The Effect of Decreased Antipseudomonal Drug Consumption on Pseudomonas aeruginosa Incidence and Antimicrobial Susceptibility Profiles over 9 Years in a Lebanese Tertiary Care Center
Abstract
1. Introduction
2. Results
2.1. General Results
2.2. Pseudomonas Isolation Density and BSI Density
2.2.1. Pseudomonas aeruginosa
2.2.2. Pseudomonas sp.
2.2.3. Pseudomonas putida
2.3. Resistance of Pseudomonas aeruginosa to Different Antibiotics
2.3.1. Difficult-to-Treat Cultures
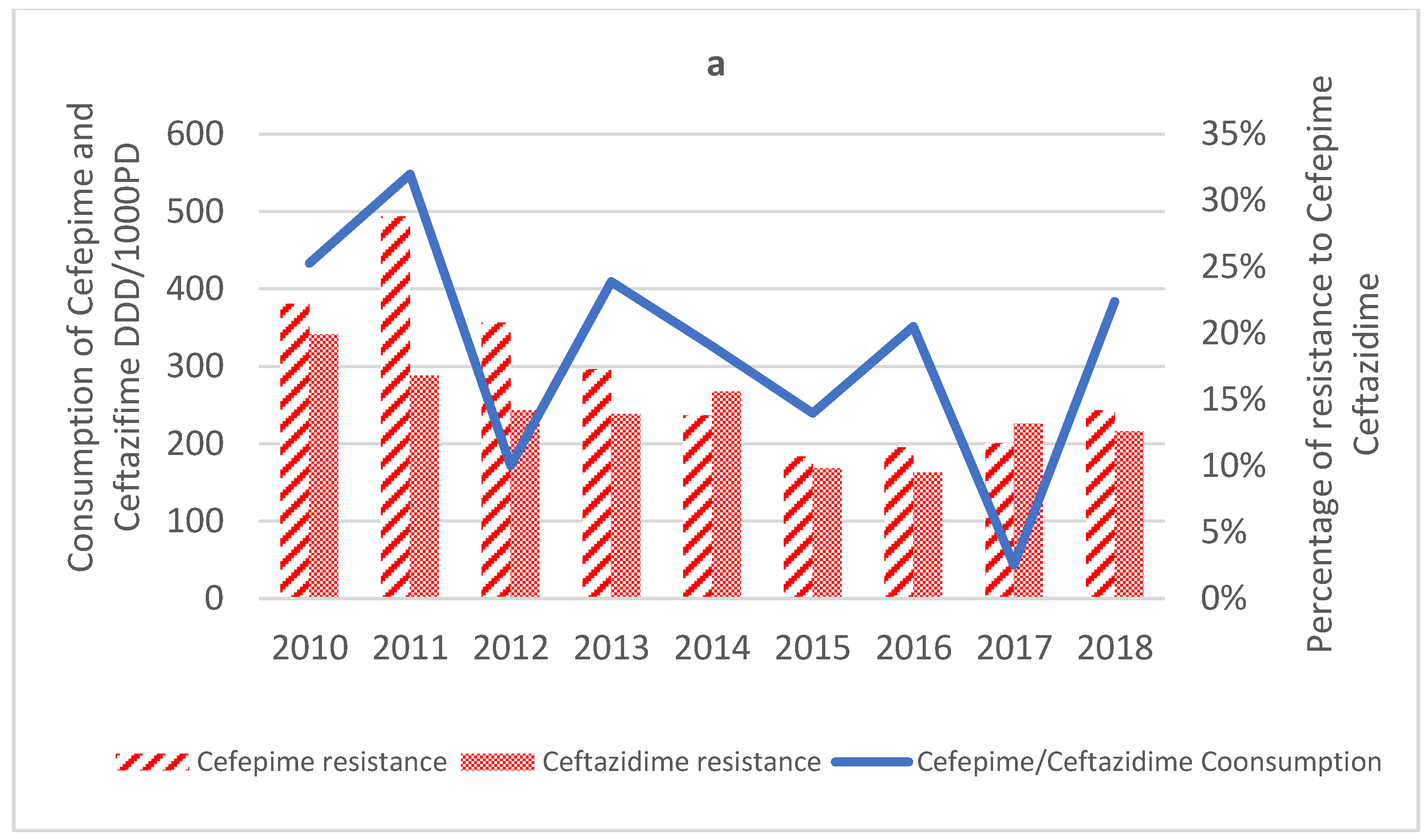
2.3.2. Cephalosporins
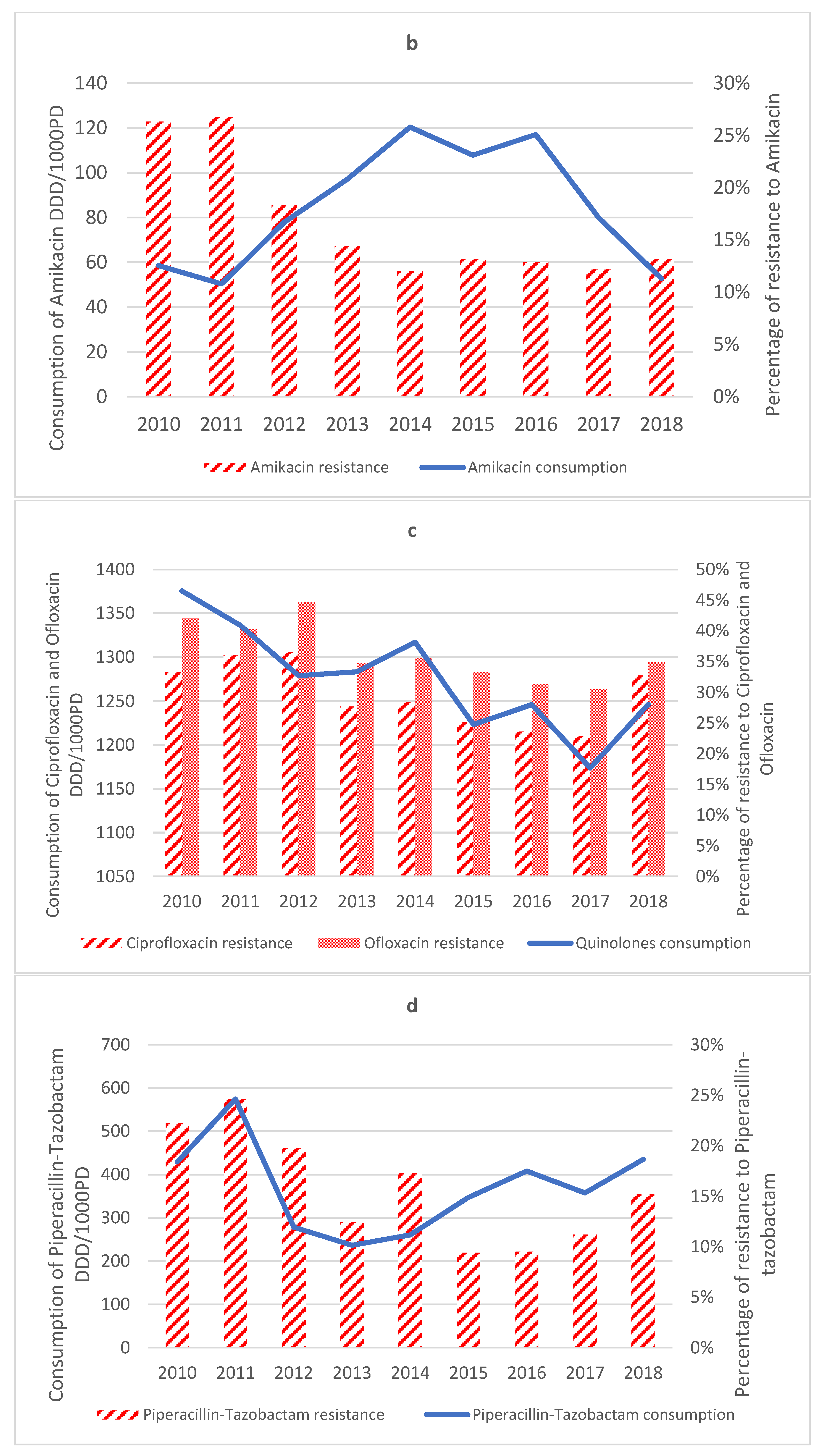
2.3.3. Aminoglycosides
2.3.4. Quinolones
2.3.5. Colistin
2.3.6. Carbapenems
2.3.7. Antipseudomonal Penicillin + β-Lactamase Inhibitors
2.4. Antimicrobial Consumption
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diekema, D.J.; Pfaller, M.; Jones, R. Survey of Bloodstream Infections Due to Gram-Negative Bacilli: Frequency of Occurrence and Antimicrobial Susceptibility of Isolates Collected in the United States, Canada, and Latin America for the SENTRY. Clin. Infect. Dis. 1999, 29, 595–607. [Google Scholar]
- Hancock, R.; Speert, D. Antibiotics for Pseudomonas and Related Infections; Dodge, J., Brock, D., Widdicombe, J., Eds.; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1996; Volume 3. [Google Scholar]
- Neuhauser, M.M.; Weinstein, R.A.; Rydman, R.; Danziger, L.H.; Karam, G.; Quinn, J.P. Antibiotic Resistance among Gram-Negative Bacilli in US Intensive Care Units: Implications for Fluoroquinolone Use. J. Am. Med. Assoc. 2003, 289, 885–888. [Google Scholar] [CrossRef]
