Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges
Abstract
1. Introduction
2. The Historical Background of H. pylori
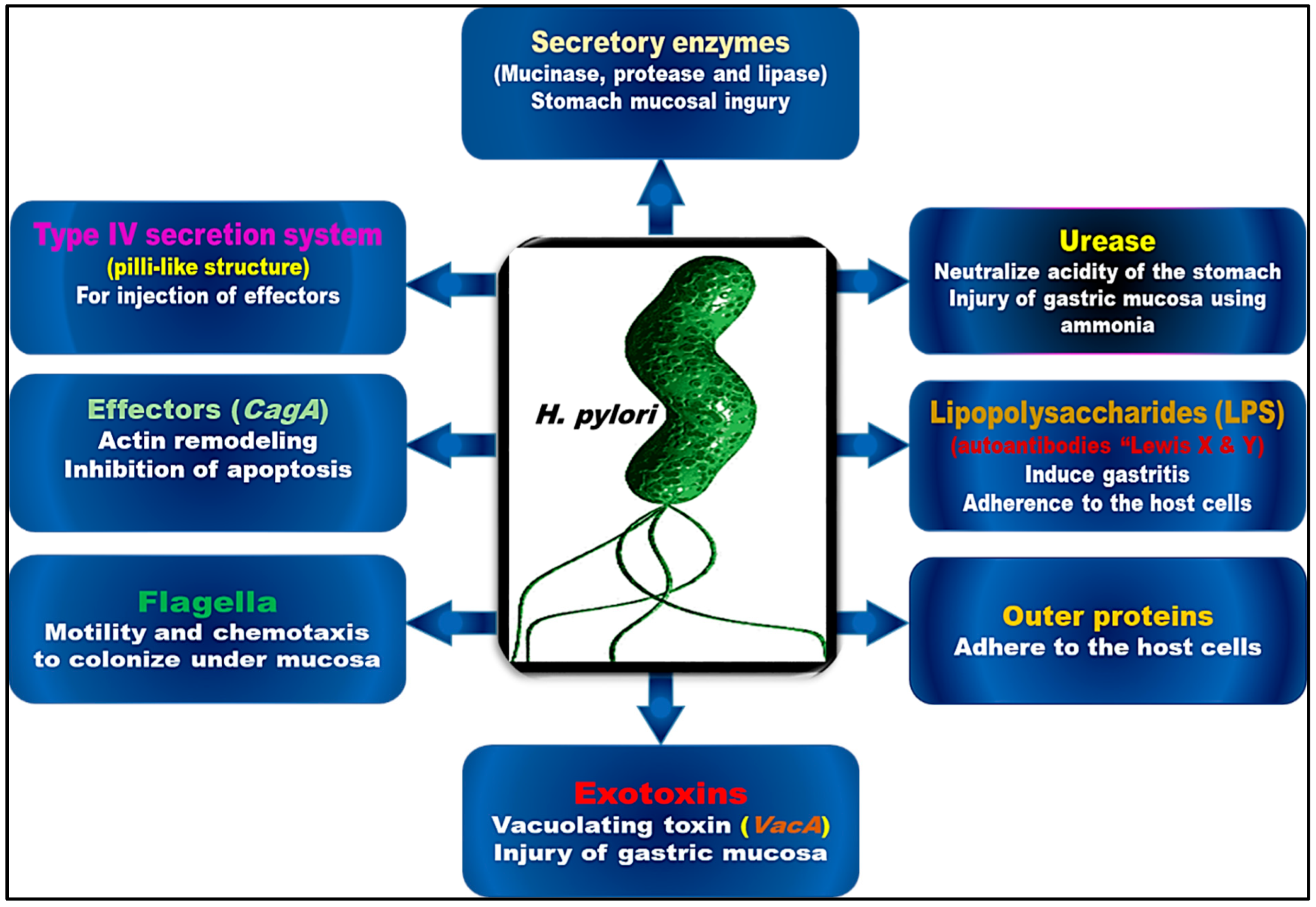
3. The Virulence and Pathogenic Pathways of H. pylori
4. H. pylori Infection and Extraintestinal Disorders
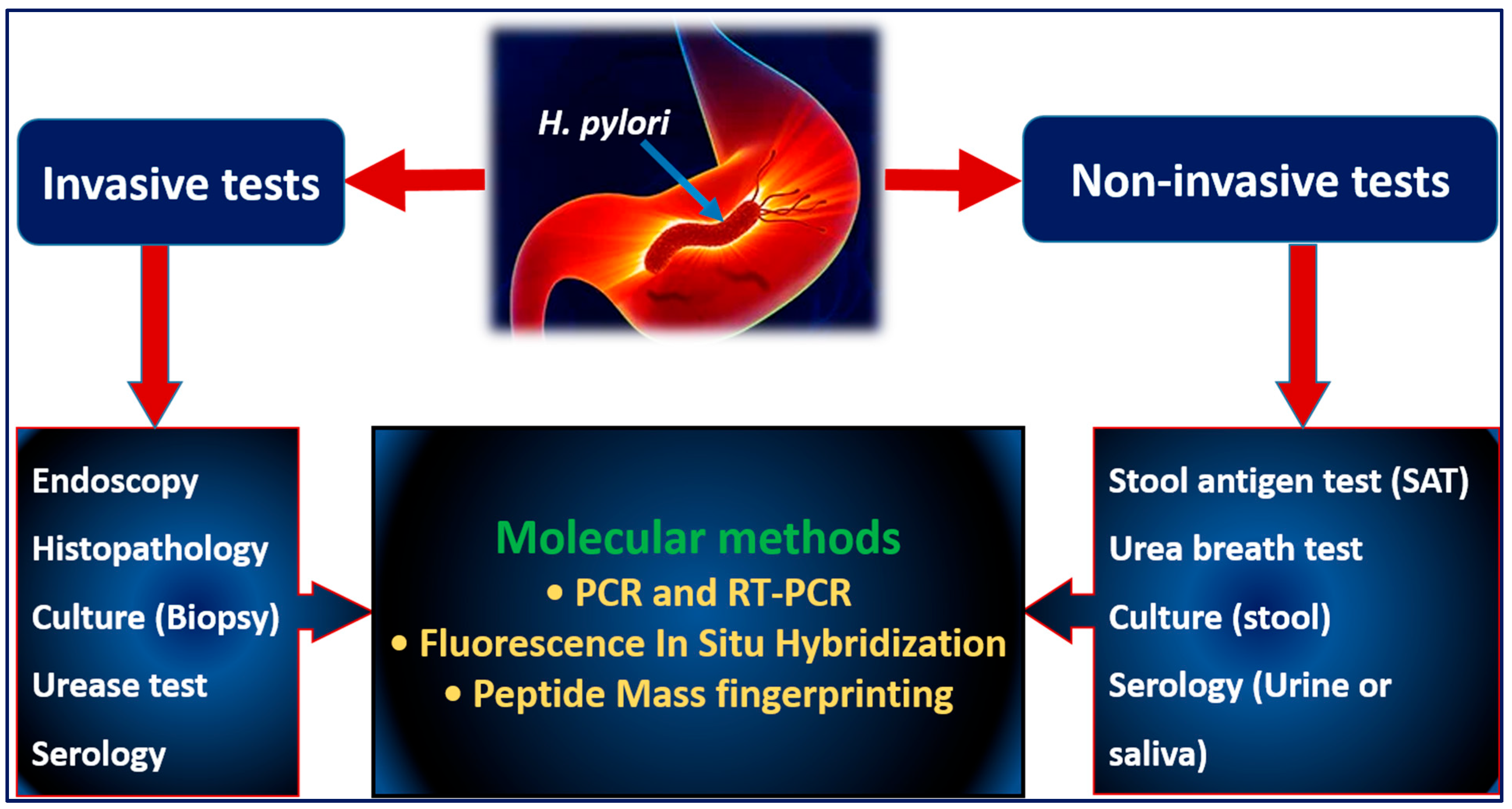
5. Diagnostic Approaches for H. pylori
5.1. Invasive Tests
5.1.1. Endoscopy
5.1.2. Histopathology
5.1.3. Culture Techniques
5.2. Noninvasive Tests
5.2.1. Stool Antigen Tests (SATs)
5.2.2. Urea Breath Test (UBT)
5.2.3. Serological Testing
5.3. Molecular Tests
5.3.1. PCR and Real-Time PCR Testing
5.3.2. Peptide Mass Fingerprinting Technology
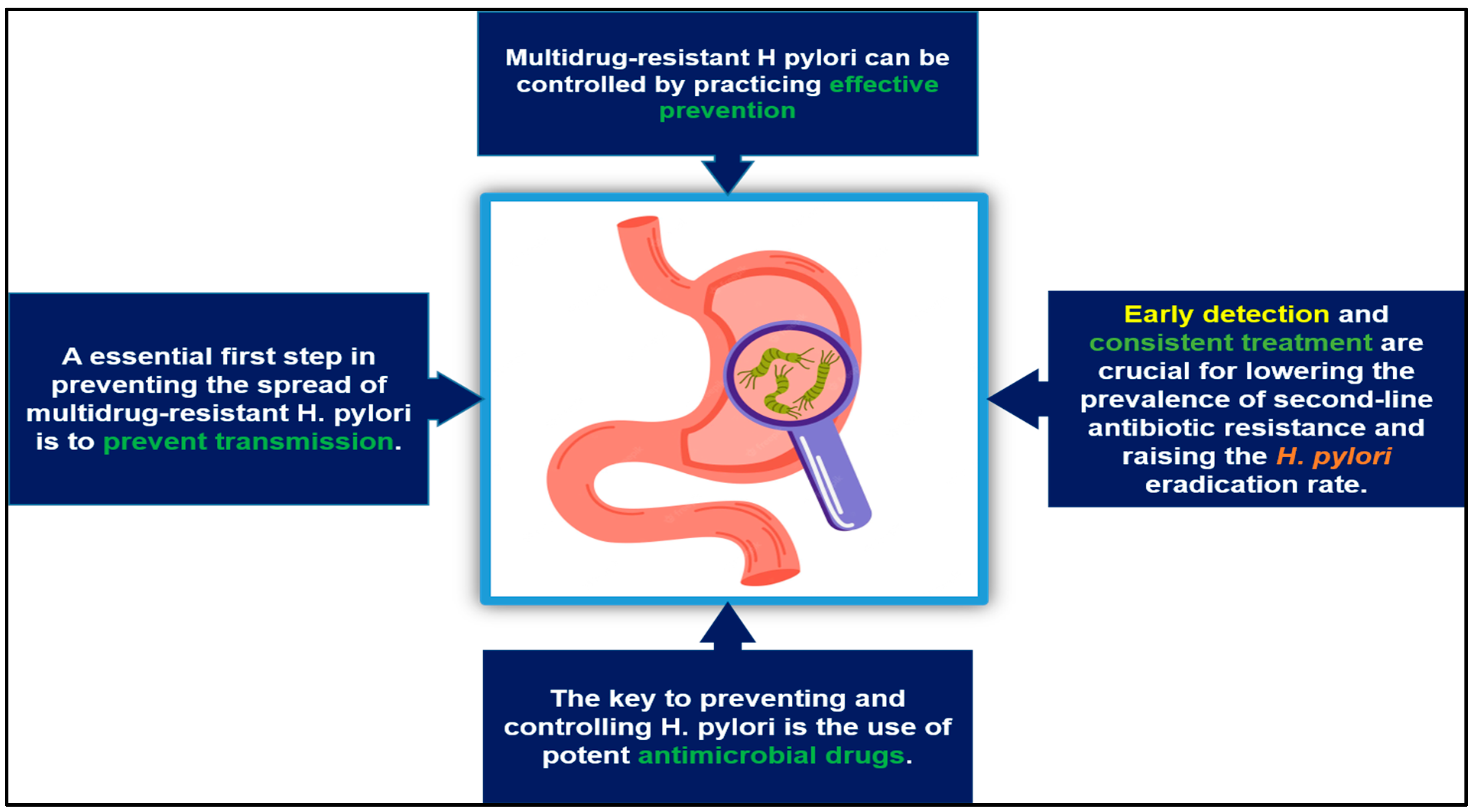
6. The Prevention and Control of Multidrug-Resistant H. pylori
7. Recent Advancements in Diagnostics and Treatment
8. The Impacts of Compliance and Resistance on the Success of H. pylori Treatment
9. Family-Based H. pylori Eradication Strategies
10. The Potential Use of Vaccines
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bizzozero, G.; der Eidechsen, D. Ueber die schlauchförmigen Drüsen des Magendarmkanals und die Beziehungen ihres. Arch. Mikrosk. Anat. 1893, 42, 82. [Google Scholar] [CrossRef]
- Krienitz, W. Ueber das Auftreten von Spirochäten verschiedener Form im Mageninhalt bei Carcinoma ventriculi. DMW-Dtsch. Med. Wochenschr. 1906, 32, 872. [Google Scholar] [CrossRef]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.; Bazzoli, F.; El-Omar, E.; Graham, D.; Hunt, R.; Rokkas, T.; Vakil, N.; Kuipers, E.J. Current concepts in the management of Helicobacter pylori infection: The Maastricht III Consensus Report. Gut 2007, 56, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Jemilohun, A.C.; Otegbayo, J.A. Helicobacter pylori infection: Past, present and future. Pan Afr. Med. J. 2016, 23, 1–15. [Google Scholar] [CrossRef]
- Talley, N.J.; Vakil, N. Guidelines for the management of dyspepsia. Off. J. Am. Coll. Gastroenterol. 2005, 100, 2324–2337. [Google Scholar] [CrossRef]
- Shatila, M.; Thomas, A.S. Current and Future Perspectives in the Diagnosis and Management of Helicobacter pylori Infection. J. Clin. Med. 2022, 11, 5086. [Google Scholar] [CrossRef]
- Fong, I.W. Current Trends and Concerns in Infectious Diseases; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- WHO. Media Centre. News Release. WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. 2017. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5447202/pdf/SaudiMedJ-38-444.pdf (accessed on 27 August 2022).
- Hooi, J.K.; Lai, W.Y.; Ng, W.K.; Suen, M.M.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.; Wu, J.C. Global prevalence of Helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Stefano, K.; Marco, M.; Federica, G.; Laura, B.; Barbara, B.; Gioacchino, L.; Gian, L.d.A. Helicobacter pylori, transmission routes and recurrence of infection: State of the art. Acta Bio Med. Atenei Parm. 2018, 89, 72. [Google Scholar]
- FitzGerald, R.; Smith, S.M. An overview of Helicobacter pylori infection. In Helicobacter pylori; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–14. [Google Scholar]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23, e12514. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology 2018, 155, 1372–1382.e17. [Google Scholar] [CrossRef]
- Reshetnyak, V.I.; Burmistrov, A.I.; Maev, I.V. Helicobacter pylori: Commensal, symbiont or pathogen? World J. Gastroenterol. 2021, 27, 545. [Google Scholar] [CrossRef]
- Burucoa, C.; Axon, A. Epidemiology of Helicobacter pylori infection. Helicobacter 2017, 22, e12403. [Google Scholar] [CrossRef]
- Pucułek, M.; Machlowska, J.; Wierzbicki, R.; Baj, J.; Maciejewski, R.; Sitarz, R. Helicobacter pylori associated factors in the development of gastric cancer with special reference to the early-onset subtype. Oncotarget 2018, 9, 31146. [Google Scholar] [CrossRef]
- Sharndama, H.C.; Mba, I.E. Helicobacter pylori: An up-to-date overview on the virulence and pathogenesis mechanisms. Braz. J. Microbiol. 2022, 53, 1–18. [Google Scholar] [CrossRef]
- Saxena, A.; Mukhopadhyay, A.K.; Nandi, S.P. Helicobacter pylori: Perturbation and restoration of gut microbiome. J. Biosci. 2020, 45, 1–15. [Google Scholar] [CrossRef]
- Cardos, A.I.; Maghiar, A.; Zaha, D.C.; Pop, O.; Fritea, L.; Miere, F.; Cavalu, S. Evolution of Diagnostic Methods for Helicobacter pylori Infections: From Traditional Tests to High Technology, Advanced Sensitivity and Discrimination Tools. Diagnostics 2022, 12, 508. [Google Scholar] [CrossRef]
- Keskin, M.; Yavuz, A. A novel rapid and accurate method for detecting Helicobacter pylori: The modified antigen test. Eur. Rev. Med. Pharm. Sci. 2022, 26, 1148–1155. [Google Scholar]
- Chey, W.; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar]
- Korkmaz, H.; Findik, D.; Ugurluoglu, C.; Terzi, Y. Reliability of stool antigen tests: Investigation of the diagnostic value of a new immunochromatographic Helicobacter pylori approach in dyspeptic patients. Asian Pac. J. Cancer Prev. 2015, 16, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Pichon, M.; Pichard, B.; Barrioz, T.; Plouzeau, C.; Croquet, V.; Fotsing, G.; Chéron, A.; Vuillemin, É.; Wangermez, M.; Haineaux, P.-A. Diagnostic accuracy of a noninvasive test for detection of Helicobacter pylori and resistance to clarithromycin in stool by the Amplidiag H. pylori+ ClariR real-time PCR assay. J. Clin. Microbiol. 2020, 58, e01787-19. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Forma, A.; Sitarz, M.; Portincasa, P.; Garruti, G.; Krasowska, D.; Maciejewski, R. Helicobacter pylori virulence factors—Mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Korona-Głowniak, I.; Forma, A.; Maani, A.; Sitarz, E.; Rahnama-Hezavah, M.; Radzikowska, E.; Portincasa, P. Mechanisms of the epithelial–mesenchymal transition and tumor microenvironment in Helicobacter pylori-induced gastric cancer. Cells 2020, 9, 1055. [Google Scholar] [CrossRef]
- Palamides, P.; Jolaiya, T.; Idowu, A.; Loell, E.; Onyekwere, C.; Ugiagbe, R.; Agbo, I.; Lesi, O.; Ndububa, D.; Adekanle, O. Helicobacter pylori patient isolates from South Africa and Nigeria differ in virulence factor pathogenicity profile and associated gastric disease outcome. Sci. Rep. 2020, 10, 11409. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, C.; Zhang, L.; Liu, X.; Chan, M.T.V.; Wu, W.K.K.; Chen, H. Host Cell Antimicrobial Responses against Helicobacter pylori Infection: From Biological Aspects to Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 10941. [Google Scholar] [CrossRef]
