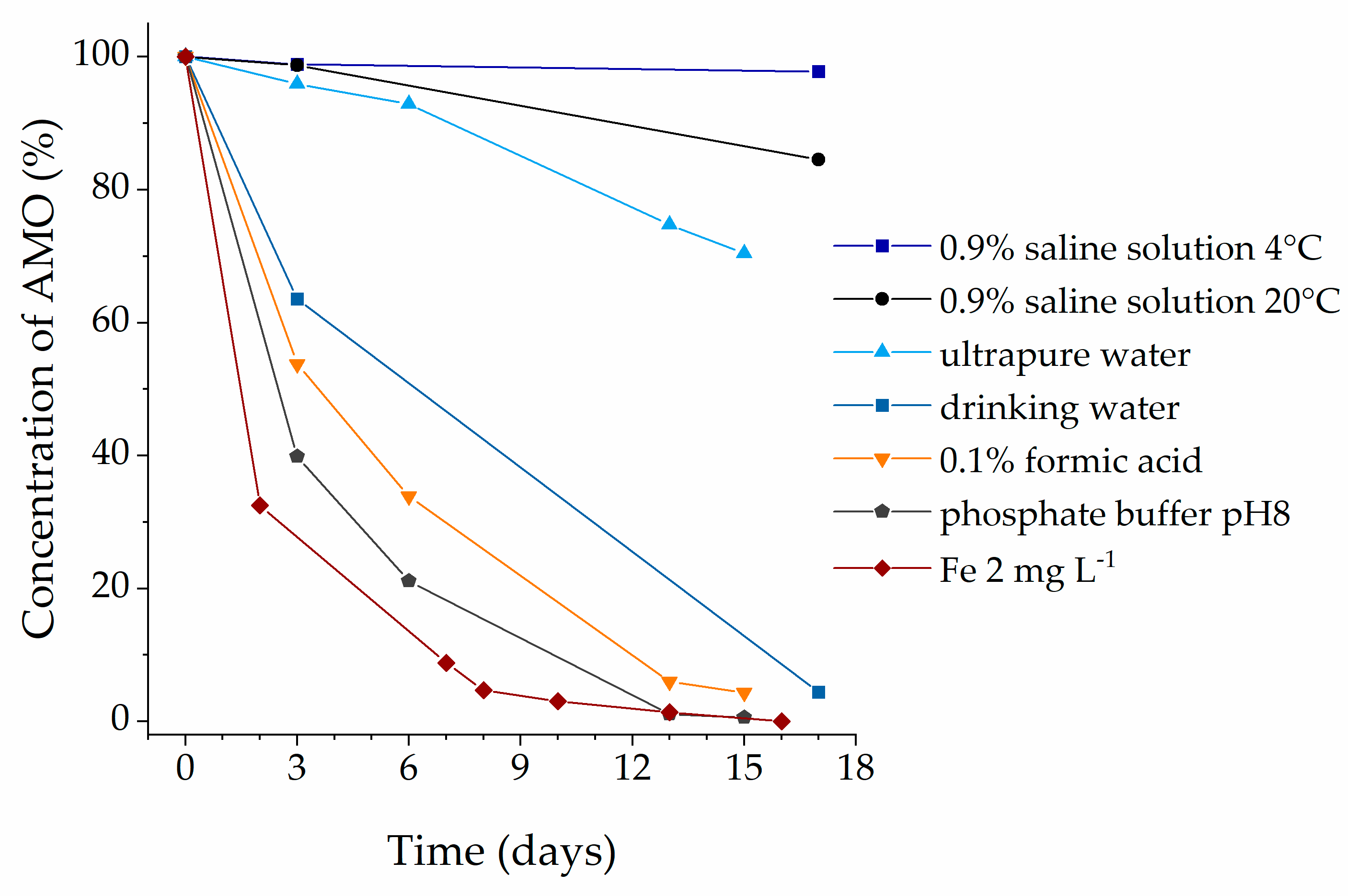
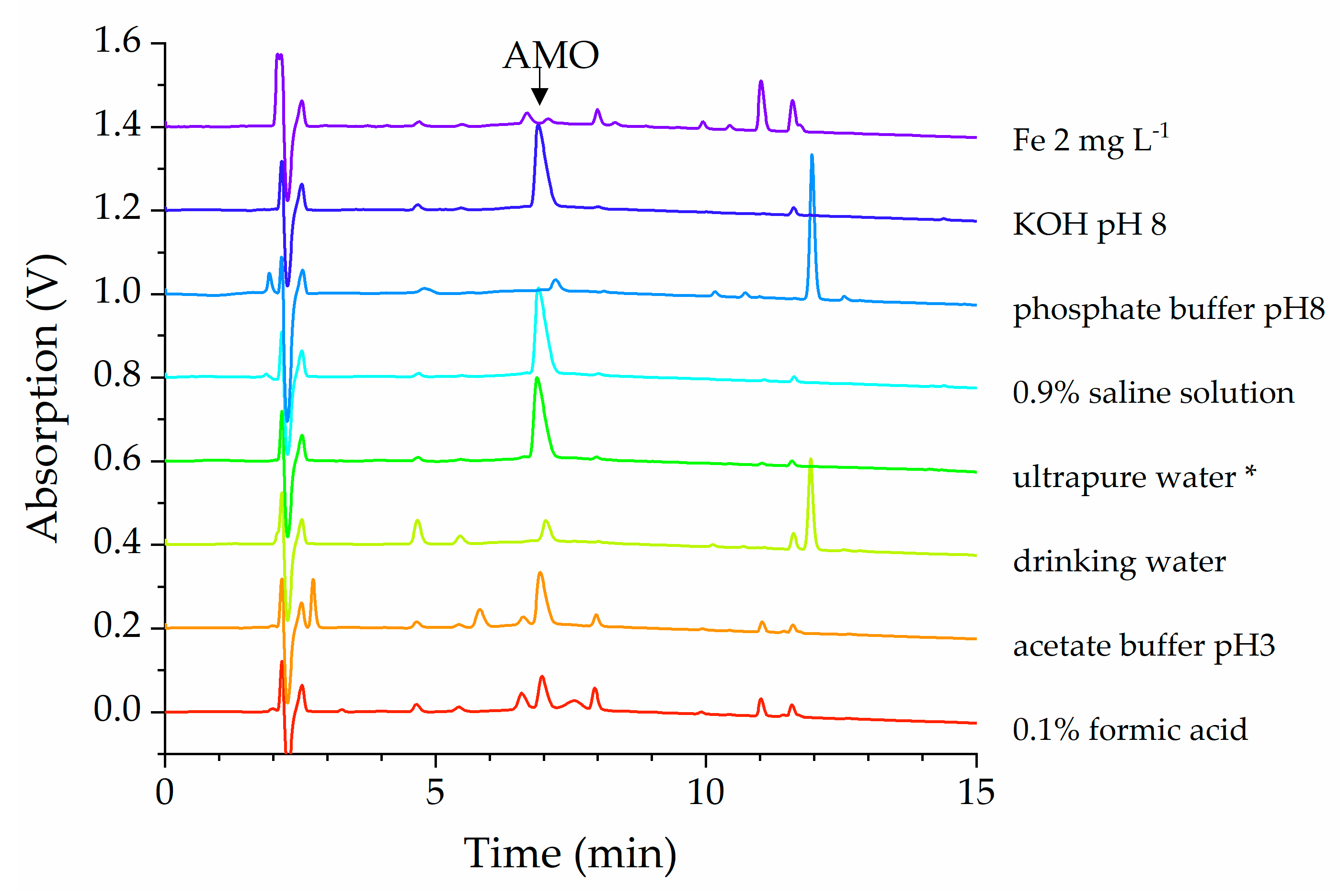
2.2. Stability of Amoxicillin (AMO) Solution
3. Discussion
4. Final Conclusions and Future Perspectives
5. Materials and Methods
5.1. Reagents and Materials
5.2. Chemical Analysis
5.3. Sample Preparation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Health at a Glance 2021: OECD Indicators; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- González Peña, O.I.; López Zavala, M.Á.; Cabral Ruelas, H. Pharmaceuticals Market, Consumption Trends and Disease Incidence Are Not Driving the Pharmaceutical Research on Water and Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 2532. [Google Scholar] [CrossRef] [PubMed]
- Bundesverband der Pharmazeutischen Industrie e.V. (BPI) Pharma-Daten 2020. Available online: https://www.bpi.de/fileadmin/user_upload/Downloads/Publikationen/Pharma-Daten/Pharma-Daten_2020_DE.pdf (accessed on 15 January 2023).
