Novel Antibacterial Agents SAAP-148 and Halicin Combat Gram-Negative Bacteria Colonizing Catheters
Abstract
1. Introduction
2. Results
2.1. Identification of the Bacterial Strains Colonizing Catheters
2.2. Bacterial Resistance to Antibiotics
2.3. Bacterial Biofilm Formation and Virulence Gene Profiles
2.4. Bactericidal Efficacies of SAAP-148 and Halicin on Selected Strains
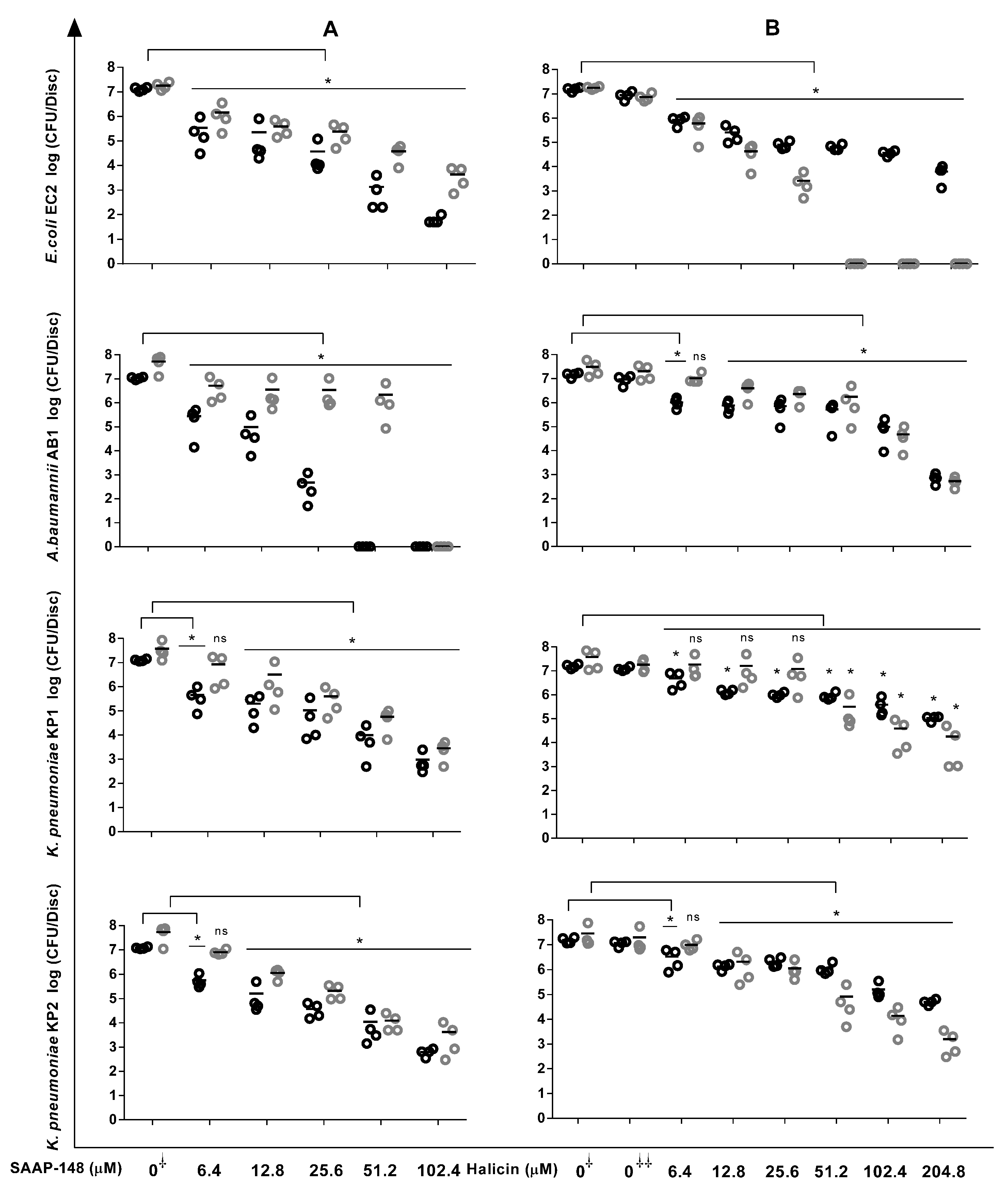
2.5. Reduction in Bacterial Counts in Biofilms by SAAP-148 and Halicin
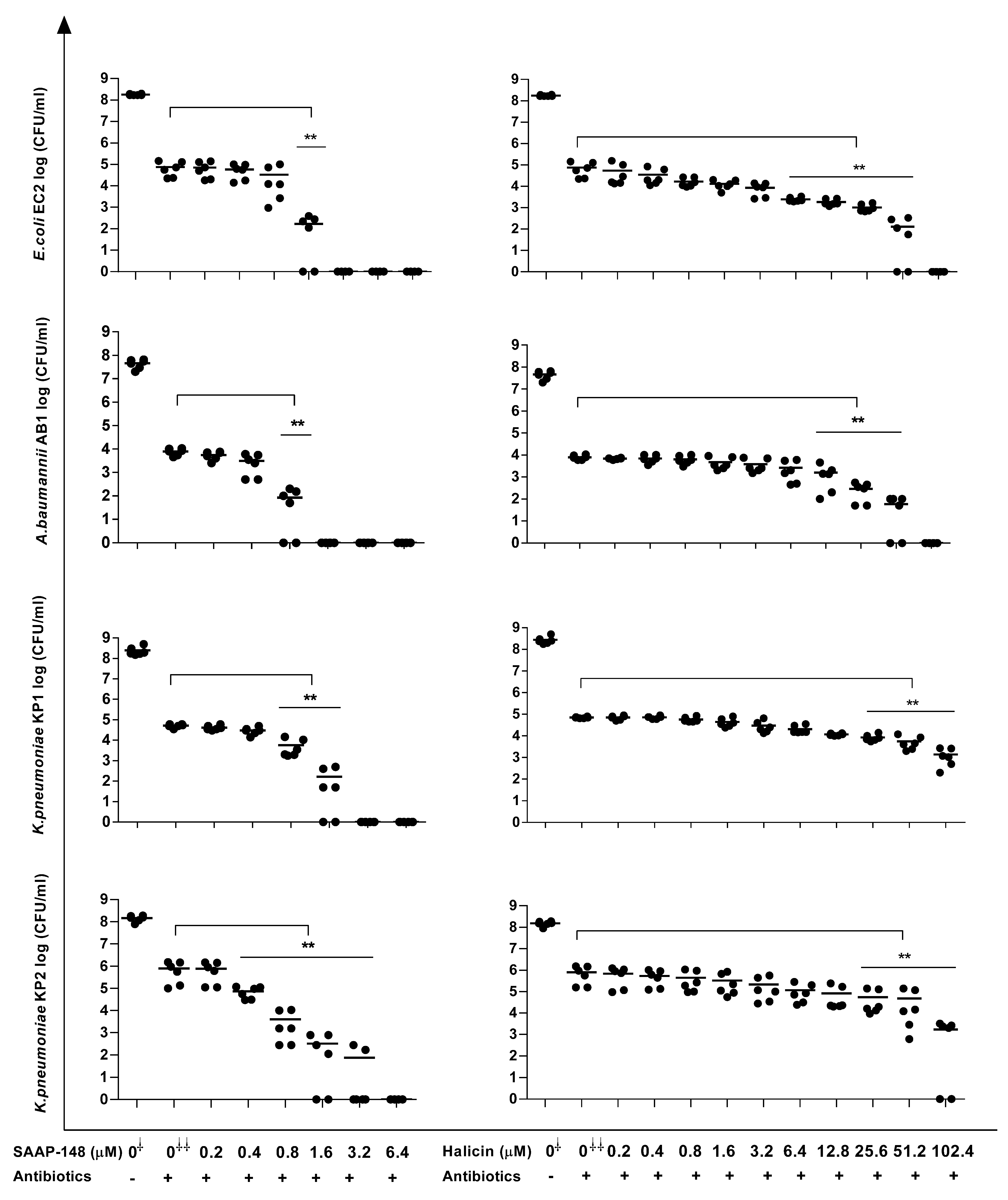
2.6. Effects of SAAP-148 and Halicin on Persisters Derived from Antibiotic-Exposed Mature Biofilms
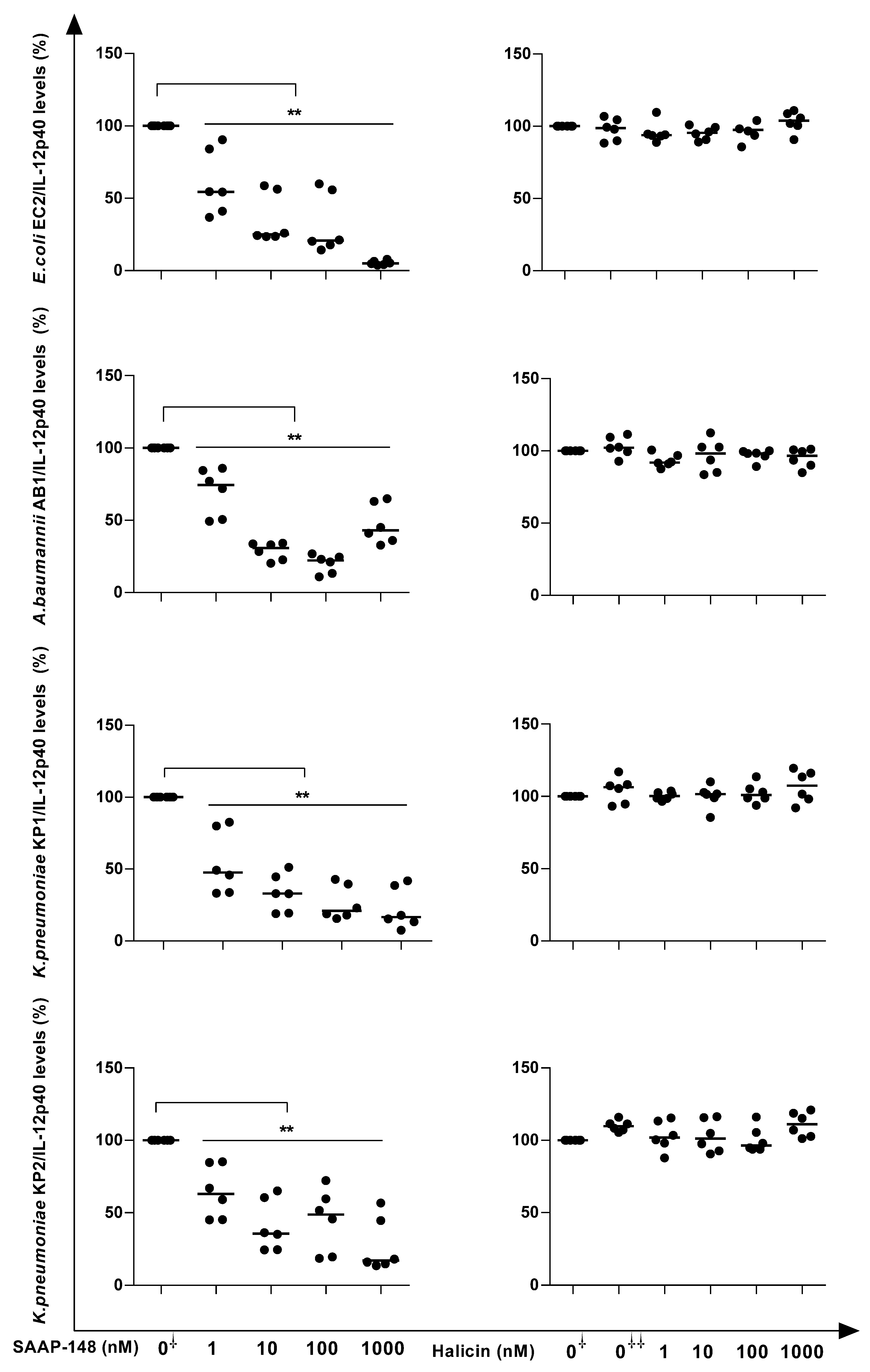
2.7. SAAP-148 and Halicin Neutralize GNB-Induced IL-12p40 Production by Human Blood Leukocytes
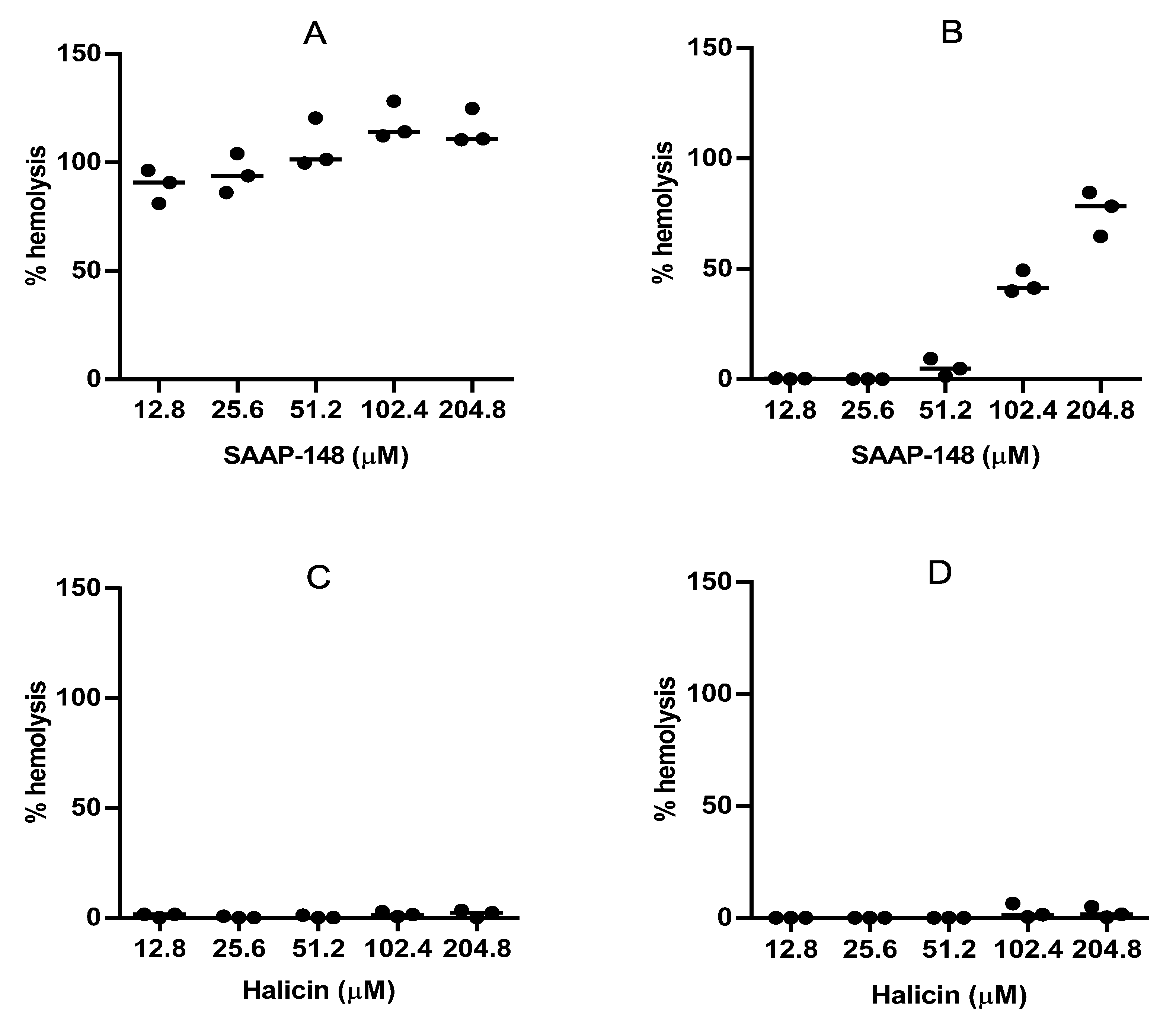
2.8. Hemolytic Activities of SAAP-148 and Halicin
2.9. Interactions between SAAP-148 and Halicin
3. Discussion
4. Materials and Methods
4.1. Isolation of Bacteria from Catheters
4.2. Identification of the Strains
4.3. Antibiotic Susceptibility Testing
4.4. Detection of Bacterial Virulence and Adhesion Genes
4.5. Biofilm Formation on Polystyrene Microplates
4.6. Novel Antibacterial Agents
4.7. In Vitro Killing Assay
4.8. Anti-Biofilm Assay
4.9. Checkerboard Assay for Determination of SAAP-148 and Halicin Synergy
4.10. Anti-Persister Assay
4.11. Assay for Bacterial Endotoxin Neutralization Capacities of SAAP-148 and Halicin
4.12. Hemolysis Assay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patel, A.R.; Patel, A.R.; Singh, S.; Singh, S.; Khawaja, I. Central line catheters and associated complications: A review. Cureus 2019, 11, e4717. [Google Scholar] [CrossRef]
- Jatczak, L.; Puton, R.C.; Proença, A.J.L.; Rubin, L.C.; Borges, L.B.; Saleh, J.N.; Corrêa, M.P. Complications of central venous catheterization at a vascular surgery service in a teaching hospital: A prospective cohort study. J. Vasc. Bras. 2023, 22, e20230070. [Google Scholar] [CrossRef]
- VanEpps, J.S.; Younger, J.G. Implantable device-related infection. Shock 2016, 46, 597–608. [Google Scholar] [CrossRef]
- Dadi, N.C.T.; Radochová, B.; Vargová, J.; Bujdáková, H. Impact of healthcare-associated infections connected to medical devices—An update. Microorganisms 2021, 9, 2332. [Google Scholar] [CrossRef]
- Drugeon, B.; Guenezan, J.; Pichon, M.; Devos, A.; Fouassin, X.; Neveu, A.; Boinot, L.; Pratt, V.; Mimoz, O. Incidence, complications, and costs of peripheral venous catheter-related bacteraemia: A retrospective, single-centre study. J. Hosp. Infect. 2023, 135, 67–73. [Google Scholar] [CrossRef]
- Voidazan, S.; Albu, S.; Toth, R.; Grigorescu, B.; Rachita, A.; Moldovan, I. Healthcare associated infections—A new pathology in medical practice? Int. J. Environ. Res. Public Health 2020, 17, 760. [Google Scholar] [CrossRef]
- Gahlot, R.; Nigam, C.; Kumar, V.; Yadav, G.; Anupurba, S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014, 4, 162–167. [Google Scholar] [CrossRef]
- Feneley, R.C.; Hopley, I.B.; Wells, P.N. Urinary catheters: History, current status, adverse events and research agenda. J. Med. Eng. Technol. 2015, 39, 459–470. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liang, X.; Vorstius, J.; Keatch, R.; Corner, G.; Nabi, G.; Davidson, F.; Gadd, G.M.; Zhao, Q. Enhanced antibacterial and antiadhesive activities of silver-PTFE nanocomposite coating for urinary catheters. ACS. Biomater. Sci. Eng. 2019, 5, 2804–2814. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Solis-Velazquez, O.A.; Gutiérrez-Lomelí, M.; Guerreo-Medina, P.J.; de Lourdes Rosas-García, M.; Iñiguez-Moreno, M.; Avila-Novoa, M.G. Nosocomial pathogen biofilms on biomaterials: Different growth medium conditions and components of biofilms produced in vitro. J. Microbiol. Immunol. Infect. 2021, 54, 1038–1047. [Google Scholar] [CrossRef]
- Thorarinsdottir, H.R.; Rockholt, M.; Klarin, B.; Broman, M.; Fraenkel, C.J.; Kander, T. Catheter-related infections: A Scandinavian observational study on the impact of a simple hygiene insertion bundle. Acta Anaesthesiol. Scand. 2020, 64, 224–231. [Google Scholar] [CrossRef]
