Fast and Sensitive Analysis of Cefiderocol in Human Plasma Microsamples by Liquid Chromatography-Isotope Dilution Tandem Mass Spectrometry for Therapeutic Drug Monitoring
Abstract
1. Introduction
2. Results
2.1. Optimization of LC-MS/MS Conditions
2.2. Method Validation
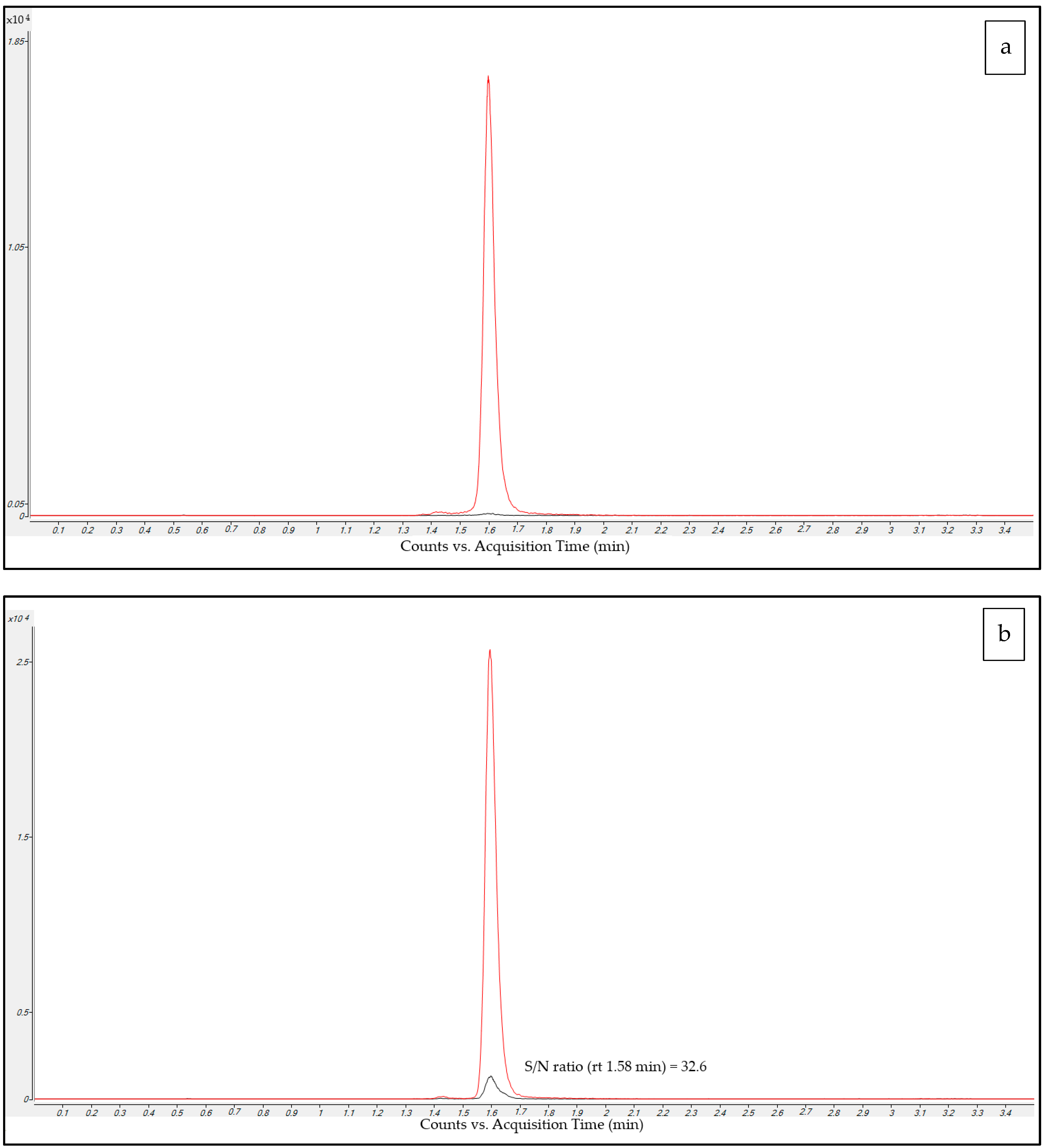
2.2.1. Sensitivity
2.2.2. Selectivity and Carry-Over