- Correa, P.; Piazuelo, M.B. Evolutionary history of the Helicobacter pylori genome: Implications for gastric carcinogenesis. Gut 2012, 6, 21. [Google Scholar] [CrossRef]
- Linz, B.; Balloux, F.; Moodley, Y.; Manica, A.; Liu, H.; Roumagnac, P.; Falush, D.; Stamer, C.; Prugnolle, F.; van der Merwe, S.W. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007, 445, 915–918. [Google Scholar] [CrossRef]
- Blaser, M.J. An endangered species in the stomach. Sci. Am. 2005, 292, 38–45. [Google Scholar] [CrossRef]
- Palmer, E.D. Investigation of the gastric mucosa spirochetes of the human. Gastroenterology 1954, 27, 218–220. [Google Scholar] [CrossRef]
- Steer, H. Ultrastructure of cell migration throught the gastric epithelium and its relationship to bacteria. J. Clin. Pathol. 1975, 28, 639–646. [Google Scholar] [CrossRef]
- Marshall, B.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 323, 1311–1315. [Google Scholar] [CrossRef]
- Atwood, K.C. Bacteria, Ulcers, and Ostracism? H. pylori and the Making of a Myth. Skept. Inq. 2004, 28, 27–34. [Google Scholar]
- Berg, A.O. Helicobacter pylori in peptic ulcer disease: Report of an NIH consensus conference. J. Am. Board Fam. Pract. 1996, 9, 205–207. [Google Scholar]
- Hunt, R.; Xiao, S.; Megraud, F.; Leon-Barua, R.; Bazzoli, F.; Van Der Merwe, S.; Coelho, L.; Fock, M.; Fedail, S.; Cohen, H. World Gastroenterology Organisation Global Guideline Helicobacter pylori in Developing Countries. J. Clin. Gastroenterol. 2011, 45, 383–388. [Google Scholar]
- Amieva, M.; Peek, R.M., Jr. Pathobiology of Helicobacter pylori–induced gastric cancer. Gastroenterology 2016, 150, 64–78. [Google Scholar] [CrossRef]
- Elhariri, M.; Hamza, D.; Elhelw, R.; Hamza, E. Occurrence of cagA+ vacA s1a m1 i1 Helicobacter pylori in farm animals in Egypt and ability to survive in experimentally contaminated UHT milk. Sci. Rep. 2018, 8, 14260. [Google Scholar] [CrossRef]
- Camilo, V.; Sugiyama, T.; Touati, E. Pathogenesis of Helicobacter pylori infection. Helicobacter 2017, 22, e12405. [Google Scholar] [CrossRef]
- Denic, M.; Touati, E.; De Reuse, H. Pathogenesis of Helicobacter pylori infection. Helicobacter 2020, 25, e12736. [Google Scholar] [CrossRef]
- Hanyu, H.; Engevik, K.A.; Matthis, A.L.; Ottemann, K.M.; Montrose, M.H.; Aihara, E. Helicobacter pylori uses the TlpB receptor to sense sites of gastric injury. Infect. Immun. 2019, 87, e00202–e00219. [Google Scholar] [CrossRef] [PubMed]
- Idowu, S.; Bertrand, P.P.; Walduck, A.K. Gastric organoids: Advancing the study of H. pylori pathogenesis and inflammation. Helicobacter 2022, 27, e12891. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; So, P.; Erramilli, S. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, S.; Hiraga, T.; Ikehara, Y.; Kudo, T.; Iwasaki, H.; Morozumi, K.; Akamatsu, S.; Tachikawa, T.; Hisashi, N. Molecular mechanisms of expression of Lewis b antigen and other type I Lewis antigens in human colorectal cancer. Glycobiology 1999, 9, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Soejima, M.; Koda, Y. Molecular mechanisms of Lewis antigen expression. Leg. Med. 2005, 7, 266–269. [Google Scholar] [CrossRef]
- Odenbreit, S.; Faller, G.; Haas, R. Role of the alpAB proteins and lipopolysaccharide in adhesion of Helicobacter pylori to human gastric tissue. Int. J. Med. Microbiol. 2002, 292, 247–256. [Google Scholar] [CrossRef]
- Mahdavi, J.; Borén, T.; Vandenbroucke-Grauls, C.; Appelmelk, B.J. Limited role of lipopolysaccharide Lewis antigens in adherence of Helicobacter pylori to the human gastric epithelium. Infect. Immun. 2003, 71, 2876–2880. [Google Scholar] [CrossRef]
- Alm, R.A.; Bina, J.; Andrews, B.M.; Doig, P.; Hancock, R.E.; Trust, T.J. Comparative genomics of Helicobacter pylori: Analysis of the outer membrane protein families. Infect. Immun. 2000, 68, 4155–4168. [Google Scholar] [CrossRef]
- Xu, C.; Soyfoo, D.M.; Wu, Y.; Xu, S. Virulence of Helicobacter pylori outer membrane proteins: An updated review. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1821–1830. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kido, Y.; Yamaoka, Y. Helicobacter pylori outer membrane protein-related pathogenesis. Toxins 2017, 9, 101. [Google Scholar] [CrossRef]
- Tamrakar, A.; Singh, R.; Kumar, A.; Makde, R.D.; Kodgire, P. Biophysical characterization of the homodimers of HomA and HomB, outer membrane proteins of Helicobacter pylori. Sci. Rep. 2021, 11, 24471. [Google Scholar] [CrossRef]
- Hage, N.; Howard, T.; Phillips, C.; Brassington, C.; Overman, R.; Debreczeni, J.; Gellert, P.; Stolnik, S.; Winkler, G.S.; Falcone, F.H. Structural basis of Lewisb antigen binding by the Helicobacter pylori adhesin BabA. Sci. Adv. 2015, 1, e1500315. [Google Scholar] [CrossRef]
- Doohan, D.; Rezkitha, Y.A.A.; Waskito, L.A.; Yamaoka, Y.; Miftahussurur, M. Helicobacter pylori BabA–SabA key roles in the adherence phase: The synergic mechanism for successful colonization and disease development. Toxins 2021, 13, 485. [Google Scholar] [CrossRef]
- Unemo, M.; Aspholm-Hurtig, M.; Ilver, D.; Bergström, J.; Borén, T.; Danielsson, D.; Teneberg, S. The sialic acid binding SabA adhesin of Helicobacter pylori is essential for nonopsonic activation of human neutrophils. J. Biol. Chem. 2005, 280, 15390–15397. [Google Scholar] [CrossRef]
- Giraldi, L.; Michelazzo, M.B.; Arzani, D.; Persiani, R.; Pastorino, R.; Boccia, S. MUC1, MUC5AC, and MUC6 polymorphisms, Helicobacter pylori infection, and gastric cancer: A systematic review and meta-analysis. Eur. J. Cancer Prev. 2018, 27, 323–330. [Google Scholar] [CrossRef]
- Kalali, B.; Mejías-Luque, R.; Javaheri, A.; Gerhard, M. H. pylori virulence factors: Influence on immune system and pathology. Mediat. Inflamm. 2014, 2014, 426309. [Google Scholar]
- Backert, S.; Tegtmeyer, N. Type IV secretion and signal transduction of Helicobacter pylori CagA through interactions with host cell receptors. Toxins 2017, 9, 115. [Google Scholar] [CrossRef]