- Ripa, M.; Morata, L.; Rodríguez-Núñez, O.; Cardozo, C.; Puerta-Alcalde, P.; Hernández-Meneses, M.; Ambrosioni, J.; Linares, L.; Bodro, M.; Valcárcel, A.; et al. Short-term peripheral venous catheter-related bloodstream infections: Evidence for increasing prevalence of Gram-negative microorganisms from a 25-Year prospective observational study. Antimicrob. Agents Chemother. 2018, 62, e00892-18. [Google Scholar] [CrossRef]
- Tsuboi, M.; Hayakawa, K.; Mezaki, K.; Katanami, Y.; Yamamoto, K.; Kutsuna, S.; Takeshita, N.; Ohmagari, N. Comparison of the epidemiology and microbiology of peripheral line-and central line associated bloodstream infections. Am. J. Infect. Control 2019, 47, 8–10. [Google Scholar] [CrossRef]
- Surapat, B.; Montakantikul, P.; Malathum, K.; Kiertiburanakul, S.; Santanirand, P.; Chindavijak, B. Microbial epidemiology and risk factors for relapse in Gram-negative bacteria catheter-related bloodstream infection with a pilot prospective study in patients with catheter removal receiving short-duration of antibiotic therapy. BMC. Infect. Dis. 2020, 20, 604. [Google Scholar] [CrossRef]
- Lendak, D.; Puerta-Alcalde, P.; Moreno-García, E.; Chumbita, M.; García-Pouton, N.; Cardozo, C.; Morata, L.; Suárez-Lledó, M.; Hernández-Meneses, M.; Ghiglione, L.; et al. Changing epidemiology of catheter-related bloodstream infections in neutropenic oncohematological patients. PLoS ONE 2021, 16, e0251010. [Google Scholar] [CrossRef]
- Mandolfo, S.; Anesi, A.; Rognoni, V. The epidemiology of central venous catheter-related bloodstream infection in our renal units is changing. J. Vasc. Access 2022, 23, 328–329. [Google Scholar] [CrossRef]
- Neoh, K.G.; Li, M.; Kang, E.T.; Chiong, E.; Tambyah, P.A. Surface modification strategies for combating catheter-related complications: Recent advances and challenges. J. Mater. Chem. B 2017, 5, 2045–2067. [Google Scholar] [CrossRef]
- Felix, L.; Whitely, C.; Tharmalingam, N.; Mishra, B.; Vera-Gonzalez, N.; Mylonakis, E.; Shukla, A.; Fuchs, B.B. Auranofin coated catheters inhibit bacterial and fungal biofilms in a murine subcutaneous model. Front. Cell. Infect. Microbiol. 2023, 13, 1135942. [Google Scholar] [CrossRef]
- Bassetti, M.; Montero, J.G.; Paiva, J.A. When antibiotic treatment fails. Intensive Care Med. 2017, 44, 73–75. [Google Scholar] [CrossRef]
- Köves, B.; Magyar, A.; Tenke, P. Spectrum and antibiotic resistance of catheter-associated urinary tract infections. GMS. Infect. Dis. 2017, 5, Doc06. [Google Scholar] [CrossRef]
- Cepas, V.; López, Y.; Muñoz, E.; Rolo, D.; Ardanuy, C.; Martí, S.; Xercavins, M.; Horcajada, J.P.; Bosch, J.; Soto, S.M. Relationship between biofilm formation and antimicrobial resistance in Gram-Negative Bacteria. Microb. Drug Resist. 2018, 25, 72–79. [Google Scholar] [CrossRef]
- Windels, E.M.; Michiels, J.E.; Van den Bergh, B.; Fauvart, M.; Michiels, J. Antibiotics: Combatting tolerance to stop resistance. mBio 2019, 10, e02095-19. [Google Scholar] [CrossRef]
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The current knowledge on the pathogenesis of tissue and medical device-related biofilm infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef]
- Chernysh, S.; Gordya, N.; Tulin, D.; Yakovlev, A. Biofilm infections between Scylla and Charybdis: Interplay of host antimicrobial peptides and antibiotics. Infect. Drug Resist. 2018, 11, 501–514. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Zapotoczna, M.; Forde, É.; Hogan, S.; Humphreys, H.; O’Gara, J.P.; Fitzgerald-Hughes, D.; Devocelle, M.; O’Neill, E. Eradication of Staphylococcus aureus biofilm infections using synthetic antimicrobial peptides. J. Infect. Dis. 2017, 215, 975–983. [Google Scholar] [CrossRef]
- de Breij, A.; Riool, M.; Cordfunke, R.A.; Malanovic, N.; de Boer, L.; Koning, R.I.; Ravensbergen, E.; Franken, M.; Van der Heijde, T.; Boekema, B.K.; et al. The antimicrobial peptide SAAP-148 combats drug-resistant bacteria and biofilms. Sci. Transl. Med. 2018, 10, eaan4044. [Google Scholar] [CrossRef]
- Van der Does, A.M.; Hiemstra, P.S.; Mookherjee, N. Antimicrobial host defense peptides: Immunomodulatory functions and translational prospects. Adv. Exp. Med. Biol. 2019, 1117, 149–171. [Google Scholar] [CrossRef]