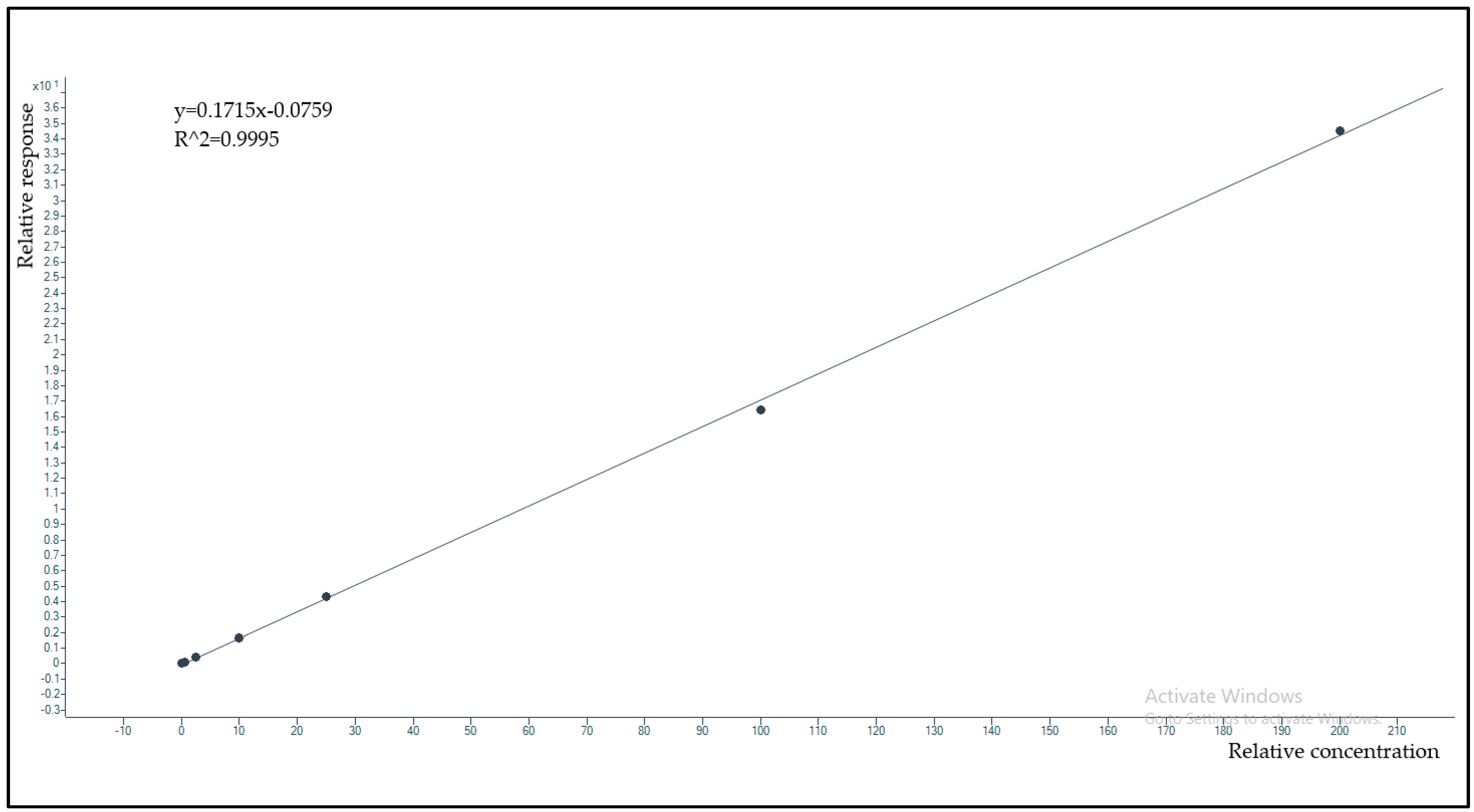
2.2.3. Linearity and Limit of Quantification (LOQ)
2.2.4. Accuracy and Precision
2.2.5. Matrix Effect and Extraction Recovery
2.2.6. Stability
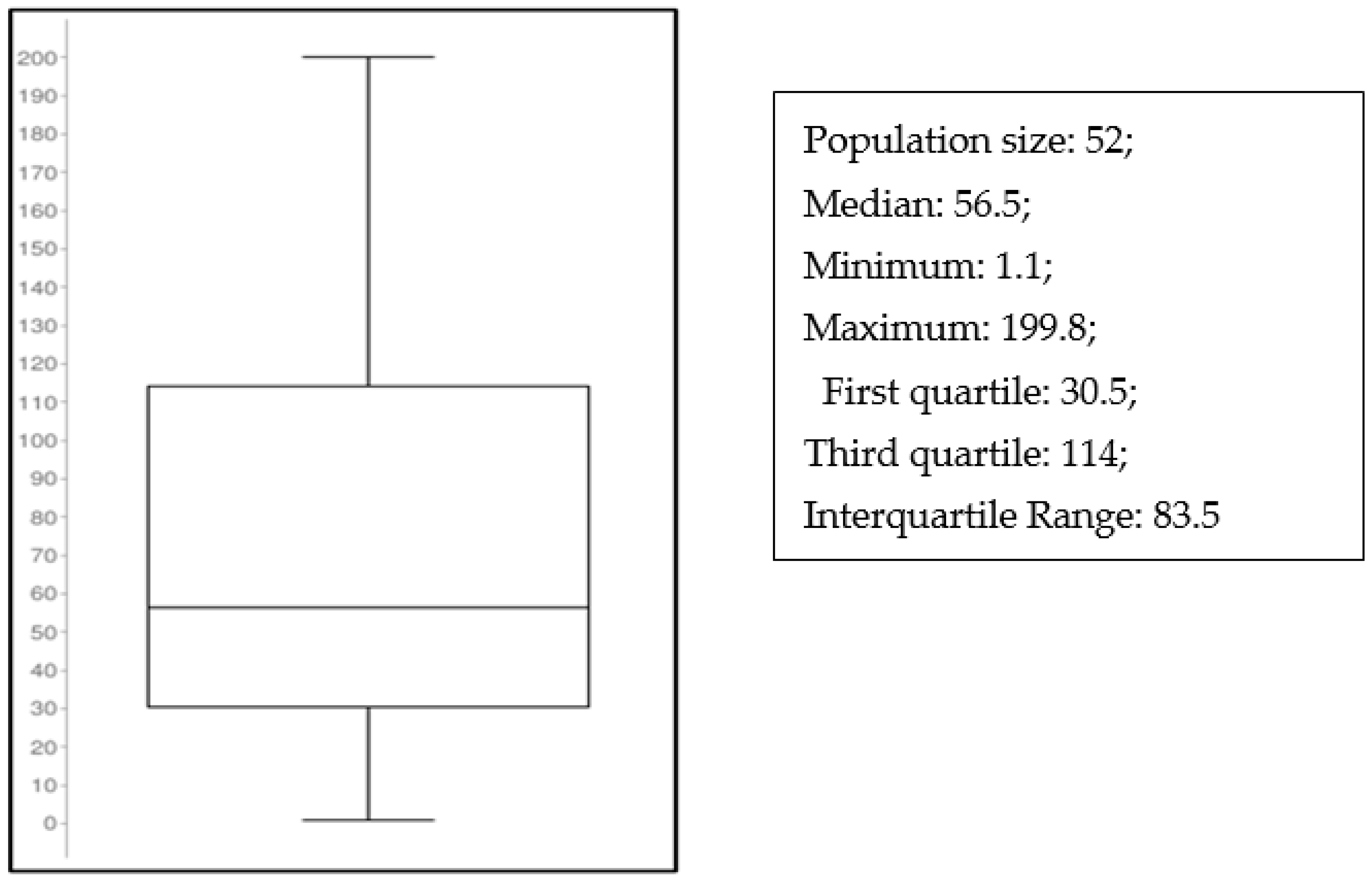
2.3. Clinical Application
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Stock Solutions, Standards and Quality Controls
4.3. Instrumentation
4.4. Sample Pre-Treatment
4.5. Method Validation
4.5.1. Selectivity and Carry-Over
4.5.2. Linearity and Limit of Quantification (LOQ)
4.5.3. Precision and Accuracy
4.5.4. Matrix Effect and Extraction Recovery
4.5.5. Stability
- extracts on board kept at 10 °C during 24 h;
- extracts kept at −20 °C during 24 h;
- matrix samples after three complete freeze and thaw cycles from −80 °C to 25 °C.