- Anderson, D.J.; Miller, B.; Marfatia, R.; Drew, R. Ability of an Antibiogram to Predict Pseudomonas Aeruginosa Susceptibility to Targeted Antimicrobials Based on Hospital Day of Isolation. Infect. Control Hosp. Epidemiol. 2012, 33, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Antibiotic Consumption and Its Relationship with Bacterial Resistance Profiles in ESKAPE Pathogens in a Peruvian Hospital—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8532675/ (accessed on 24 October 2022).
- Rossolini, G.M.; Mantengoli, E. Treatment and Control of Severe Infections Caused by Multiresistant Pseudomonas Aeruginosa. Clin. Microbiol. Infect. Suppl. 2005, 11, 17–32. [Google Scholar] [CrossRef]
- Lister, P.D.; Wolter, D.J.; Hanson, N.D. Antibacterial-Resistant Pseudomonas Aeruginosa: Clinical Impact and Complex Regulation of Chromosomally Encoded Resistance Mechanisms. Clin. Microbiol. Rev. 2009, 22, 582–610. [Google Scholar] [CrossRef]
- Tóth, H.; Buchholcz, G.; Fésüs, A.; Balázs, B.; Nagy, J.B.; Majoros, L.; Szarka, K.; Kardos, G. Evolution of the Gram-Negative Antibiotic Resistance Spiral over Time: A Time-Series Analysis. Antibiotics 2021, 10, 734. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.-J.; Cheng, Z. Antibiotic Resistance in Pseudomonas Aeruginosa: Mechanisms and Alternative Therapeutic Strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Dabbousi, A.A.; Dabboussi, F.; Hamze, M.; Osman, M.; Kassem, I.I. The Emergence and Dissemination of Multidrug Resistant Pseudomonas Aeruginosa in Lebanon: Current Status and Challenges during the Economic Crisis. Antibiotics 2022, 11, 687. [Google Scholar] [CrossRef]