- Graham, D.Y. Helicobacter pylori update: Gastric cancer, reliable therapy, and possible benefits. Gastroenterology 2015, 148, 719–731.e713. [Google Scholar] [CrossRef]
- Tsay, F.-W.; Hsu, P.-I. H. pylori infection and extra-gastroduodenal diseases. J. Biomed. Sci. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Mărginean, C.D.; Mărginean, C.O.; Meliț, L.E. Helicobacter pylori-Related Extraintestinal Manifestations—Myth or Reality. Children 2022, 9, 1352. [Google Scholar] [CrossRef]
- Suzuki, H.; Franceschi, F.; Nishizawa, T.; Gasbarrini, A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter 2011, 16, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Banić, M.; Franceschi, F.; Babić, Z.; Gasbarrini, A. Extragastric M anifestations of Helicobacter pylori I nfection. Helicobacter 2012, 17, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Li, Y.; Zhang, Y.; Bai, L.; Yang, P. Helicobacter pylori infection and lung cancer: A review of an emerging hypothesis. Carcinogenesis 2013, 34, 1189–1195. [Google Scholar] [CrossRef]
- Buzás, G.M. Metabolic consequences of Helicobacter pylori infection and eradication. World J. Gastroenterol. WJG 2014, 20, 5226. [Google Scholar] [CrossRef] [PubMed]
- Chmiela, M.; Gajewski, A.; Rudnicka, K. Helicobacter pylori vs. coronary heart disease-searching for connections. World J. Cardiol. 2015, 7, 187. [Google Scholar] [CrossRef]
- Tseng, D.S.; Li, D.; Cholleti, S.M.; Wei, J.C.; Jodesty, Y.; Pham, H.-V. Effect of Helicobacter pylori treatment on unexplained iron deficiency anemia. Perm. J. 2019, 23, 18–195. [Google Scholar] [CrossRef]
- Blecker, U.; Renders, F.; Lanciers, S.; Vandenplas, Y. Syncopes leading to the diagnosis of a Helicobacter pylori positive chronic active haemorrhagic gastritis. Eur. J. Pediatr. 1991, 150, 560–561. [Google Scholar] [CrossRef]
- Qu, X.-H.; Huang, X.-L.; Xiong, P.; Zhu, C.-Y.; Huang, Y.-L.; Lu, L.-G.; Sun, X.; Rong, L.; Zhong, L.; Sun, D.-Y. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J. Gastroenterol. WJG 2010, 16, 886. [Google Scholar]
- Monzón, H.; Forné, M.; Esteve, M.; Rosinach, M.; Loras, C.; Espinós, J.C.; Viver, J.M.; Salas, A.; Fernández-Bañares, F. Helicobacter pylori infection as a cause of iron deficiency anaemia of unknown origin. World J. Gastroenterol. WJG 2013, 19, 4166. [Google Scholar] [CrossRef]
- Gasbarrini, A.; Franceschi, F.; Tartaglione, R.; Landolfi, R.; Pola, P.; Gasbarrini, G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet 1998, 352, 878. [Google Scholar] [CrossRef]
- García, A. Resolution of an autoimmune thrombocytopenic purpura after eradicating treatment of Helicobacter pylori. Sangre 1999, 44, 387–388. [Google Scholar]
- Stasi, R.; Sarpatwari, A.; Segal, J.B.; Osborn, J.; Evangelista, M.L.; Cooper, N.; Provan, D.; Newland, A.; Amadori, S.; Bussel, J.B. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: A systematic review. Blood J. Am. Soc. Hematol. 2009, 113, 1231–1240. [Google Scholar] [CrossRef]
- Tan, H.J.; Goh, K.L. Extragastrointestinal manifestations of Helicobacter pylori infection: Facts or myth? A critical review. J. Dig. Dis. 2012, 13, 342–349. [Google Scholar] [CrossRef]
- O’Connor, H.; Axon, A.; Dixon, M. Campylobacter-like organisms unusual in type A (pernicious anaemia) gastritis. Lancet 1984, 324, 1091. [Google Scholar] [CrossRef]
- Stabler, S.P. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef]
- Sarari, A.S.; Farraj, M.A.; Hamoudi, W.; Essawi, T.A. Helicobacter pylori, a causative agent of vitamin B12 deficiency. J. Infect. Dev. Ctries 2008, 2, 346–349. [Google Scholar]
- Rahman, Y.A.; Hafez, R.M.M.; Ahmed, R.M.M. Helicobacter pylori and its hematological effect. Egypt. J. Intern. Med. 2019, 31, 332–342. [Google Scholar] [CrossRef]
- Erkan, Y.; Köksal, F. Autoimmune extraintestinal manifestations of Helicobacter pylori infection: A bundle of conflicts. J. Immunol. Clin. Microbiol. 2016, 1, 22–30. [Google Scholar]
- Devrajani, B.R.; Shah, S.Z.A.; Soomro, A.A.; Devrajani, T. Type 2 diabetes mellitus: A risk factor for Helicobacter pylori infection: A hospital based case-control study. Int. J. Diabetes Dev. Ctries 2010, 30, 22. [Google Scholar] [CrossRef]
- Bener, A.; Micallef, R.; Afifi, M.; Derbala, M.; Al-Mulla, H.M.; Usmani, M.A. Association between type 2 diabetes mellitus and Helicobacter pylori infection. Turk. J. Gastroenterol. 2007, 18, 225–229. [Google Scholar]
- Anastasios, R.; Goritsas, C.; Papamihail, C.; Trigidou, R.; Garzonis, P.; Ferti, A. Helicobacter pylori infection in diabetic patients: Prevalence and endoscopic findings. Eur. J. Intern. Med. 2002, 13, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Nasif, W.A.; Mukhtar, M.H.; Nour Eldein, M.M.; Ashgar, S.S. Oxidative DNA damage and oxidized low density lipoprotein in Type II diabetes mellitus among patients with Helicobacter pylori infection. Diabetol. Metab. Syndr. 2016, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Toda, A.; Yamamoto-Honda, R.; Arase, Y.; Sone, H. Association between Helicobacter pylori infection, eradication and diabetes mellitus. J. Diabetes Investig. 2019, 10, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Cai, C.; Jin, Q.; Chen, X.; Yu, C. The efficacy of Helicobacter pylori eradication in diabetics and its effect on glycemic control: A systematic review and meta-analysis. Helicobacter 2021, 26, e12781. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, R.J.; Dobbs, S.M.; Weller, C.; Charlett, A.; Bjarnason, I.T.; Curry, A.; Ellis, D.S.; Ibrahim, M.A.; McCrossan, M.V.; O’Donohue, J. Helicobacter hypothesis for idiopathic parkinsonism: Before and beyond. Helicobacter 2008, 13, 309–322. [Google Scholar] [CrossRef]