4.6. Clinical Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Giske, C.G.; Grāmatniece, A.; Abodakpi, H.; Tam, V.H.; Leibovici, L. New β-Lactam–β-Lactamase Inhibitor Combinations. Clin. Microbiol. Rev. 2020, 34, e00115-20. [Google Scholar] [CrossRef] [PubMed]
- Katsube, T.; Echols, R.; Wajima, T. Pharmacokinetic and Pharmacodynamic Profiles of Cefiderocol, a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, S552–S558. [Google Scholar] [CrossRef] [PubMed]
- Syed, Y.Y. Cefiderocol: A Review in Serious Gram-Negative Bacterial Infections. Drugs 2021, 81, 1559–1571. [Google Scholar] [CrossRef]
- Bassetti, M.; Echols, R.; Matsunaga, Y.; Ariyasu, M.; Doi, Y.; Ferrer, R.; Lodise, T.P.; Naas, T.; Niki, Y.; Paterson, D.L.; et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): A randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 2021, 21, 226–240. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America Guidance on the Treatment of AmpC β-Lactamase–Producing Enterobacterales, Carbapenem-Resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia Infections. Clin. Infect. Dis. 2022, 74, 2089–2114. [Google Scholar] [CrossRef]
- Pascale, R.; Pasquini, Z.; Bartoletti, M.; Caiazzo, L.; Fornaro, G.; Bussini, L.; Volpato, F.; Marchionni, E.; Rinaldi, M.; Trapani, F.; et al. Cefiderocol treatment for carbapenem-resistant Acinetobacter baumannii infection in the ICU during the COVID-19 pandemic: A multicentre cohort study. JAC-Antimicrob. Resist. 2021, 3, dlab174. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Leonildi, A.; Della Sala, L.; Vecchione, A.; Barnini, S.; Farcomeni, A.; Menichetti, F. Cefiderocol- Compared to Colistin-Based Regimens for the Treatment of Severe Infections Caused by Carbapenem-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2022, 66, e0214221. [Google Scholar] [CrossRef]
- Gatti, M.; Bartoletti, M.; Cojutti, P.G.; Gaibani, P.; Conti, M.; Giannella, M.; Viale, P.; Pea, F. A descriptive case series of pharmacokinetic/pharmacodynamic target attainment and microbiological outcome in critically ill patients with documented severe extensively drug-resistant Acinetobacter baumannii bloodstream infection and/or ventilator-associated pneumonia treated with cefiderocol. J. Glob. Antimicrob. Resist. 2021, 27, 294–298. [Google Scholar] [CrossRef]
- Zimmer, J.; Röhr, A.; Kluge, S.; Faller, J.; Frey, O.; Wichmann, D.; König, C. Validation and Application of an HPLC-UV Method for Routine Therapeutic Drug Monitoring of Cefiderocol. Antibiotics 2021, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Mernissi, T.; Bodeau, S.; André, C.; Zahr, N.; Mary, A.; Dupont, H.; Kontar, L.; Lemaire-Hurtel, A.-S.; Bennis, Y. An HPLC assay for the therapeutic drug monitoring of cefiderocol in critically ill patients. J. Antimicrob. Chemother. 2021, 76, 1643–1646. [Google Scholar] [CrossRef]
- McLeod, J.; Stadler, E.; Wilson, R.; Holmes, A.; O’Hare, D. Electrochemical detection of cefiderocol for therapeutic drug monitoring. Electrochem. Commun. 2021, 133, 107147. [Google Scholar] [CrossRef]
- Katsube, T.; Echols, R.; Ferreira, J.C.A.; Krenz, H.K.; Berg, J.K.; Galloway, C. Cefiderocol, a Siderophore Cephalosporin for Gram-Negative Bacterial Infections: Pharmacokinetics and Safety in Subjects with Renal Impairment. J. Clin. Pharmacol. 2017, 57, 584–591. [Google Scholar] [CrossRef]