- Kim, Y.M.; Son, H.P.S.C.; Lee, J.K.; Jang, M.K.; Lee, J.R. Anti-biofilm effects of rationally designed peptides against planktonic cells and pre-formed Biofilm of Pseudomonas aeruginosa. Antibiotics 2023, 12, 349. [Google Scholar] [CrossRef]
- di Luca, M.; Maccari, G.; Nifosì, R. Treatment of microbial biofilms in the post-antibiotic era: Prophylactic and therapeutic use of antimicrobial peptides and their design by bioinformatics tools. Pathog. Dis. 2014, 70, 257–270. [Google Scholar] [CrossRef]
- Jaśkiewicz, M.; Neubauer, D.; Kazor, K.; Bartoszewska, S.; Kamysz, W. Antimicrobial activity of selected antimicrobial peptides against planktonic culture and biofilm of Acinetobacter baumannii. Probiotics Antimicrob. Proteins 2018, 11, 317–324. [Google Scholar] [CrossRef]
- Feng, X.; Sambanthamoorthy, K.; Palys, T.; Paranavitana, C. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 2013, 49, 131–137. [Google Scholar] [CrossRef]
- Luo, Y.; McLean, D.T.F.; Linden, G.J.; McAuley, D.F.; McMullan, R.; Lundy, F.T. The naturally occurring host defenses peptide, LL-37, and its truncated mimetics KE-18 and KR-12 have selected biocidal and antibiofilm activities against Candida albicans, Staphylococcus aureus, and Escherichia coli in vitro. Front. Microbiol. 2017, 8, 544. [Google Scholar] [CrossRef]
- Spencer, J.J.; Pitts, R.E.; Pearson, R.A.; King, L.B. The effects of antimicrobial peptides WAM-1 and LL-37 on multidrug-resistant Acinetobacter baumannii. Pathog. Dis. 2018, 76, fty007. [Google Scholar] [CrossRef]
- van Gent, M.E.; van der Reijden, T.J.K.; Lennard, P.R.; de Visser, A.W.; Schonkeren-Ravensbergen, B.; Dolezal, N.; Cordfunke, R.A.; Drijfhout, J.W.; Nibbering, P.H. Synergism between the synthetic antibacterial and antibiofilm peptide (SAAP)-148 and halicin. Antibiotics 2022, 11, 673. [Google Scholar] [CrossRef]
- Scheper, H.; Wubbolts, J.M.; Verhagen, J.A.M.; de Visser, A.W.; van der Wall, R.J.P.; Visser, L.G.; de Boer, M.G.J.; Nibbering, P.H. SAAP-148 eradicates MRSA persisters within mature biofilm models simulating prosthetic joint infection. Front. Microbiol. 2020, 12, 625952. [Google Scholar] [CrossRef]
- Verheul, M.; Drijfhout, J.W.; Pijls, B.G.; Nibbering, P.H. Non-contact induction heating and SAAP-148 eliminate persisters within MRSA biofilms mimicking a metal implant infection. Eur. Cells. Mater. 2021, 43, 34–42. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A deep learning approach to antibiotic discovery. Cell 2020, 18, 688–702.e13. [Google Scholar] [CrossRef]
- Booq, R.Y.; Tawfik, E.A.; Alfassam, H.A.; Alfahad, A.J.; Alyamani, E.J. Assessment of the antibacterial efficacy of halicin against pathogenic bacteria. Antibiotics 2021, 10, 1480. [Google Scholar] [CrossRef]
- Aburayan, W.S.; Booq, R.Y.; BinSaleh, N.S.; Alfassam, H.A.; Bakr, A.A.; Bukhary, H.A.; Alyamani, E.J.; Tawfik, E.A. The delivery of the novel drug ‘Halicin’ using electrospun fibers for the treatment of pressure ulcer against pathogenic bacteria. Pharmaceutics 2020, 12, 1189. [Google Scholar] [CrossRef]
- Koppen, B.C.; Mulder, P.P.G.; de Boer, L.; Riool, M.; Drijfhout, J.W.; Zaat, S.A.J. Synergistic microbicidal effect of cationic antimicrobial peptides and teicoplanin against planktonic and biofilm-encased Staphylococcus aureus. Int. J. Antimicrob. Agents 2019, 53, 143–151. [Google Scholar] [CrossRef]
- Dijksteel, G.S.; Ulrich, M.M.W.; Vlig, M.; Nibbering, P.H.; Cordfunke, R.A.; Drijfhout, J.W.; Middelkoop, E.; Boekema, B.K.H.L. Potential factors contributing to the poor antimicrobial efficacy of SAAP-148 in a rat wound infection model. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 38. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Liu, Y.; Pengfei, S.; Yong, W.U. Antibacterial effects of small molecule antidiabetic agent Halicin against Staphylococcus aureus. Chin. J. Lab. Med. 2021, 44, 1029–1034. [Google Scholar] [CrossRef]
- Higashihira, S.; Simpson, S.J.; Collier, C.D.; Natoli, R.M.; Kittaka, M.; Greenfield, E.M. Halicin is effective against Staphylococcus aureus biofilms in vitro. Clin. Orthop. Relat. Res. 2022, 480, 1476–1487. [Google Scholar] [CrossRef]