- Tan, A.H.; Mahadeva, S.; Marras, C.; Thalha, A.M.; Kiew, C.K.; Yeat, C.M.; Ng, S.W.; Ang, S.P.; Chow, S.K.; Loke, M.F.J.P.; et al. Helicobacter pylori infection is associated with worse severity of Parkinson’s disease. Park. Relat. Disord. 2015, 21, 221–225. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.Y.; Mahadeva, S.; Loke, M.F.; Tan, J.Y.; Ang, B.H.; Chin, K.P.; Mohammad Adnan, A.F.; Ong, S.M.C.; Ibrahim, A.I.; et al. Helicobacter pylori eradication in Parkinson’s disease: A randomized placebo-controlled trial. Mov. Disord. 2020, 35, 2250–2260. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Ikeda, Y.; Murakami, H.; Hori, S.-i.; Hino, K.; Sasaki, C.; Nishikawa, M. Classification of atrophic mucosal patterns on Blue LASER Imaging for endoscopic diagnosis of Helicobacter pylori-related gastritis: A retrospective, observational study. PLoS ONE 2018, 13, e0193197. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, F.; Zhou, Y.; Xia, G.L.; Dong, L.; He, W.H.; Xiao, B. Blue laser magnifying endoscopy in the diagnosis of chronic gastritis. Exp. Ther. Med. 2019, 18, 1993–2000. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’morain, C.; Gisbert, J.; Kuipers, E.; Axon, A.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y. Management of Helicobacter pylori infection—The Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- White, J.R.; Sami, S.S.; Reddiar, D.; Mannath, J.; Ortiz-Fernández-Sordo, J.; Beg, S.; Scott, R.; Thiagarajan, P.; Ahmad, S.; Parra-Blanco, A. Narrow band imaging and serology in the assessment of premalignant gastric pathology. Scand. J. Gastroenterol. 2018, 53, 1611–1618. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Kuo, F.-C.; Liu, C.-J.; Wu, M.-C.; Shih, H.-Y.; Wang, S.S.; Wu, J.-Y.; Kuo, C.-H.; Huang, Y.-K.; Wu, D.-C. Diagnosis of Helicobacter pylori infection: Current options and developments. World J. Gastroenterol. WJG 2015, 21, 11221. [Google Scholar] [CrossRef]
- Tongtawee, T.; Kaewpitoon, S.; Kaewpitoon, N.; Dechsukhum, C.; Leeanansaksiri, W.; Loyd, R.A.; Matrakool, L.; Panpimanmas, S. Diagnosis of Helicobacter pylori infection. Asian Pac. J. Cancer Prev. 2016, 17, 1631–1635. [Google Scholar] [CrossRef]
- Graham, D.Y.; Miftahussurur, M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J. Adv. Res. 2018, 13, 51–57. [Google Scholar] [CrossRef]
- Dechant, F.-X.; Dechant, R.; Kandulski, A.; Selgrad, M.; Weber, F.; Reischl, U.; Wilczek, W.; Mueller, M.; Weigand, K. Accuracy of different rapid urease tests in comparison with histopathology in patients with endoscopic signs of gastritis. Digestion 2020, 101, 184–190. [Google Scholar] [CrossRef]
- Kuhns, L.G.; Benoit, S.L.; Bayyareddy, K.; Johnson, D.; Orlando, R.; Evans, A.L.; Waldrop, G.L.; Maier, R.J. Carbon fixation driven by molecular hydrogen results in chemolithoautotrophically enhanced growth of Helicobacter pylori. J. Bacteriol. 2016, 198, 1423–1428. [Google Scholar] [CrossRef]
- Dolak, W.; Bilgilier, C.; Stadlmann, A.; Leiner, J.; Püspök, A.; Plieschnegger, W.; Siebert, F.; Wewalka, F.; Schöfl, R.; Huber-Schönauer, U. A multicenter prospective study on the diagnostic performance of a new liquid rapid urease test for the diagnosis of Helicobacter pylori infection. Gut Pathog. 2017, 9, 1–5. [Google Scholar] [CrossRef]
- Hortelano, I.; Moreno, Y.; Vesga, F.J.; Ferrús, M.A. Evaluation of different culture media for detection and quantification of H. pylori in environmental and clinical samples. Int. Microbiol. 2020, 23, 481–487. [Google Scholar] [CrossRef]
- Leszczyńska, K.; Namiot, A.; Namiot, Z.; Leszczyńska, J.; Jakoniuk, P.; Chilewicz, M.; Namiot, D.; Kemona, A.; Milewski, R.; Bucki, R. Patient factors affecting culture of Helicobacter pylori isolated from gastric mucosal specimens. Adv. Med. Sci. 2010, 55, 161–166. [Google Scholar] [CrossRef]
- Best, L.M.; Takwoingi, Y.; Siddique, S.; Selladurai, A.; Gandhi, A.; Low, B.; Yaghoobi, M.; Gurusamy, K.S. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst. Rev. 2018, 3, CD012080. [Google Scholar]
- Nguyen, T.V.H.; Bengtsson, C.; Nguyen, G.K.; Granström, M. Evaluation of a novel monoclonal-based antigen-in-stool enzyme immunoassay (Premier Platinum HpSA PLUS) for diagnosis of Helicobacter pylori infection in Vietnamese children. Helicobacter 2008, 13, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Hasosah, M. Accuracy of invasive and noninvasive methods of Helicobacter pylori infection diagnosis in Saudi children. Saudi J. Gastroenterol. Off. J. Saudi Gastroenterol. Assoc. 2019, 25, 126. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; De La Morena, F.; Abraira, V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: A systematic review and meta-analysis. Off. J. Am. Coll. Gastroenterol. ACG 2006, 101, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Raguza, D.; Machado, R.S.; Ogata, S.K.; Granato, C.F.H.; Patrício, F.R.S.; Kawakami, E. Validation of a monoclonal stool antigen test for diagnosing Helicobacter pylori infection in young children. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Lario, S.; Ramírez-Lázaro, M.J.; Montserrat, A.; Quílez, M.E.; Junquera, F.; Martinez-Bauer, E.; Sanfeliu, I.; Brullet, E.; Campo, R.; Segura, F. Diagnostic accuracy of three monoclonal stool tests in a large series of untreated Helicobacter pylori infected patients. Clin. Biochem. 2016, 49, 682–687. [Google Scholar] [CrossRef]
- Qiu, E.; Li, Z.; Han, S. Methods for detection of Helicobacter pylori from stool sample: Current options and developments. Braz. J. Microbiol. 2021, 52, 2057–2062. [Google Scholar] [CrossRef]
- El-Shabrawi, M.; Abd El-Aziz, N.; El-Adly, T.Z.; Hassanin, F.; Eskander, A.; Abou-Zekri, M.; Mansour, H.; Meshaal, S. Stool antigen detection versus 13 C-urea breath test for non-invasive diagnosis of pediatric Helicobacter pylori infection in a limited resource setting. Arch. Med. Sci. 2018, 14, 69–73. [Google Scholar] [CrossRef]
- Korkmaz, H.; Kesli, R.; Karabagli, P.; Terzi, Y. Comparison of the diagnostic accuracy of five different stool antigen tests for the diagnosis of Helicobacter pylori infection. Helicobacter 2013, 18, 384–391. [Google Scholar] [CrossRef]
- Miftahussurur, M.; Yamaoka, Y. Diagnostic methods of Helicobacter pylori infection for epidemiological studies: Critical importance of indirect test validation. BioMed Res. Int. 2016, 2016, 4819423. [Google Scholar] [CrossRef]
- Shimoyama, T.; Sawaya, M.; Ishiguro, A.; Hanabata, N.; Yoshimura, T.; Fukuda, S. Applicability of a rapid stool antigen test, using monoclonal antibody to catalase, for the management of Helicobacter pylori infection. J. Gastroenterol. 2011, 46, 487–491. [Google Scholar] [CrossRef]