- Llopis, B.; Bleibtreu, A.; Schlemmer, D.; Robidou, P.; Paccoud, O.; Tissot, N.; Noé, G.; Junot, H.; Luyt, C.; Funck-Brentano, C.; et al. Simple and accurate quantitative analysis of cefiderocol and ceftobiprole in human plasma using liquid chromatography-isotope dilution tandem mass spectrometry: Interest for their therapeutic drug monitoring and pharmacokinetic studies. Clin. Chem. Lab. Med. 2021, 59, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Tey, H.Y.; See, H.H. A review of recent advances in microsampling techniques of biological fluids for therapeutic drug monitoring. J. Chromatogr. A 2021, 1635, 461731. [Google Scholar] [CrossRef] [PubMed]
- Rincón, J.P.; Meesters, R.J.W. Evaluation of peripheral blood microsampling techniques in combination with liquid chromatography-high resolution mass spectrometry for the determination of drug pharmacokinetics in clinical studies. Drug Test. Anal. 2014, 6, 568–577. [Google Scholar] [CrossRef]
- Ruggiero, C.; Ramirez, S.; Ramazzotti, E.; Mancini, R.; Muratori, R.; Raggi, M.A.; Conti, M. Multiplexed therapeutic drug monitoring of antipsychotics in dried plasma spots by LC-MS/MS. J. Sep. Sci. 2020, 43, 1440–1449. [Google Scholar] [CrossRef]
- Spooner, N.; Denniff, P.; Michielsen, L.; De Vries, R.; Ji, Q.C.; Arnold, M.E.; Woods, K.; Woolf, E.J.; Xu, Y.; Boutet, V.; et al. A device for dried blood microsampling in quantitative bioanalysis: Overcoming the issues associated blood hematocrit. Bioanalysis 2015, 7, 653–659. [Google Scholar] [CrossRef]
- da Silva, A.C.C.; de Lima Feltraco Lizot, L.; Bastiani, M.F.; Antunes, M.V.; Brucker, N.; Linden, R. Dried plasma spots for therapeutic monitoring of amikacin: Validation of an UHPLC-MS/MS assay and pharmacokinetic application. J. Pharm. Biomed. Anal. 2020, 184, 113201. [Google Scholar] [CrossRef]
- Saisho, Y.; Katsube, T.; White, S.; Fukase, H.; Shimada, J. Pharmacokinetics, Safety, and Tolerability of Cefiderocol, a Novel Siderophore Cephalosporin for Gram-Negative Bacteria, in Healthy Subjects. Antimicrob. Agents Chemother. 2018, 62, e02163-17. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Singley, C.M.; Hoover, J.; Nakamura, R.; Echols, R.; Rittenhouse, S.; Tsuji, M.; Yamano, Y. Efficacy of Cefiderocol against Carbapenem-Resistant Gram-Negative Bacilli in Immunocompetent-Rat Respiratory Tract Infection Models Recreating Human Plasma Pharmacokinetics. Antimicrob. Agents Chemother. 2017, 61, e00700-17. [Google Scholar] [CrossRef] [PubMed]
- Loeuille, G.; Vigneron, J.; D’Huart, E.; Charmillon, A.; Demoré, B. Physicochemical stability of cefiderocol, a novel siderophore cephalosporin, in syringes at 62.5 mg/mL for continuous administration in intensive care units. Eur. J. Hosp. Pharm. 2021. [Google Scholar] [CrossRef] [PubMed]
- Neuner, E.A.; Gallagher, J.C. Pharmacodynamic and pharmacokinetic considerations in the treatment of critically Ill patients infected with carbapenem-resistant Enterobacteriaceae. Virulence 2017, 8, 440–452. [Google Scholar] [CrossRef]
- EMA. Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation (accessed on 26 October 2022).