- Kemper, N. Veterinary Antibiotics in the Aquatic and Terrestrial Environment. Ecol. Indic. 2008, 8, 1–13. [Google Scholar] [CrossRef]
- Haupt, R.; Heinemann, C.; Hayer, J.J.; Schmid, S.M.; Guse, M.; Bleeser, R.; Steinhoff-Wagner, J. Critical Discussion of the Current Environmental Risk Assessment (ERA) of Veterinary Medicinal Products (VMPs) in the European Union, Considering Changes in Animal Husbandry. Environ. Sci. Eur. 2021, 33, 1–21. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Guideline on Excipients in the Dossier for Application for Marketing Authorisation of a Medicinal Product; European Medicines Agency: Amsterdam, The Netherlands, 2008.
- Kamphues, J. Risks Due to the Medication of Feed and Water in Animal Facilities. Dtsch. Tierarztl. Wochenschr. 1996, 103, 250–256. [Google Scholar] [PubMed]
- Richter, A.; Hafez, H.M.; Böttner, A.; Gangl, A.; Hartmann, K.; Kaske, M.; Kehrenberg, C.; Kietzmann, M.; Klarmann, D.; Klein, G.; et al. Verabreichung von Antibiotika in Geflügelbeständen. Tierarztl. Prax. Ausgabe G Grosstiere Nutztiere 2009, 37, 321–329. [Google Scholar] [CrossRef]
- Vermeulen, B.; De Backer, P.; Remon, J.P. Drug Administration to Poultry. Adv. Drug Deliv. Rev. 2002, 54, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Microbiological Effects of Sublethal Levels of Antibiotics. Nat. Rev. Microbiol. 2014, 12, 465–478. [Google Scholar] [CrossRef]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem Response to Antibiotics Entering the Aquatic Environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. The Review on Antimicrobial Resistance. 2014. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 15 January 2023).
- Skandalis, N.; Maeusli, M.; Papafotis, D.; Miller, S.; Lee, B.; Theologidis, I.; Luna, B. Environmental Spread of Antibiotic Resistance. Antibiotics 2021, 10, 640. [Google Scholar] [CrossRef]
- G7 Health Ministers Declaration of the G7 Health Ministers, Berlin Declaration on Antimicrobial Resistance—Global Union for Antibiotics Research and Development (GUARD). Available online: https://www.bundesgesundheitsministerium.de/fileadmin/Dateien/3_Downloads/G/G7/G7_Health_Ministers_Declaration_AMR_and_EBOLA.pdf (accessed on 15 January 2023).