- Ferwana, M.; Abdulmajeed, I.; Alhajiahmed, A.; Madani, W.; Firwana, B.; Hasan, R.; Altayar, O.; Limburg, P.J.; Murad, M.H.; Knawy, B. Accuracy of urea breath test in Helicobacter pylori infection: Meta-analysis. World J. Gastroenterol. WJG 2015, 21, 1305. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, L.; Ai, Y.; Pan, Z.; Guo, M.; Han, J. Diagnostic accuracy of the 14C-urea breath test in Helicobacter pylori infections: A meta-analysis. Wien. Klin. Wochenschr. 2017, 129, 38–45. [Google Scholar] [CrossRef]
- Shimoyama, T. Stool antigen tests for the management of Helicobacter pylori infection. World J. Gastroenterol. WJG 2013, 19, 8188. [Google Scholar] [CrossRef]
- Lopes, A.I.; Vale, F.F.; Oleastro, M. Helicobacter pylori infection-recent developments in diagnosis. World J. Gastroenterol. WJG 2014, 20, 9299. [Google Scholar]
- Roth, D.E.; Taylor, D.N.; Gilman, R.H.; Meza, R.; Katz, U.; Bautista, C.; Cabrera, L.; Velapatiño, B.; Lebron, C.; Razúri, M. Posttreatment follow-up of Helicobacter pylori infection using a stool antigen immunoassay. Clin. Diagn. Lab. Immunol. 2001, 8, 718–723. [Google Scholar] [CrossRef]
- Lottspeich, C.; Schwarzer, A.; Panthel, K.; Koletzko, S.; Rüssmann, H. Evaluation of the novel Helicobacter pylori ClariRes real-time PCR assay for detection and clarithromycin susceptibility testing of H. pylori in stool specimens from symptomatic children. J. Clin. Microbiol. 2007, 45, 1718–1722. [Google Scholar] [CrossRef]
- Pohl, D.; Keller, P.M.; Bordier, V.; Wagner, K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J. Gastroenterol. 2019, 25, 4629. [Google Scholar] [CrossRef]
- Šeligová, B.; Lukáč, Ľ.; Bábelová, M.; Vávrová, S.; Sulo, P. Diagnostic reliability of nested PCR depends on the primer design and threshold abundance of Helicobacter pylori in biopsy, stool, and saliva samples. Helicobacter 2020, 25, e12680. [Google Scholar] [CrossRef]
- Berlamont, H.; De Witte, C.; De Bruyckere, S.; Fox, J.G.; Backert, S.; Smet, A.; Boyen, F.; Haesebrouck, F. Differentiation of gastric Helicobacter species using MALDI-TOF mass spectrometry. Pathogens 2021, 10, 366. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Abdeen, E.; Al-Dubaib, M.; Alsayeqh, A.; Ibrahem, M.; Hamada, M.; Alenzi, A.; Moussa, I.; Hemeg, H.A. Proteomic characterization and discrimination of Aeromonas species recovered from meat and water samples with a spotlight on the antimicrobial resistance of Aeromonas hydrophila. Microbiologyopen 2019, 8, e782. [Google Scholar] [CrossRef]
- Elbehiry, A.; Marzouk, E.; Moussa, I.M.; Dawoud, T.M.; Mubarak, A.S.; Al-Sarar, D.; Alsubki, R.A.; Alhaji, J.H.; Hamada, M.; Abalkhail, A. Acinetobacter baumannii as a community foodborne pathogen: Peptide mass fingerprinting analysis, genotypic of biofilm formation and phenotypic pattern of antimicrobial resistance. Saudi J. Biol. Sci. 2021, 28, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Aldubaib, M.; Abalkhail, A.; Marzouk, E.; ALbeloushi, A.; Moussa, I.; Ibrahem, M.; Albazie, H.; Alqarni, A.; Anagreyyah, S. How MALDI-TOF Mass Spectrometry Technology Contributes to Microbial Infection Control in Healthcare Settings. Vaccines 2022, 10, 1881. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Al-Dubaib, M.; Marzouk, E.; Osman, S.; Edrees, H. Performance of MALDI biotyper compared with Vitek™ 2 compact system for fast identification and discrimination of Staphylococcus species isolated from bovine mastitis. MicrobiologyOpen 2016, 5, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Tanno, D.; Saito, K.; Ohashi, K.; Toyokawa, M.; Yamadera, Y.; Shimura, H. Matrix-Assisted Laser Desorption Ionization–Time-of-Flight Mass Spectrometry with Time-of-Flight Peak Analysis for Rapid and Accurate Detection of Group B Streptococcus in Pregnant Women. Microbiol. Spectr. 2022, e0173221. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Van Belkum, A.; Girard, V.; Charrier, J.-P.; Pincus, D. An update on the routine application of MALDI-TOF MS in clinical microbiology. Expert Rev. Proteom. 2019, 16, 695–710. [Google Scholar] [CrossRef]
- Oviaño, M.; Rodríguez-Sánchez, B. MALDI-TOF mass spectrometry in the 21st century clinical microbiology laboratory. Enferm. Infecc. Microbiol. Clin. 2021, 39, 192–200. [Google Scholar] [CrossRef]
- Hosseinian, H.; Ortiz Ortega, E.; Rosales López, M.J.; Rodríguez Vera, A.; Hosseini, S. Characterization Techniques for Mass Spectrometry Analysis. Mater. Charact. Tech. Appl. 2022, 47–69. [Google Scholar]
- Florio, W.; Baldeschi, L.; Rizzato, C.; Tavanti, A.; Ghelardi, E.; Lupetti, A. Detection of antibiotic-resistance by MALDI-TOF mass spectrometry: An expanding area. Front. Cell. Infect. Microbiol. 2020, 10, 572909. [Google Scholar] [CrossRef]
- Wolk, D.M.; Clark, A.E. Matrix-assisted laser desorption time of flight mass spectrometry. Clin. Lab. Med. 2018, 38, 471–486. [Google Scholar] [CrossRef]
- Matos, R.; De Witte, C.; Smet, A.; Berlamont, H.; De Bruyckere, S.; Amorim, I.; Gärtner, F.; Haesebrouck, F. Antimicrobial susceptibility pattern of Helicobacter heilmannii and Helicobacter ailurogastricus isolates. Microorganisms 2020, 8, 957. [Google Scholar] [CrossRef]
- Balážová, T.; Makovcová, J.; Šedo, O.; Slaný, M.; Faldyna, M.; Zdráhal, Z. The influence of culture conditions on the identification of Mycobacterium species by MALDI-TOF MS profiling. FEMS Microbiol. Lett. 2014, 353, 77–84. [Google Scholar] [CrossRef]
- Li, R.-J.; Dai, Y.-Y.; Qin, C.; Li, X.-H.; Qin, Y.-C.; Pan, Y.; Huang, Y.-Y.; Huang, Z.-S.; Huang, Y.-Q. Treatment strategies and preventive methods for drug-resistant Helicobacter pylori infection. World J. Meta-Anal. 2020, 8, 98–108. [Google Scholar] [CrossRef]
- Avery, L.M.; Nicolau, D.P. Investigational drugs for the treatment of infections caused by multidrug-resistant Gram-negative bacteria. Expert Opin. Investig. Drugs 2018, 27, 325–338. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y.J.C.M.R. Helicobacter pylori Infection, Its Laboratory Diagnosis, and Antimicrobial Resistance: A Perspective of Clinical Relevance. Clin. Microbiol. Rev. 2022, e0025821. [Google Scholar] [CrossRef]
- Abadi, A.T.B. Helicobacter pylori treatment: New perspectives using current experience. J. Glob. Antimicrob. Resist. 2017, 8, 123–130. [Google Scholar] [CrossRef]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y.; Chen, Y.; Wang, J.B.; Du, Y.Q.; Lu, N.H. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter 2018, 23, e12475. [Google Scholar] [CrossRef]