- Cojutti, P.G.; Rinaldi, M.; Zamparini, E.; Rossi, N.; Tedeschi, S.; Conti, M.; Pea, F.; Viale, P. Population Pharmacokinetics of Dalbavancin and Dosing Consideration for Optimal Treatment of Adult Patients with Staphylococcal Osteoarticular Infections. Antimicrob. Agents Chemother. 2021, 65, e02260-20. [Google Scholar] [CrossRef] [PubMed]
- Cojutti, P.G.; Tedeschi, S.; Gatti, M.; Zamparini, E.; Meschiari, M.; Della Siega, P.; Mazzitelli, M.; Soavi, L.; Binazzi, R.; Erne, E.M.; et al. Population Pharmacokinetic and Pharmacodynamic Analysis of Dalbavancin for Long-Term Treatment of Subacute and/or Chronic Infectious Diseases: The Major Role of Therapeutic Drug Monitoring. Antibiotics 2022, 11, 996. [Google Scholar] [CrossRef]
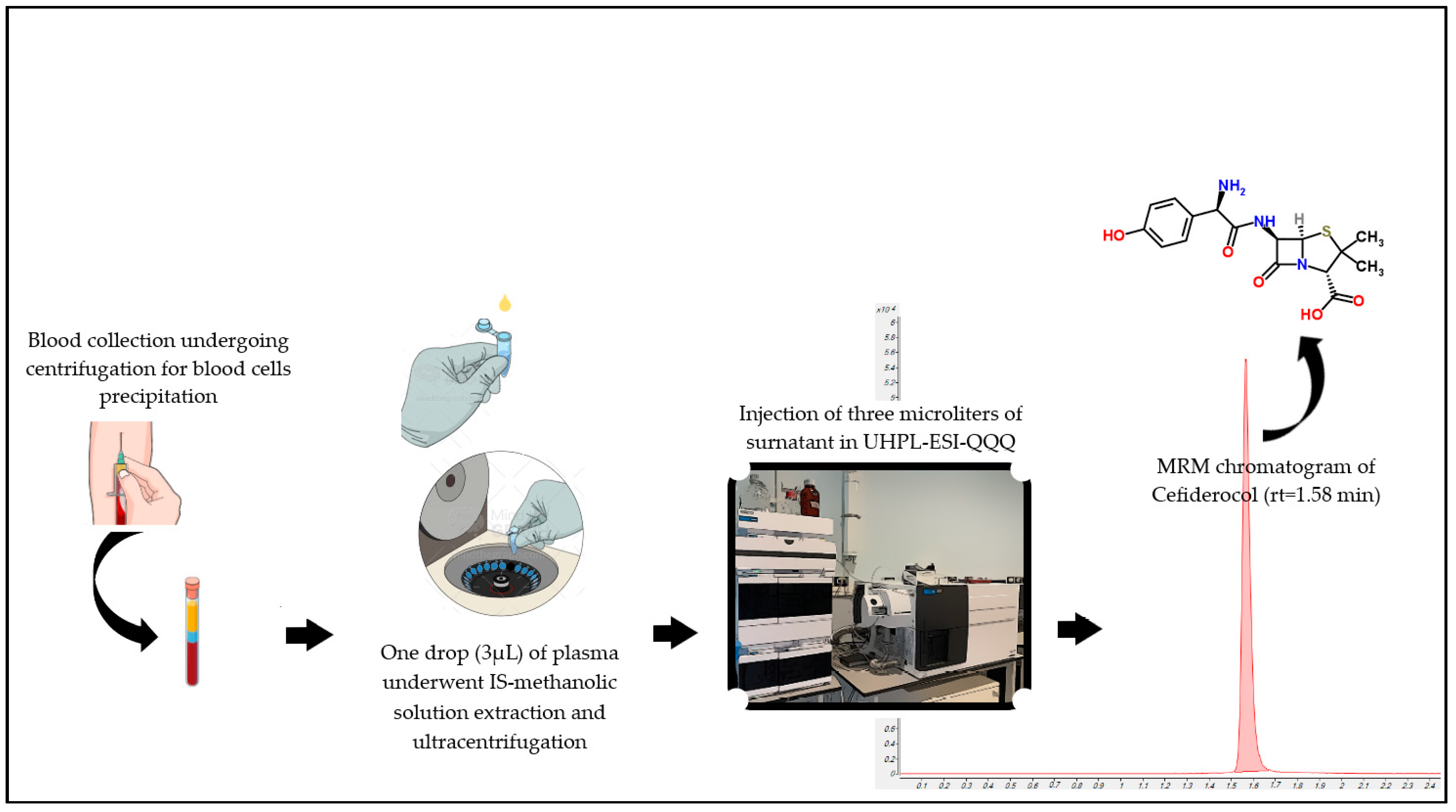

| Analyte | Retention Time (min) | Precursor Ion (m/z) | Product Ion (m/z) | Dwell Time (ms) | Fragmentator (V) | Collision Energy (V) |
|---|---|---|---|---|---|---|
| Cefiderocol | 1.58 | 752.1 | 285.2 | 50 | 166 | 20 |
| Cefiderocol-d12 | 1.58 | 764.1 | 297.3 | 50 | 166 | 20 |
| Time (min) | A (%) | B (%) | Flow (mL/min) |
|---|---|---|---|
| 0 | 95 | 5 | 0.5 |
| 2 | 5 | 95 | 0.5 |
| 2.5 | 5 | 95 | 0.5 |
| 2.51 | 95 | 5 | 0.5 |
| 4 | 95 | 5 | 0.5 |
| QC Levels | Intraday (n = 5) | Inter-Day (n = 3) | |||||
|---|---|---|---|---|---|---|---|
| Sample Name | Nominal Conc. (mg/L) | Avg Conc. (mg/L) | Avg Accuracy (Bias%) | Avg Conc. (mg/L) | Avg Conc. (mg/L) | Avg Precision (CV%) | Avg Accuracy (Bias%) |
| LOQ | 0.1 | 0.12 | 13.5 | 8.8 | 0.12 | 14.8 | 9.2 |
| LQC | 0.25 | 7.5 | 10.9 | 9.3 | 9.8 | 9.6 | 8.3 |
| MQC | 75 | 102.5 | 9.8 | 7.9 | 104.2 | 8.0 | 9.7 |
| HQC | 150 | 395.7 | 9.7 | 8.1 | 401.1 | 11.0 | 13.1 |
| Quality Control Level | N° Replicates | Average Matrix Effect (%) | Average IS-Normalized Matrix Effect (%) |
|---|---|---|---|
| LQC | 30 | 181.9 | 104.2 |