- Hussain, Z.; Pengfei, S.; Yimin, L.; Shasha, L.; Zehao, L.; Yifan, Y.; Linhui, L.; Linying, Z.; Yong, W. Study on antibacterial effect of halicin (SU3327) against Enterococcus faecalis and Enterococcus faecium. Pathog. Dis. 2022, 80, ftac037. [Google Scholar] [CrossRef]
- Van Gent, M.E.; Schonkeren-Ravensbergen, B.; Achkif, A.; Beentjes, D.; Dolezal, N.; Van Meijgaarden, K.E.; Drijfhout, J.W.; Nibbering, P.H. C-terminal PEGylation improves SAAP-148 peptide’s immunomodulatory activities. J. Innate Immun. 2023, 15, 724–738. [Google Scholar] [CrossRef]
- van Gent, M.E.; Klodzinska, S.N.; Wouter Drijfhout, J.; Nielsen, H.M.; Nibbering, P.H. Encapsulation in oleyl-modified hyaluronic acid nanogels substantially improves the clinical potential of the antimicrobial peptides SAAP-148 and Ab-Cath. Eur. J. Pharm. Biopharm. 2023, 193, 254–261. [Google Scholar] [CrossRef]
- Ali, M.; van Gent, M.E.; de Waal, A.M.; van Doodewaerd, B.R.; Bos, E.; Koning, R.I.; Cordfunke, R.A.; Drijfhout, J.W.; Nibbering, P.H. Physical and functional characterization of PLGA nanoparticles containing the antimicrobial peptide SAAP-148. Int. J. Mol. Sci. 2023, 24, 2867. [Google Scholar] [CrossRef]
- Schmitz, M.G.J.; Riool, M.; de Boer, L.; Vrehen, A.F.; Bartels, P.A.A.; Zaat, S.A.J.; Dankers, P.Y.W. Development of an antimicrobial peptide SAAP-148-functionalized supramolecular coating on Titanium to prevent biomaterial-associated infections. Adv. Mater. Technol. 2023, 8, 2201846. [Google Scholar] [CrossRef]
- Ön, A.; Vejzovic, D.; Jennings, J.; Parigger, L.; Cordfunke, R.A.; Drijfhout, J.W.; Lohner, K.; Malanovic, N. Bactericidal activity to Escherichia coli: Different modes of action of two 24-Mer peptides SAAP-148 and OP-145, both derived from human Cathelicidine LL-37. Antibiotics 2023, 12, 1163. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Buetti, N.; Lo Priore, E.; Atkinson, A.; Widmer, A.F.; Kronenberg, A.; Marschall, J.; Swiss Centre for Antibiotic Resistance (ANRESIS). Catheter-related infections: Does the spectrum of microbial causes change over time? A nationwide surveillance study. BMJ Open 2018, 8, e023824. [Google Scholar] [CrossRef]
- Namiganda, V.; Mina, Y.; Meklat, A.; Touati, D.; Bouras, N.; Barakate, M.; Sabaou, N. Antibiotic Resistance Pattern of Acinetobacter baumannii Strains Isolated from Different Clinical Specimens and Their Sensibility Against Bioactive Molecules Produced by Actinobacteria. Arab. J. Sci. Eng. 2019, 44, 6267–6275. [Google Scholar] [CrossRef]
- Benamrouche, N.; Lafer, O.; Benmahdi, L.; Benslimani, A.; Amhis, W.; Houria Ammari, H.; Assaous, F.; Azzam, A.; Rahal, K.; Tali Maamar, H.J. Phenotypic and genotypic characterization of multidrug-resistant Acinetobacter baumannii isolated in Algerian hospitals. Infect. Dev. Ctries 2020, 14, 1395–1401. [Google Scholar] [CrossRef]
- Benzaid, C.; Tichati, L.; Rouabhia, M.; Akil Dahdouh, S. Prevalence of microbial nosocomial infections in the resuscitation unit of the University Hospital of Annaba-Algeria. Ann. Biol. Clin. 2022, 80, 527–536. [Google Scholar] [CrossRef]
- Harzallah, B.; Grama, B.S.; Benabdelmalek, A.; Mekhloufi, I. Incidence of nosocomial infections in reanimation unit at the hospital of Constantine (Algeria). South Asian J. Exp. Biol. 2022, 12, 557–566. [Google Scholar] [CrossRef]
- Boulesnam, S.L.; Hamaidi-Chergui, F.; Benamara, M.; Azrou, S. Phenotypical Comparison between Environmental and Clinical Acinetobacter baumannii Strains Isolated from an Intensive Care Unit. Malays. J. Med. Sci. 2023, 30, 85–93. [Google Scholar] [CrossRef]
- Strasheim, W.; Kock, M.M.; Ueckermann, V.; Hoosien, E.; Dreyer, A.W.; Ehlers, M.M. Surveillance of catheter-related infections: The supplementary role of the microbiology laboratory. BMC Infect. Dis. 2015, 15, 5. [Google Scholar] [CrossRef][Green Version]
- Navab-Daneshmand, T.; Friedrich, M.N.D.; Gächter, M.; Montealegre, M.C.; Mlambo, L.S.; Nhiwatiwa, T.; Mosler, H.J.; Julian, T.R. Escherichia coli Contamination across Multiple Environmental Compartments (Soil, Hands, Drinking Water, and Handwashing Water) in Urban Harare: Correlations and Risk Factors. Am. J. Trop. Med. Hyg. 2018, 98, 803–813. [Google Scholar] [CrossRef]