- Moghnieh, R.A.; Kanafani, Z.A.; Tabaja, H.Z.; Sharara, S.L.; Awad, L.S.; Kanj, S.S. Epidemiology of Common Resistant Bacterial Pathogens in the Countries of the Arab League. Lancet Infect. Dis. 2018, 18, e379–e394. [Google Scholar] [CrossRef] [PubMed]
- Al-Orphaly, M.; Hadi, H.A.; Eltayeb, F.K.; Al-Hail, H.; Samuel, B.G.; Sultan, A.A.; Skariah, S. Epidemiology of Multidrug-Resistant Pseudomonas Aeruginosa in the Middle East and North Africa Region. mSphere 2021, 6, e00202-21. [Google Scholar] [CrossRef] [PubMed]
- Heidari, R.; Farajzadeh Sheikh, A.; Hashemzadeh, M.; Farshadzadeh, Z.; Salmanzadeh, S.; Saki, M. Antibiotic Resistance, Biofilm Production Ability and Genetic Diversity of Carbapenem-Resistant Pseudomonas Aeruginosa Strains Isolated from Nosocomial Infections in Southwestern Iran. Mol. Biol. Rep. 2022, 49, 3811–3822. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, Y.; Jiang, S.; Shen, P.; Lu, X.; Xiao, Y. Association between the Rate of Fluoroquinolones-Resistant Gram-Negative Bacteria and Antibiotic Consumption from China Based on 145 Tertiary Hospitals Data in 2014. BMC Infect. Dis. 2020, 20, 269. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Yu, X.; Zhou, H.; Li, B.; Chen, G.; Ye, Z.; Wang, Y.; Cui, X.; Zheng, Y.; et al. Impact of Antimicrobial Stewardship Managed by Clinical Pharmacists on Antibiotic Use and Drug Resistance in a Chinese Hospital, 2010–2016: A Retrospective Observational Study. BMJ Open 2019, 9, e026072. [Google Scholar] [CrossRef] [PubMed]