- Gozlan, I.; Rotstein, A.; Avisar, D. Amoxicillin-Degradation Products Formed under Controlled Environmental Conditions: Identification and Determination in the Aquatic Environment. Chemosphere 2013, 91, 985–992. [Google Scholar] [CrossRef]
- Zessel, K.; Mohring, S.; Hamscher, G.; Kietzmann, M.; Stahl, J. Biocompatibility and Antibacterial Activity of Photolytic Products of Sulfonamides. Chemosphere 2014, 100, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Spielmeyer, A.; Höper, H.; Hamscher, G. Long-Term Monitoring of Sulfonamide Leaching from Manure Amended Soil into Groundwater. Chemosphere 2017, 177, 232–238. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). ICH Topic Q 1 A (R2). In Stability Testing of New Drug Substances and Products; CPMP/ICH/2736/99; European Medicines Agency: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Hirte, K.; Seiwert, B.; Schüürmann, G.; Reemtsma, T. New Hydrolysis Products of the Beta-Lactam Antibiotic Amoxicillin, Their PH-Dependent Formation and Search in Municipal Wastewater. Water Res. 2016, 88, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Ecke, A.; Westphalen, T.; Retzmann, A.; Schneider, R.J. Factors Affecting the Hydrolysis of the Antibiotic Amoxicillin in the Aquatic Environment. Chemosphere 2023, 311, 136921. [Google Scholar] [CrossRef]
- Kamphues, J.; Böhm, R.; Flachowsky, G.; Lahrssen-Wiederholt, M.; Meyer, U.; Schenkel, H. Empfehlungen Zur Beurteilung Der Hygienischen Qualität von Tränkwasser Für Lebensmittel Liefernde Tiere Unter Berücksichtigung Der Gegebenen Rechtlichen Rahmenbedingungen. Landbauforsch. Völkenrode 2007, 3, 255–272. [Google Scholar]
- Białk-Bielińska, A.; Stolte, S.; Matzke, M.; Fabiańska, A.; Maszkowska, J.; Kołodziejska, M.; Liberek, B.; Stepnowski, P.; Kumirska, J. Hydrolysis of Sulphonamides in Aqueous Solutions. J. Hazard. Mater. 2012, 221–222, 264–274. [Google Scholar] [CrossRef]
- Kokoszka, K.; Wilk, J.; Felis, E.; Bajkacz, S. Application of UHPLC-MS/MS Method to Study Occurrence and Fate of Sulfonamide Antibiotics and Their Transformation Products in Surface Water in Highly Urbanized Areas. Chemosphere 2021, 283, 131189. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Ciniglia, C.; De Champdoré, M.; Lo Giudice, R.; Marotta, R.; Zuccato, E. Antibiotics in the Environment: Occurrence in Italian STPs, Fate, and Preliminary Assessment on Algal Toxicity of Amoxicillin. Environ. Sci. Technol. 2004, 38, 6832–6838. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hahne, F.; Müller, C.; Yalman, S.; Meißner, J.; Kietzmann, M.; Hamscher, G. Stability of Important Veterinary Antibiotics Amoxicillin, Sulfadiazine, and Trimethoprim in Practice-Relevant Model Solutions. Antibiotics 2023, 12, 214. https://doi.org/10.3390/antibiotics12020214
Hahne F, Müller C, Yalman S, Meißner J, Kietzmann M, Hamscher G. Stability of Important Veterinary Antibiotics Amoxicillin, Sulfadiazine, and Trimethoprim in Practice-Relevant Model Solutions. Antibiotics. 2023; 12(2):214. https://doi.org/10.3390/antibiotics12020214
Chicago/Turabian StyleHahne, Friederike, Clarissa Müller, Suzan Yalman, Jessica Meißner, Manfred Kietzmann, and Gerd Hamscher. 2023. "Stability of Important Veterinary Antibiotics Amoxicillin, Sulfadiazine, and Trimethoprim in Practice-Relevant Model Solutions" Antibiotics 12, no. 2: 214. https://doi.org/10.3390/antibiotics12020214
APA StyleHahne, F., Müller, C., Yalman, S., Meißner, J., Kietzmann, M., & Hamscher, G. (2023). Stability of Important Veterinary Antibiotics Amoxicillin, Sulfadiazine, and Trimethoprim in Practice-Relevant Model Solutions. Antibiotics, 12(2), 214. https://doi.org/10.3390/antibiotics12020214