- Daga, A.P.; Koga, V.L.; Soncini, J.G.M.; de Matos, C.M.; Perugini, M.R.E.; Pelisson, M.; Kobayashi, R.K.T.; Vespero, E.C. Escherichia coli Bloodstream Infections in Patients at a University Hospital: Virulence Factors and Clinical Characteristics. Front. Cell. Infect. Microbiol. 2019, 9, 191. [Google Scholar] [CrossRef]
- Barbadoro, P.; Labricciosa, F.M.; Recanatini, C.; Gori, G.; Tirabassi, F.; Martini, E.; Gioia, M.G.; D’Errico, M.M.; Prospero, E. Catheter-associated urinary tract infection: Role of the setting of catheter insertion. Am. J. Infect. Control 2015, 43, 707–710. [Google Scholar] [CrossRef]
- Weiner, L.; Webb, A.; Limbago, B.; Dudeck, M.; Patel, J.; Kallen, A.; Edwards, J.R.; Sievert, D. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016, 37, 1288–1301. [Google Scholar] [CrossRef]
- Dybowski, B.A.; Zapała, P.; Bres-Niewada, E.Z.L.; Miązek-Zapała, N.; Poletajew, S.; Młynarczyk, G.; Radziszewski, P. Catheter-associated bacterial flora in patients with benign prostatic hyperplasia: Shift in antimicrobial susceptibility pattern. BMC Infect. Dis. 2018, 18, 590. [Google Scholar] [CrossRef]
- Peng, D.; Li, X.; Liu, P.; Luo, M.; Chen, S.; Su, K.; Zhang, Z.; He, Q.; Qiu, J.; Li, Y. Epidemiology of pathogens and antimicrobial resistance of catheter-associated urinary tract infections in intensive care units: A systematic review and meta-analysis. Am. J. Infect. Control 2018, 46, e81–e90. [Google Scholar] [CrossRef]
- Aouf, A.; Gueddi, T.; Djeghout, B.; Ammari, H. Frequency and susceptibility pattern of uropathogenic Enterobacteriaceae isolated from patients in Algiers, Algeria. J. Infect. Dev. Ctries 2018, 12, 244–249. [Google Scholar] [CrossRef]
- Nabti, L.Z.; Sahli, F.; Radji, N.; Mezaghcha, W.; Semara, L.; Aberkane, S.; Lounnas, M.; Solassol, J.; Didelot, M.N.; Jean-Pierre, H.; et al. High prevalence of multidrug-resistant Escherichia coli in urine samples from inpatients and outpatients at a tertiary care Hospital in Setif, Algeria. Microb. Drug. Resist. 2019, 25, 386–393. [Google Scholar] [CrossRef]
- Ait-Mimoune, N.; Hassaine, H.; Boulanoir, M. Bacteriological profile of urinary tract infections and antibiotic susceptibility of Escherichia coli in Algeria. Iran. J. Microbiol. 2022, 14, 156–160. [Google Scholar] [CrossRef]
- Flores-Mireles, A.; Hreha, T.N.; Hunstad, D.A. Pathophysiology, treatment and prevention of catheter-associated urinary tract infection. Top. Spinal. Cord. Inj. Rehabil. 2019, 25, 228–240. [Google Scholar] [CrossRef]
- Alcántar-Curiel, M.D.; Ledezma-Escalante, C.A.; Jarillo-Quijada, M.A.; Gayosso-Vázquez, C.; Morfín-Otero, R.; Rodríguez-Noriega, E.; Cedillo-Ramírez, M.L.; Santos-Preciado, J.I.; Girón, J.A. Association of antibiotic resistance, cell adherence, and biofilm production with the endemicity of nosocomial Klebsiella pneumoniae. BioMed. Res. Int. 2018, 23, 7012958. [Google Scholar] [CrossRef]
- Ramos-Vivas, J.; Chapartegui-González, I.; Fernández-Martínez, M.; González-Rico, C.; Fortún, J.; Escudero, R.; Marco, F.; Linares, L.; Montejo, M.; Aranzamendi, M.; et al. Biofilm formation by multidrug resistant Enterobacteriaceae strains isolated from solid organ transplant recipients. Sci. Rep. 2019, 9, 8928. [Google Scholar] [CrossRef]
- Lin, M.F.; Lin, Y.Y.; Lan, C.Y. Characterization of biofilm production in different strains of Acinetobacter baumannii and the effects of chemical compounds on biofilm formation. PeerJ 2020, 8, e9020. [Google Scholar] [CrossRef]
- Pontes, C.; Alves, M.; Santos, C.; Ribeiro, M.H.; Gonçalves, L.; Bettencourt, A.F.; Ribeiro, I.A.C. Can Sophorolipids prevent biofilm formation on silicone catheter tubes? Int. J. Pharm. 2016, 513, 697–708. [Google Scholar] [CrossRef]
- Azam, M.W.; Zuberi, A.; Khan, A.U. bolA gene involved in curli amyloids and fimbriae production in E. coli: Exploring pathways to inhibit biofilm and amyloid formation. J. Biol. Res. 2020, 27, 10. [Google Scholar] [CrossRef]
- Colquhoun, J.M.; Rather, P.N. Insights into mechanisms of biofilm formation in Acinetobacter baumannii and implications for Uropathogenesis. Front. Cell. Infect. Microbiol. 2020, 10, 253. [Google Scholar] [CrossRef]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef]