- Barnsteiner, S.; Baty, F.; Albrich, W.C.; Flury, B.B.; Gasser, M.; Plüss-Suard, C.; Schlegel, M.; Kronenberg, A.; Kohler, P.; on behalf of the Swiss Centre for Antibiotic Resistance (ANRESIS). Antimicrobial Resistance and Antibiotic Consumption in Intensive Care Units, Switzerland, 2009 to 2018. Eurosurveillance 2021, 26, 2001537. [Google Scholar] [CrossRef]
- Diallo, O.O.; Baron, S.A.; Dubourg, G.; Chaudet, H.; Halfon, P.; Camiade, S.; Comte, B.; Joubert, S.; François, A.; Seyral, P.; et al. Major Discrepancy between Factual Antibiotic Resistance and Consumption in South of France: Analysis of 539,037 Bacterial Strains. Sci. Rep. 2020, 10, 18262. [Google Scholar] [CrossRef]
- Álvarez-Lerma, F.; Olaechea-Astigarraga, P.; Gimeno, R.; Catalan, M.; Nuvials, X.; Gracia-Arnilla, M.P.; Palomar-Martínez, M.; Seijas-Betolaza, I.; Martínez-Alonso, M. ENVIN-HELICS Study Group Changes of Resistance Rates in Pseudomonas Aeruginosa Strains Are Unrelated to Antimicrobial Consumption in ICU Populations with Invasive Device-Related Infection. Med. Intensiv. (Engl. Ed.) 2020, 44, 399–408. [Google Scholar] [CrossRef]
- Association between Consumption of Fluoroquinolones and Carbapenems and Their Resistance Rates in Pseudomonas Aeruginosa in Argentina—PMC. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8828346/ (accessed on 21 October 2022).