- Guevara, B.; Cogdill, A.G. Helicobacter pylori: A review of current diagnostic and management strategies. Dig. Dis. Sci. 2020, 65, 1917–1931. [Google Scholar] [CrossRef]
- Fallone, C.A.; Chiba, N.; van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69.e14. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Off. J. Am. Coll. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Gisbert, J.P.; Alcedo, J.; Amador, J.; Bujanda, L.; Calvet, X.; Castro-Fernández, M.; Fernández-Salazar, L.; Gené, E.; Lanas, Á.; Lucendo, A.J. V Conferencia Española de Consenso sobre el tratamiento de la infección por Helicobacter pylori. Rev. Española Enferm. Dig. 2021, 113, 740–763. [Google Scholar]
- Shah, S.C.; Iyer, P.G.; Moss, S.F. AGA clinical practice update on the management of refractory Helicobacter pylori infection: Expert review. Gastroenterology 2021, 160, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guan, L.; Hu, B. Detection and Treatment of Helicobacter pylori: Problems and Advances. Gastroenterol. Res. Pract. 2022, 2022, 4710964. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Rabitti, S.; Eusebi, L.H.; Bazzoli, F. Treatment of Helicobacter pylori infection: A clinical practice update. Eur. J. Clin. Investig. 2018, 48, e12857. [Google Scholar] [CrossRef] [PubMed]
- Zagari, R.M.; Frazzoni, L.; Marasco, G.; Fuccio, L.; Bazzoli, F. Treatment of Helicobacter pylori infection: A clinical practice update. Minerva Med. 2020, 112, 281–287. [Google Scholar]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance—From biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar] [CrossRef]
- Hathroubi, S.; Zerebinski, J.; Clarke, A.; Ottemann, K.M. Helicobacter pylori biofilm confers antibiotic tolerance in part via a protein-dependent mechanism. Antibiotics 2020, 9, 355. [Google Scholar] [CrossRef]
- Kadkhodaei, S.; Siavoshi, F.; Akbari Noghabi, K. Mucoid and coccoid Helicobacter pylori with fast growth and antibiotic resistance. Helicobacter 2020, 25, e12678. [Google Scholar] [CrossRef]
- Aruru, M.; Truong, H.-A.; Clark, S. Pharmacy Emergency Preparedness and Response (PEPR): A proposed framework for expanding pharmacy professionals’ roles and contributions to emergency preparedness and response during the COVID-19 pandemic and beyond. Res. Soc. Adm. Pharm. 2021, 17, 1967–1977. [Google Scholar] [CrossRef]
- Liu, N.; Zhou, N.; Chai, N.; Liu, X.; Jiang, H.; Wu, Q.; Li, Q. Helicobacter pylori promotes angiogenesis depending on Wnt/beta-catenin-mediated vascular endothelial growth factor via the cyclooxygenase-2 pathway in gastric cancer. BMC Cancer 2016, 16, 321. [Google Scholar] [CrossRef]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Teng, T.Z.J.; Sudharsan, M.; Yau, J.W.K.; Tan, W.; Shelat, V.G. Helicobacter pylori knowledge and perception among multi-ethnic Asians. Helicobacter 2021, 26, e12794. [Google Scholar] [CrossRef]
- Wang, Y.-x.; Zou, J.-y.; Hu, L.-f.; Liu, Q.; Huang, R.-l.; Tang, T.; Yue, Q.-q.; Sun, Y.-x.; Xiao, Q.; Zeng, X. What is the general Chinese public’s awareness of and attitudes towards Helicobacter pylori screening and associated health behaviours? A cross-sectional study. BMJ Open 2022, 12, e057929. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Liu, T.-S.; Fan, X.-M.; Dong, L.; Fang, G.-T.; Tu, C.-T.; Gu, X.-Y.; Wang, J.-Y. Epidemiological study of Helicobacter pylori infection and its risk factors in Shanghai. Zhonghua Yi Xue Za Zhi 2005, 85, 802–806. [Google Scholar]
- Xia, P.; Ma, M.-F.; Wang, W. Status of Helicobacter pylori infection among migrant workers in Shijiazhuang, China. Asian Pac. J. Cancer Prev. 2012, 13, 1167–1170. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Choi, K.S.; Shin, H.-R.; Bang, Y.-J. Public awareness of gastric cancer risk factors and disease screening in a high risk region: A population-based study. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2009, 41, 59–66. [Google Scholar] [CrossRef]
- Shin, D.W.; Cho, J.; Kim, S.H.; Kim, Y.J.; Choi, H.C.; Son, K.Y.; Park, S.M.; Park, J.H.; Park, M.S.; Cho, B. Preferences for the “screen and treat” strategy of Helicobacter pylori to prevent gastric cancer in healthy Korean populations. Helicobacter 2013, 18, 262–269. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Knowledge about causes of peptic ulcer disease—United States, March–April 1997. MMWR. Morb. Mortal. Wkly. Rep. 1997, 46, 985–987. [Google Scholar]
- Chi, D.L. Reducing Alaska Native paediatric oral health disparities: A systematic review of oral health interventions and a case study on multilevel strategies to reduce sugar-sweetened beverage intake. Int. J. Circumpolar Health 2013, 72, 21066. [Google Scholar] [CrossRef]
- Driscoll, L.J.; Brown, H.E.; Harris, R.B.; Oren, E. Population knowledge, attitude, and practice regarding Helicobacter pylori transmission and outcomes: A literature review. Front. Public Health 2017, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Wynne, A.; Hastings, E.V.; Colquhoun, A.; Chang, H.-J.; Goodman, K.J.; Grp, C. Untreated water and Helicobacter pylori: Perceptions and behaviors in a Northern Canadian community. Circumpol. Health 2013, 72, 704–705. [Google Scholar]
- Abebaw, W.; Kibret, M.; Abera, B. Prevalence and risk factors of H. pylori from dyspeptic patients in northwest Ethiopia: A hospital based cross-sectional study. Asian Pac. J. Cancer Prev. 2014, 15, 4459–4463. [Google Scholar]
- Ahmed, K.S.; Khan, A.A.; Ahmed, I.; Tiwari, S.K.; Habeeb, A.; Ahi, J.; Abid, Z.; Ahmed, N.; Habibullah, C.M. Impact of household hygiene and water source on the prevalence and transmission of Helicobacter pylori: A South Indian perspective. Singap. Med. J. 2007, 48, 543. [Google Scholar]
- Lee, Y.Y.; Ismail, A.W.; Mustaffa, N.; Musa, K.I.; Majid, N.A.; Choo, K.E.; Mahendra Raj, S.; Derakhshan, M.H.; Malaty, H.M.; Graham, D.Y.J.H. Sociocultural and dietary practices among Malay subjects in the north-eastern region of Peninsular Malaysia: A region of low prevalence of Helicobacter pylori infection. Helicobacter 2012, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.d.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 1–33. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Saxena, K.; Chauhan, N.; Jain, U. Advances in diagnosis of Helicobacter pylori through biosensors: Point of care devices. Anal. Biochem. 2021, 630, 114325. [Google Scholar] [CrossRef]