- Pompilio, A.; Scribano, D.; Sarshar, M.; Di Bonaventura, G.; Palamara, A.T.; Ambrosi, C. Gram-negative bacteria holding together in a biofilm: The Acinetobacter baumannii way. Microorganisms 2021, 9, 1353. [Google Scholar] [CrossRef]
- Ferreira, R.L.; da Silva, B.; Rezende, G.S.; Nakamura-Silva, R.; Pitondo-Silva, A.; Campanini, E.B.; Brito, M.; da Silva, E.; Freire, C.; da Cunha, A.F.; et al. High prevalence of multidrug-resistant Klebsiella pneumoniae harboring several virulence and β-Lactamase encoding genes in a Brazilian intensive care unit. Front. Microbiol. 2019, 9, 3198. [Google Scholar] [CrossRef]
- Ayad, A.; Drissi, M.; de Curraize, C.; Dupont, C.; Hartmann, A.; Solanas, S.; Siebor, E.; Amoureux, L.; Neuwirth, C. Occurence of ArmA and RmtB aminoglycoside resistance 16S rRNA methylases in extended-spectrum β-Lactamases producing Escherichia coli in Algerian Hospitals. Front. Microbiol. 2016, 7, 1409. [Google Scholar] [CrossRef][Green Version]
- Bourafa, N.; Chaalal, W.; Bakour, S.; Lalaoui, R.; Boutefnouchet, N.; Diene, S.M.; Rolain, J.M. Molecular characterization of carbapenem-resistant Gram-negative bacilli clinical isolates in Algeria. Infect. Drug Resist. 2018, 11, 735–742. [Google Scholar] [CrossRef]
- Zenati, F.; Barguigua, A.; Nayme, K.; Benbelaïd, F.; Khadir, A.; Bellahsene, C.; Bendahou, M.; Hassaine, H.; Timinouni, M. Characterization of uropathogenic ESBL-producing Escherichia coli isolated from hospitalized patients in western Algeria. J. Infect. Dev. Ctries 2019, 13, 291–302. [Google Scholar] [CrossRef]
- Shenkutie, A.M.; Yao, M.Z.; Siu, G.K.; Wong, B.; Leung, P.H. Biofilm-Induced Antibiotic Resistance in Clinical Acinetobacter baumannii Isolates. Antibiotics 2020, 9, 817. [Google Scholar] [CrossRef]
- Olivares, E.; Badel-Berchoux, S.; Provot, C.; Prévost, G.; Bernardi, T.; Jehl, F. Clinical Impact of Antibiotics for the Treatment of Pseudomonas aeruginosa Biofilm Infections. Front. Microbiol. 2020, 10, 2894. [Google Scholar] [CrossRef]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Derived Depolymerases against Bacterial Biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef]
- Zhao, A.; Sun, J.; Liu, Y. Understanding bacterial biofilms: From definition to treatment strategies. Front. Cell. Infect. Microbiol. 2023, 13, 1137947. [Google Scholar] [CrossRef]
- Alves, M.J.; Barreira, J.C.; Carvalho, I.; Trinta, L.; Perreira, L.; Ferreira, I.C.F.R.; Pintado, M. Propensity for biofilm formation by clinical isolates from urinary tract infections: Developing a multifactorial predictive model to improve antibiotherapy. J. Med. Microbiol. 2014, 63, 471–477. [Google Scholar] [CrossRef]
- Poursina, F.; Sepehrpour, S.; Mobasherizadeh, S. Biofilm Formation in Nonmultidrug-resistant Escherichia coli Isolated from Patients with Urinary Tract Infection in Isfahan, Iran. Adv. Biomed. Res. 2018, 7, 40. [Google Scholar] [CrossRef]
- Osthoff, M.; McGuinness, S.L.; Wagen, A.Z.; Eisen, D.P. Urinary tract infections due to extended-spectrum beta-lactamase-producing Gram-negative bacteria: Identification of risk factors and outcome predictors in an Australian tertiary referral hospital. Int. J. Infect. Dis. 2015, 34, 79–83. [Google Scholar] [CrossRef]
- Ranjbar, R.; Fatahian Kelishadrokhi, A.; Chehelgerdi, M. Molecular characterization, serotypes and phenotypic and genotypic evaluation of antibiotic resistance of the Klebsiella pneumoniae strains isolated from different types of hospital-acquired infections. Infect. Drug Resist. 2019, 12, 603–611. [Google Scholar] [CrossRef]
- Caneiras, C.; Lito, L.; Melo-Cristino, J.; Duarte, A. Community- and hospital-acquired Klebsiella pneumoniae urinary tract infections in Portugal: Virulence and antibiotic resistance. Microorganisms 2019, 7, 138. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Caffrey, A.R.; Daffinee, K.E.; Luther, M.K.; Lopes, V.; LaPlante, K.L. Weak biofilm formation among carbapenem-resistant Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2019, 95, 114877. [Google Scholar] [CrossRef]