- Álvarez-Marín, R.; López-Cerero, L.; Guerrero-Sánchez, F.; Palop-Borras, B.; Rojo-Martín, M.D.; Ruiz-Sancho, A.; Herrero-Rodríguez, C.; García, M.V.; Lazo-Torres, A.M.; López, I.; et al. Do Specific Antimicrobial Stewardship Interventions Have an Impact on Carbapenem Resistance in Gram-Negative Bacilli? A Multicentre Quasi-Experimental Ecological Study: Time-Trend Analysis and Characterization of Carbapenemases. J. Antimicrob. Chemother. 2021, 76, 1928–1936. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Chen, L.H.; Tian, Y.; Wei, R.; Im, T.; Yu, K.; Rieg, G.; Bider-Canfield, Z.; Wong, F.; Takhar, H.S.; et al. Do Inpatient Antimicrobial Stewardship Programs Help Us in the Battle Against Antimicrobial Resistance? Clin. Infect. Dis. 2020, 73, e4454–e4462. [Google Scholar] [CrossRef] [PubMed]
- Paiboonvong, T.; Tedtaisong, P.; Montakantikul, P.; Gorsanan, S.; Tantisiriwat, W. Correlation between Carbapenem Consumption and Carbapenems Susceptibility Profiles of Acinetobacter Baumannii and Pseudomonas Aeruginosa in an Academic Medical Center in Thailand. Antibiotics 2022, 11, 143. [Google Scholar] [CrossRef]
- Popović, R.; Tomić, Z.; Tomas, A.; Anđelić, N.; Vicković, S.; Jovanović, G.; Bukumirić, D.; Horvat, O.; Sabo, A. Five-Year Surveillance and Correlation of Antibiotic Consumption and Resistance of Gram-Negative Bacteria at an Intensive Care Unit in Serbia. J. Chemother. 2020, 32, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Messadi, A.A.; Lamia, T.; Kamel, B.; Salima, O.; Monia, M.; Saida, B.R. Association between Antibiotic Use and Changes in Susceptibility Patterns of Pseudomonas Aeruginosa in an Intensive Care Burn Unit: A 5-Year Study, 2000–2004. Burns 2008, 34, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Baditoiu, L.; Axente, C.; Lungeanu, D.; Muntean, D.; Horhat, F.; Moldovan, R.; Hogea, E.; Bedreag, O.; Sandesc, D.; Licker, M. Intensive Care Antibiotic Consumption and Resistance Patterns: A Cross-Correlation Analysis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-M.; Jiang, R.-J.; Wu, J.-L.; Weng, X.-B.; Ling, L.-P. Antibiotic Resistance Determinants and Virulence Factors of Hypervirulent and Carbapenem Non-Susceptible Pseudomonas Aeruginosa. Clin. Lab. 2022, 68. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Chen, Y.; Jiang, S.; Shen, P.; Lu, X.; Xiao, Y. Association between Antibiotic Consumption and the Rate of Carbapenem-Resistant Gram-Negative Bacteria from China Based on 153 Tertiary Hospitals Data in 2014. Antimicrob. Resist. Infect. Control. 2018, 7, 137. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Huang, H.-W.; Liu, H.-Y.; Chuang, H.-C.; Chen, B.-L.; Wang, E.-Y.; Tsao, L.-H.; Ai, M.-Y.; Lee, Y.-J. Correlation between Antibiotic Consumption and Resistance of Pseudomonas Aeruginosa in a Teaching Hospital Implementing an Antimicrobial Stewardship Program: A Longitudinal Observational Study. J. Microbiol. Immunol. Infect. 2022. [Google Scholar] [CrossRef]
- M100Ed32; Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed. Clinical Laboratory and Standards Institute: Wayne, PA, USA, 2022.
- Chamieh, A.; Zgheib, R.; El-Sawalhi, S.; Yammine, L.; El-Hajj, G.; Zmerli, O.; Afif, C.; Rolain, J.-M.; Azar, E. Trends of Multidrug-Resistant Pathogens, Difficult to Treat Bloodstream Infections, and Antimicrobial Consumption at a Tertiary Care Center in Lebanon from 2015–2020: COVID-19 Aftermath. Antibiotics 2021, 10, 1016. [Google Scholar] [CrossRef]
- Addinsoft XLSTAT 2014: Data Analysis and Statistical Solution for Microsoft Excel; Addinsoft: Paris, France, 2014.