- Pramanik, P.K.D.; Solanki, A.; Debnath, A.; Nayyar, A.; El-Sappagh, S.; Kwak, K.-S. Advancing modern healthcare with nanotechnology, nanobiosensors, and internet of nano things: Taxonomies, applications, architecture, and challenges. IEEE Access 2020, 8, 65230–65266. [Google Scholar] [CrossRef]
- Yadav, A.K.; Verma, D.; Chaudhary, N.; Kumar, A.; Solanki, P.R. Aptamer based switches: A futuristic approach for Helicobacter pylori detection. Mater. Lett. 2022, 308, 131239. [Google Scholar] [CrossRef]
- Safarov, T.; Kiran, B.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S. An overview of nanotechnology-based treatment approaches against Helicobacter pylori. Expert Rev. Anti-Infect. Ther. 2019, 17, 829–840. [Google Scholar] [CrossRef]
- Argueta, E.A.; Moss, S.F. How we approach difficult to eradicate Helicobacter pylori. Gastroenterology 2022, 162, 32–37. [Google Scholar] [CrossRef]
- Fu, J.; Sun, C.-F.; He, H.-Y.; Ojha, S.C.; Shi, H.; Deng, C.-L.; Sheng, Y.-J. The effect of CYP2C19 gene polymorphism on the eradication rate of Helicobacter pylori by proton pump inhibitors-containing regimens in Asian populations: A meta-analysis. Pharmacogenomics 2021, 22, 859–879. [Google Scholar] [CrossRef]
- Zihlif, M.; Bashaireh, B.; Rashid, M.; Almadani, Z.; Jarrar, Y. Effect of major CYP2C19 genetic polymorphisms on Helicobacter pylori eradication based on different treatment regimens. Biomed. Rep. 2022, 16, 1–6. [Google Scholar] [CrossRef]
- Clyne, M.; Rowland, M. The role of host genetic polymorphisms in Helicobacter pylori mediated disease outcome. In Helicobacter pylori in Human Diseases; Springer: Berlin/Heidelberg, Germany, 2019; pp. 151–172. [Google Scholar]
- Karbalaei, M.; Khorshidi, M.; Sisakht-pour, B.; Ghazvini, K.; Farsiani, H.; Youssefi, M.; Keikha, M. What are the effects of IL-1β (rs1143634), IL-17A promoter (rs2275913) and TLR4 (rs4986790) gene polymorphism on the outcomes of infection with H. pylori within as Iranian population; A systematic review and meta-analysis. Gene Rep. 2020, 20, 100735. [Google Scholar] [CrossRef]
- Eed, E.M.; Hawash, Y.A.; Khalifa, A.S.; Alsharif, K.F.; Alghamdi, S.A.; Almalki, A.A.; Almehmadi, M.M.; Ismail, K.A.; Taha, A.A.; Saber, T. Association of toll-like receptors 2, 4, 9 and 10 genes polymorphisms and Helicobacter pylori-related gastric diseases in Saudi patients. Indian J. Med. Microbiol. 2020, 38, 94–100. [Google Scholar] [CrossRef]
- Rokkas, T.; Gisbert, J.P.; Malfertheiner, P.; Niv, Y.; Gasbarrini, A.; Leja, M.; Megraud, F.; O’Morain, C.; Graham, D. Comparative effectiveness of multiple different first-line treatment regimens for Helicobacter pylori infection: A network meta-analysis. Gastroenterology 2021, 161, 495–507.e494. [Google Scholar] [CrossRef]
- Shinozaki, S.; Kobayashi, Y.; Osawa, H.; Sakamoto, H.; Hayashi, Y.; Lefor, A.K.; Yamamoto, H. Effectiveness and safety of vonoprazan versus proton pump inhibitors for second-line Helicobacter pylori eradication therapy: Systematic review and meta-analysis. Digestion 2021, 102, 319–325. [Google Scholar] [CrossRef]
- Kiyotoki, S.; Nishikawa, J.; Sakaida, I. Efficacy of vonoprazan for Helicobacter pylori eradication. Intern. Med. 2020, 59, 153–161. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Viertel, T.M.; Ritter, K.; Horz, H.P. Viruses versus bacteria—Novel approaches to phage therapy as a tool against multidrug-resistant pathogens. J. Antimicrob. Chemother. 2014, 69, 2326–2336. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A. Review: Treatment of Helicobacter pylori infection 2022. Microbiota Inhealth Dis. 2022, 4, e713. [Google Scholar]
- Chua, B.Q.Y.; Chong, V.W.S.; Teng, T.Z.J.; Chia, C.T.W.; Aung, M.O.; Shelat, V.G. Does technology-enhanced communication improve Helicobacter pylori eradication outcomes? A meta-analysis. Helicobacter 2022, 27, e12890. [Google Scholar] [CrossRef] [PubMed]
- Zha, J.; Li, Y.y.; Qu, J.y.; Yang, X.x.; Han, Z.x.; Zuo, X. Effects of enhanced education for patients with the Helicobacter pylori infection: A systematic review and meta-analysis. Helicobacter 2022, 27, e12880. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, X.h.; Zhang, L.l.; Wu, L.m. Application of WeChat platform in the management of patients infected with Helicobacter pylori. Helicobacter 2021, 26, e12832. [Google Scholar] [CrossRef]
- Zhou, B.G.; Yan, X.L.; Wan, L.Y.; Zhang, Q.; Li, B.; Ai, Y.W. Effect of enhanced patient instructions on Helicobacter pylori eradication: A systematic review and meta-analysis of randomized controlled trials. Helicobacter 2022, 27, e12869. [Google Scholar] [CrossRef]
- Schubert, J.P.; Gehlert, J.; Rayner, C.K.; Roberts-Thomson, I.C.; Costello, S.; Mangoni, A.A.; Bryant, R.V. Antibiotic resistance of Helicobacter pylori in Australia and New Zealand: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1450–1456. [Google Scholar] [CrossRef]
- Kouhsari, E.; Sadeghifard, N.; Khadiv, A.; Sayadi, H.; Amiriani, T.; Ghafourian, S.; Valadbeigi, H.; Krutova, M. Heteroresistance to clarithromycin and metronidazole in patients with a Helicobacter pylori infection: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2022, 21, 1–8. [Google Scholar] [CrossRef]
- Ding, S.-Z. Global whole family based-Helicobacter pylori eradication strategy to prevent its related diseases and gastric cancer. World J. Gastroenterol. 2020, 26, 995. [Google Scholar] [CrossRef]
- Ding, S. Focusing on whole family based-Helicobacter pylori infection management and clinical research to prevent gastric mucosal diseases and gastric cancer. Zhonghua Yi Xue Za Zhi 2019, 99, 1446–1448. [Google Scholar]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. Response to Georgopoulos et al. Off. J. Am. Coll. Gastroenterol. ACG 2017, 112, 1169–1170. [Google Scholar] [CrossRef]
- Liou, J.; Malfertheiner, P.; Lee, Y.; Sheu, B.; Sugano, K.; Cheng, H.; Yeoh, K.; Hsu, P.; Goh, K.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef]
- (NICE), N.I.f.H.C.E. Gastro-Oesophageal Reflux Disease and Dyspepsia in Adults: Investigation and Management. 2014. Available online: https://www.nice.org.uk/Guidance/CG184 (accessed on 18 October 2019).