- Khorsi, K.; Messai, Y.; Hamidi, M.; Ammari, H.; Bakour, R. High prevalence of multidrug-resistance in Acinetobacter baumannii and dissemination of carbapenemase-encoding genes blaOXA-23-like, blaOXA-24-like and blaNDM-1 in Algiers hospitals. Asian Pac. J. Trop. Med. 2015, 8, 438–446. [Google Scholar] [CrossRef]
- Bakour, S.; Olaitan, A.O.; Ammari, H.; Touati, A.; Saoudi, S.; Saoudi, K.; Rolain, J.M. Emergence of Colistin- and Carbapenem-resistant Acinetobacter baumannii ST2 clinical isolate in Algeria: First Case Report. Microb. Drug Resist. 2015, 21, 279–285. [Google Scholar] [CrossRef]
- de Breij, A.; Riool, M.; Kwakman, P.H.; de Boer, L.; Cordfunke, R.A.; Drijfhout, J.W.; Cohen, O.; Emanuel, N.; Zaat, S.A.; Nibbering, P.H.; et al. Prevention of Staphylococcus aureus biomaterial-associated infections using a polymer-lipid coating containing the antimicrobial peptide OP-145. J. Control. Release 2016, 222, 1–8. [Google Scholar] [CrossRef]
- Nussbaumer-Pröll, A.; Zeitlinger, M. Use of Supplemented or Human Material to Simulate PD Behavior of Antibiotics at the Target Site In vitro. Pharmaceutics 2020, 12, 773. [Google Scholar] [CrossRef]
- Wu, K.C.; Hua, K.F.; Yu, Y.H.; Cheng, Y.H.; Cheng, T.T.; Huang, Y.K.; Chang, H.W.; Chen, W.J. Antibacterial and Antibiofilm Activities of Novel Antimicrobial Peptides against Multidrug-Resistant Enterotoxigenic Escherichia Coli. Int. J. Mol. Sci. 2021, 22, 3926. [Google Scholar] [CrossRef]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef]
- Soares, A.; Roussel, V.; Pestel-Caron, M.; Barreau, M.; Caron, F.; Bouffartigues, E.; Chevalier, S.; Etienne, M. Understanding Ciprofloxacin Failure in Pseudomonas aeruginosa Biofilm: Persister Cells Survive Matrix Disruption. Front. Microbiol. 2019, 10, 2603. [Google Scholar] [CrossRef]
- Wang, L.; Di Luca, M.; Tkhilaishvili, T.; Trampuz, A.; Gonzalez Moreno, M. Synergistic Activity of Fosfomycin, Ciprofloxacin, and Gentamicin against Escherichia coli and Pseudomonas aeruginosa Biofilms. Front. Microbiol. 2019, 10, 2522. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial biofilm and its role in the pathogenesis of disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Bi, Y.; Xia, G.; Shi, C.; Wan, J.; Liu, L.; Chen, Y.; Wu, Y.; Zhang, W.; Zhou, M.; He, H.; et al. Therapeutic strategies against bacterial biofilms. Fundam. Res. 2021, 1, 193–212. [Google Scholar] [CrossRef]
- Jang, M.; Kim, J.; Choi, Y.; Bang, J.; Kim, Y. Antiseptic Effect of Ps-K18: Mechanism of Its Antibacterial and Anti-Inflammatory Activities. Int. J. Mol. Sci. 2019, 20, 4895. [Google Scholar] [CrossRef]
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880. [Google Scholar] [CrossRef]
- Heinbockel, L.; Weindl, G.; Martinez-de-Tejada, G.; Correa, W.; Sanchez-Gomez, S.; Bárcena-Varela, S.; Goldmann, T.; Garidel, P.; Gutsmann, T.; Brandenburg, K. Inhibition of Lipopolysaccharide- and Lipoprotein-Induced Inflammation by Antitoxin Peptide Pep19-2.5. Front. Immunol. 2018, 9, 1704. [Google Scholar] [CrossRef]
- Ridyard, K.E.; Overhage, J. The Potential of Human Peptide LL-37 as an Antimicrobial and Anti-Biofilm Agent. Antibiotics 2021, 10, 650. [Google Scholar] [CrossRef]
- Krishnan, M.; Choi, J.; Choi, S.; Kim, Y. Anti-Endotoxin 9-Meric Peptide with Therapeutic Potential for the Treatment of Endotoxemia. J. Microbiol. Biotechnol. 2021, 31, 25–32. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Ebbensgaard, A.; Mordhorst, H.; Overgaard, M.T.; Aarestrup, F.M.; Hansen, E.B. Dissection of the antimicrobial and hemolytic activity of Cap18: Generation of Cap18 derivatives with enhanced specificity. PLoS ONE 2018, 13, e0197742. [Google Scholar] [CrossRef]
- Guo, X.; Yan, T.; Rao, J.; Yue, X.; Pei, X.; Deng, J.; Sun, W.; Yang, W.; Zhang, B.; Xie, J. Potent Antimicrobial and Antibiofilm Activities of Feleucin-K3 Analogs Modified by α-(4-Pentenyl)-Ala against Multidrug-Resistant Bacteria. Biomolecules 2021, 11, 761. [Google Scholar] [CrossRef]