| Antibiotic Resistance | Gradient | 95% CI | R-Squared | p-Value | Trend |
|---|---|---|---|---|---|
| Cefepime (n = 1818) | −0.018 | −0.03 to −0.01 | 0.662 | 0.048 | ↓ |
| Ceftazidime (n = 1817) | −0.009 | −0.02 to −0.003 | 0.557 | 0.016 | ↓ |
| Ciprofloxacin (n = 1821) | −0.014 | −0.03 to −0.001 | 0.377 | 0.076 | ↔ |
| Colistin (n = 55) | 0.000 | −0.03 to 0.0 | 0.313 | 0.316 | ↔ |
| Gentamicin (n = 1827) | −0.010 | −0.03 to −0.004 | 0.629 | 0.006 | ↓ |
| Imipenem (n = 1823) | 0.001 | −0.01 to 0.01 | 0.00003 | 0.834 | ↔ |
| Meropenem (n = 1285) | −0.004 | −0.04 to 0.01 | 0.303 | 0.548 | ↔ |
| TZP (n = 1827) | −0.014 | −0.03 to −0.003 | 0.561 | 0.076 | ↔ |
| Aztreonam (n = 1796) | −0.022 | −0.04 to −0.003 | 0.566 | 0.059 | ↔ |
| Amikacin (n = 1827) | −0.015 | −0.03 to −0.003 | 0.69 | 0.036 | ↓ |
| Ofloxacin (n = 1802) | −0.0163 | −0.02 to −0.01 | 0.646 | 0.029 | ↓ |
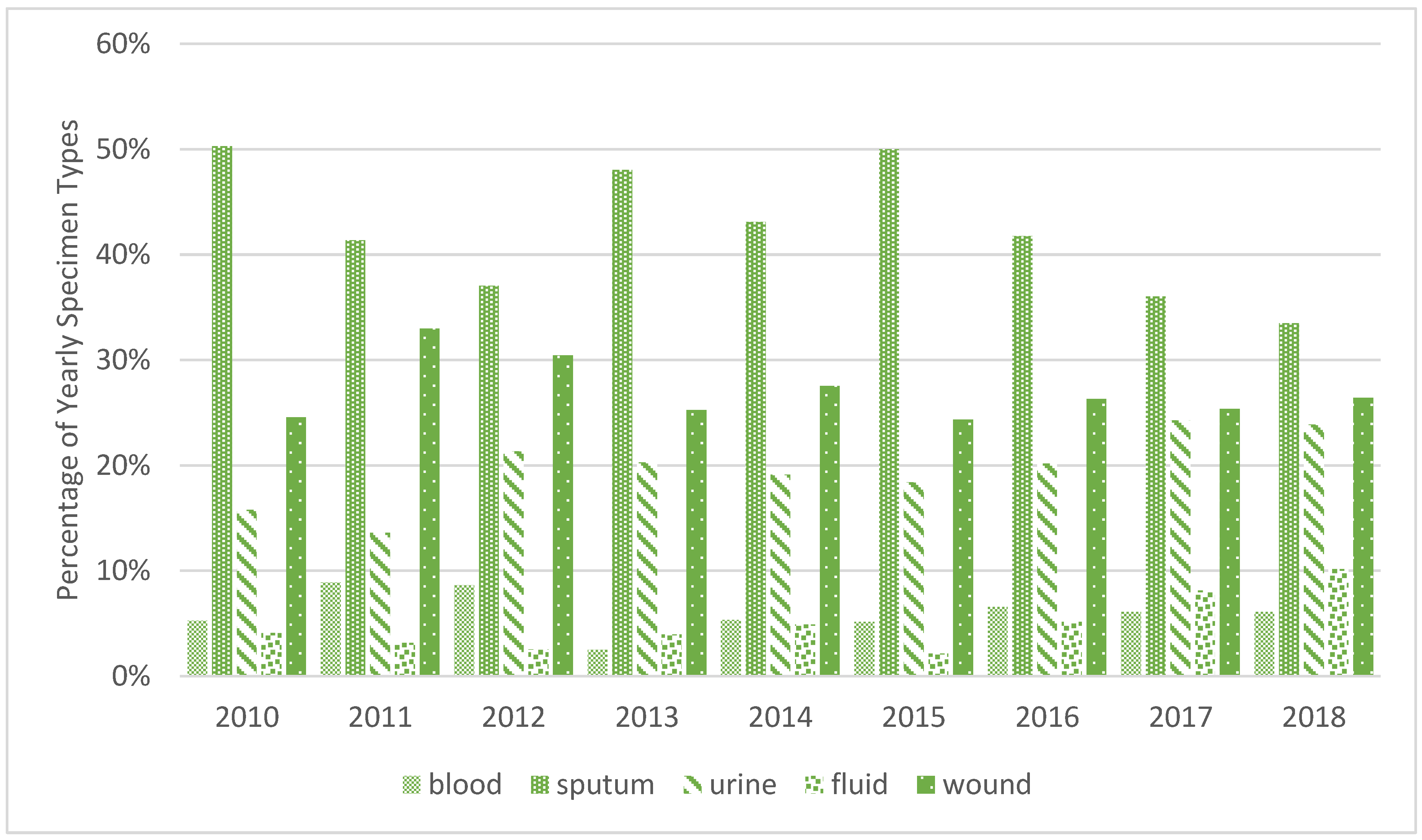
| Year | Blood | Sputum | Urine | Wound |
|---|---|---|---|---|
| 2010 | 0 | 2 | 0 | 0 |
| 2011 | 0 | 8 | 0 | 2 |
| 2012 | 0 | 1 | 2 | 2 |
| 2013 | 0 | 4 | 2 | 1 |
| 2014 | 0 | 3 | 3 | 3 |
| 2015 | 0 | 3 | 1 | 1 |
| 2016 | 0 | 2 | 2 | 1 |
| 2017 | 0 | 2 | 2 | 2 |
| 2018 | 1 | 5 | 5 | 1 |
| Total | 1 | 30 | 17 | 13 |
| Antibiotic Resistance | Gradient | 95% CI | R−Squared | p-Value | Trend |
|---|---|---|---|---|---|
| Cephalosporins | −0.006 | −0.01 to −0.003 | 0.21 | 0.000 | ↔ |
| Quinolones | −0.004 | −0.01 to −0.001 | 0.702 | 0.013 | ↓ |
| Colistin | 0.000 | 0 to 0.001 | 0.084 | 0.029 | ↔ |
| Imipenem | −0.017 | −0.020 to −0.015 | 0.908 | <0.0001 | ↓ |
| Meropenem | 0.001 | −0.01 to 0.01 | 0.00006 | 0.764 | ↔ |
| Carbapenems | −0.017 | −0.02 to −0.01 | 0.399 | <0.0001 |  * * |