- Zhang, X.; Arnold, I.C.; Müller, A. Mechanisms of persistence, innate immune activation and immunomodulation by the gastric pathogen Helicobacter pylori. Curr. Opin. Microbiol. 2020, 54, 1–10. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J. Helicobacter pylori recrudescence and its influencing factors. J. Cell. Mol. Med. 2019, 23, 7919–7925. [Google Scholar] [CrossRef]
- Blanchard, T.G.; Czinn, S.J. Identification of Helicobacter pylori and the evolution of an efficacious childhood vaccine to protect against gastritis and peptic ulcer disease. Pediatr. Res. 2017, 81, 170–176. [Google Scholar] [CrossRef]
- Banga Ndzouboukou, J.L.; Lei, Q.; Ullah, N.; Zhang, Y.; Hao, L.; Fan, X. Helicobacter pylori adhesins: HpaA a potential antigen in experimental vaccines for H. pylori. Helicobacter 2021, 26, e12758. [Google Scholar] [CrossRef]
- Dos Santos Viana, I.; Cordeiro Santos, M.L.; Santos Marques, H.; Lima de Souza Goncalves, V.; Bittencourt de Brito, B.; Franca da Silva, F.A.; Oliveira e Silva, N.; Dantas Pinheiro, F.; Fernandes Teixeira, A.; Tanajura Costa, D. Vaccine development against Helicobacter pylori: From ideal antigens to the current landscape. Expert Rev. Vaccines 2021, 20, 989–999. [Google Scholar] [CrossRef]
- Guo, L.; Hong, D.; Wang, S.; Zhang, F.; Tang, F.; Wu, T.; Chu, Y.; Liu, H.; He, M.; Yang, H. Therapeutic protection against H. pylori infection in Mongolian gerbils by oral immunization with a tetravalent epitope-based vaccine with polysaccharide adjuvant. Front. Immunol. 2019, 10, 1185. [Google Scholar] [CrossRef]
- Pan, X.; Ke, H.; Niu, X.; Li, S.; Lv, J.; Pan, L. Protection against Helicobacter pylori infection in BALB/c mouse model by oral administration of multivalent epitope-based vaccine of cholera toxin B subunit-HUUC. Front. Immunol. 2018, 9, 1003. [Google Scholar] [CrossRef]
- Czinn, S.J.; Blanchard, T. Vaccinating against Helicobacter pylori infection. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Ma, J.; Dong, Q.; Liu, Q. Live bacterial vaccine vector and delivery strategies of heterologous antigen: A review. Immunol. Lett. 2018, 197, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, J.; Ji, Q.; Liu, Q. Evaluation of an attenuated Listeria monocytogenes as a vaccine vector to control Helicobacter pylori infection. Immunol. Lett. 2021, 238, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Stubljar, D.; Jukic, T.; Ihan, A. How far are we from vaccination against Helicobacter pylori infection? Expert Rev. Vaccines 2018, 17, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Mao, X.-H.; Li, J.-X.; Tong, W.-D.; Wang, B.; Zhang, Y.-J.; Guo, G.; Zhao, Z.-J.; Li, L.; Wu, D.-L. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015, 386, 1457–1464. [Google Scholar] [CrossRef]
- Poonyam, P.; Chotivitayatarakorn, P.; Vilaichone, R.-K. High effective of 14-day high-dose PPI-bismuth-containing quadruple therapy with probiotics supplement for Helicobacter pylori eradication: A double blinded-randomized placebo-controlled study. Asian Pac. J. Cancer Prev. 2019, 20, 2859. [Google Scholar] [CrossRef]
- Butorova, L.; Ardatskaya, M.; Osadchuk, M.; Kadnikova, N.; Lukianova, E.; Plavnik, R.; Sayutina, E.; Topchiy, T.; Tuayeva, E. Comparison of clinical-metabolic efficacy of pre-and probiotics in the conducted optimized protocols of eradication therapy of Helicobacter pylori infection. Ter. Arkhiv 2020, 92, 64–69. [Google Scholar] [CrossRef]
- Neshani, A.; Zare, H.; Akbari Eidgahi, M.R.; Hooshyar Chichaklu, A.; Movaqar, A.; Ghazvini, K. Review of antimicrobial peptides with anti-Helicobacter pylori activity. Helicobacter 2019, 24, e12555. [Google Scholar] [CrossRef]
- Sukri, A.; Lopes, B.S.; Hanafiah, A. The Emergence of multidrug-resistant Helicobacter pylori in Southeast Asia: A systematic review on the trends and intervention strategies using antimicrobial peptides. Antibiotics 2021, 10, 1061. [Google Scholar] [CrossRef]
- Im, B.N.; Shin, H.; Lim, B.; Lee, J.; Kim, K.S.; Park, J.M.; Na, K. Helicobacter pylori-targeting multiligand photosensitizer for effective antibacterial endoscopic photodynamic therapy. Biomaterials 2021, 271, 120745. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.N.; Tang, G.Y.; Li, S.; Gan, R.Y.; Li, H.B. Natural products for the prevention and management of Helicobacter pylori infection. Compr. Rev. Food Sci. Food Saf. 2018, 17, 937–952. [Google Scholar] [CrossRef]
- Sousa, C.; Ferreira, R.; Azevedo, N.F.; Oleastro, M.; Azeredo, J.; Figueiredo, C.; Melo, L.D. Helicobacter pylori infection: From standard to alternative treatment strategies. Crit. Rev. Microbiol. 2022, 48, 376–396. [Google Scholar] [CrossRef]
- Sutton, P.; Boag, J.M. Status of vaccine research and development for Helicobacter pylori. Vaccines 2019, 37, 7295–7299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbehiry, A.; Marzouk, E.; Aldubaib, M.; Abalkhail, A.; Anagreyyah, S.; Anajirih, N.; Almuzaini, A.M.; Rawway, M.; Alfadhel, A.; Draz, A.; et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics 2023, 12, 191. https://doi.org/10.3390/antibiotics12020191
Elbehiry A, Marzouk E, Aldubaib M, Abalkhail A, Anagreyyah S, Anajirih N, Almuzaini AM, Rawway M, Alfadhel A, Draz A, et al. Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics. 2023; 12(2):191. https://doi.org/10.3390/antibiotics12020191
Chicago/Turabian StyleElbehiry, Ayman, Eman Marzouk, Musaad Aldubaib, Adil Abalkhail, Sulaiman Anagreyyah, Nuha Anajirih, Abdulaziz M. Almuzaini, Mohammed Rawway, Abdulmajeed Alfadhel, Abdelmaged Draz, and et al. 2023. "Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges" Antibiotics 12, no. 2: 191. https://doi.org/10.3390/antibiotics12020191
APA StyleElbehiry, A., Marzouk, E., Aldubaib, M., Abalkhail, A., Anagreyyah, S., Anajirih, N., Almuzaini, A. M., Rawway, M., Alfadhel, A., Draz, A., & Abu-Okail, A. (2023). Helicobacter pylori Infection: Current Status and Future Prospects on Diagnostic, Therapeutic and Control Challenges. Antibiotics, 12(2), 191. https://doi.org/10.3390/antibiotics12020191