| MQC | 30 | 185.7 | 105.1 |
| HQC | 30 | 187.2 | 98.3 |
| Quality Control | LQC | MQC | HQC | |
|---|---|---|---|---|
| Types of sample | Tested conditions | Avg Accuracy (Bias%) | Avg Accuracy (Bias%) | Avg Accuracy (Bias%) |
| extracts | autosampler post 2 h | −22.1 | −29.1 | −24.6 |
| freezer post 24 h | −16.5 | −16.7 | −22.1 | |
| plasma samples | freeze-thaw stability | |||
| 1 cycle | −15.8 | −15.1 | −15.4 | |
| 2 cycle | −35.1 | −39.0 | −32.6 | |
| 3 cycle | −77.4 | −75.2 | −76.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barone, R.; Conti, M.; Cojutti, P.G.; Gatti, M.; Viale, P.; Pea, F. Fast and Sensitive Analysis of Cefiderocol in Human Plasma Microsamples by Liquid Chromatography-Isotope Dilution Tandem Mass Spectrometry for Therapeutic Drug Monitoring. Antibiotics 2023, 12, 213. https://doi.org/10.3390/antibiotics12020213
Barone R, Conti M, Cojutti PG, Gatti M, Viale P, Pea F. Fast and Sensitive Analysis of Cefiderocol in Human Plasma Microsamples by Liquid Chromatography-Isotope Dilution Tandem Mass Spectrometry for Therapeutic Drug Monitoring. Antibiotics. 2023; 12(2):213. https://doi.org/10.3390/antibiotics12020213
Chicago/Turabian StyleBarone, Rossella, Matteo Conti, Pier Giorgio Cojutti, Milo Gatti, Pierluigi Viale, and Federico Pea. 2023. "Fast and Sensitive Analysis of Cefiderocol in Human Plasma Microsamples by Liquid Chromatography-Isotope Dilution Tandem Mass Spectrometry for Therapeutic Drug Monitoring" Antibiotics 12, no. 2: 213. https://doi.org/10.3390/antibiotics12020213
APA StyleBarone, R., Conti, M., Cojutti, P. G., Gatti, M., Viale, P., & Pea, F. (2023). Fast and Sensitive Analysis of Cefiderocol in Human Plasma Microsamples by Liquid Chromatography-Isotope Dilution Tandem Mass Spectrometry for Therapeutic Drug Monitoring. Antibiotics, 12(2), 213. https://doi.org/10.3390/antibiotics12020213