| Piperacillin−tazobactam | 0.000 | −0.003 to 0.003 | 0.013 | 0.926 | ↔ |
| Amikacin | 0.001 | 0.0 to 0.001 | 0.067 | 0.079 | ↔ |
| All Anti-pseudomonal antibiotics | −0.0407 | −0.05 to −0.03 | 0.485 | <0.0001 |  * * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Basst, R.; Saliba, S.; Saleh, L.; Saoud, N.; Azar, E.; Zalloua, P.; Chamieh, A. The Effect of Decreased Antipseudomonal Drug Consumption on Pseudomonas aeruginosa Incidence and Antimicrobial Susceptibility Profiles over 9 Years in a Lebanese Tertiary Care Center. Antibiotics 2023, 12, 192. https://doi.org/10.3390/antibiotics12020192
El-Basst R, Saliba S, Saleh L, Saoud N, Azar E, Zalloua P, Chamieh A. The Effect of Decreased Antipseudomonal Drug Consumption on Pseudomonas aeruginosa Incidence and Antimicrobial Susceptibility Profiles over 9 Years in a Lebanese Tertiary Care Center. Antibiotics. 2023; 12(2):192. https://doi.org/10.3390/antibiotics12020192
Chicago/Turabian StyleEl-Basst, Rima, Sanaa Saliba, Lama Saleh, Nicolas Saoud, Eid Azar, Pierre Zalloua, and Amanda Chamieh. 2023. "The Effect of Decreased Antipseudomonal Drug Consumption on Pseudomonas aeruginosa Incidence and Antimicrobial Susceptibility Profiles over 9 Years in a Lebanese Tertiary Care Center" Antibiotics 12, no. 2: 192. https://doi.org/10.3390/antibiotics12020192
APA StyleEl-Basst, R., Saliba, S., Saleh, L., Saoud, N., Azar, E., Zalloua, P., & Chamieh, A. (2023). The Effect of Decreased Antipseudomonal Drug Consumption on Pseudomonas aeruginosa Incidence and Antimicrobial Susceptibility Profiles over 9 Years in a Lebanese Tertiary Care Center. Antibiotics, 12(2), 192. https://doi.org/10.3390/antibiotics12020192