- Oddo, A.; Hansen, P.R. Hemolytic Activity of Antimicrobial Peptides. Methods Mol. Biol. 2017, 1548, 427–435. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.; Costa, R.C.; Cordeiro, J.M.; Nagay, B.E.; de Almeida, A.B.; Retamal-Valdes, B.; Nociti, F.H.; Feres, M.; Rangel, E.C.; et al. Targeting Pathogenic Biofilms: Newly Developed Superhydrophobic Coating Favors a Host-Compatible Microbial Profile on the Titanium Surface. ACS App. Mater. Interfaces 2020, 12, 10118–10129. [Google Scholar] [CrossRef] [PubMed]
- Piller, P.; Wolinski, H.; Cordfunke, R.A.; Drijfhout, J.W.; Keller, S.; Lohner, K.; Malanovic, N. Membrane Activity of LL-37 Derived Antimicrobial Peptides against Enterococcus hirae: Superiority of SAAP-148 over OP-145. Biomolecules 2022, 12, 523. [Google Scholar] [CrossRef] [PubMed]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Kim, Y.G.; Lee, J.H.; Lee, S.K.; Chae, J.D.; Son, B.K.; Seo, C.H.; Park, Y. Synergistic effects and antibiofilm properties of chimeric peptides against multidrug-resistant Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 2014, 58, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente-Nuñez, C.; Reffuveille, F.; Mansour, S.C.; Reckseidler-Zenteno, S.L.; Hernandez, D.; Brackman, G.; Coenye, T.; Hancock, R.E.W. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 2015, 22, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Jorge, P.; Grzywacz, D.; Kamysz, W.; Lourenço, A.; Pereira, M.O. Searching for new strategies against biofilm infections: Colistin-AMP combinations against Pseudomonas aeruginosa and Staphylococcus aureus single- and double-species biofilms. PLoS ONE 2017, 12, e0174654. [Google Scholar] [CrossRef] [PubMed]
- Swedan, S.; Shubair, Z.; Almaaytah, A. Synergism of cationic antimicrobial peptide WLBU2 with antibacterial agents against biofilms of multi-drug resistant Acinetobacter baumannii and Klebsiella pneumoniae. Infect. Drug Resist. 2019, 12, 2019–2030. [Google Scholar] [CrossRef]
- Kalsy, M.; Tonk, M.; Hardt, M.; Dobrindt, U.; Zdybicka-Barabas, A.; Cytrynska, M.; Vilcinskas, A.; Mukherjee, K. The insect antimicrobial peptide cecropin A disrupts uropathogenic Escherichia coli biofilms. NPJ Biofilms Microbiomes 2020, 6, 6. [Google Scholar] [CrossRef]
- Duong, L.; Gross, S.P.; Siryaporn, A. Developing antimicrobial synergy with AMPs. Front. Med. Technol. 2021, 3, 640981. [Google Scholar] [CrossRef]
- Van Praagh, A.D.G.; Li, T.; Zhang, S.; Arya, A.; Chen, L.; Zhang, X.X.; Bertolami, S.; Mortin, L.I. Dapptomucin antibiotic lock therapy in a rat model of staphylococcal central venous catheter biofilm interactions. Antimicrob. Agents Chemother. 2011, 55, 4081–4089. [Google Scholar] [CrossRef]
- Signorino, C.; Fusco, E.; Galli, L.; Chiappini, E. Effectiveness of Antimicrobial Lock Therapy for the Treatment of Catheter-Related and Central-Line-Associated Bloodstream Infections in Children: A Single Center Retrospective Study. Antibiotics 2023, 12, 800. [Google Scholar] [CrossRef] [PubMed]
- Riool, M.; de Beij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Antimicrobial peptides in biomedical device manufacturing. Front. Chem. 2017, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Bita, B.; Groza, A. Polymeric Coatings and Antimicrobial Peptides as Efficient Systems for Treating Implantable Medical Devices Associated-Infections. Polymers 2022, 14, 1611. [Google Scholar] [CrossRef] [PubMed]
- Copling, A.; Akantibila, M.; Kumaresan, R.; Fleischer, G.; Cortes, D.; Tripathi, R.S.; Carabetta, V.J.; Vega, S.L. Recent Advances in Antimicrobial Peptide Hydrogels. Int. J. Mol. Sci. 2023, 24, 7563. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.J.; Flores-Mireles, A.L. Urinary Catheter Coating Modifications: The Race against Catheter-Associated Infections. Coatings 2020, 10, 23. [Google Scholar] [CrossRef]
- Gefter Shenderovich, J.; Zaks, B.; Kirmayer, D.; Lavy, E.; Steinberg, D.; Friedman, M. Chlorhexidine sustained-release varnishes for catheter coating—Dissolution kinetics and antibiofilm properties. Eur. J. Pharm. Sci. 2018, 112, 1–7. [Google Scholar] [CrossRef]
- Menezes, F.G.; Correa, L.; Medina-Pestana, J.O.; Aguiar, W.F.; Camargo, L.F.A. A randomized clinical trial comparing Nitrofurazone-coated and uncoated urinary catheters in kidney transplant recipients: Results from a pilot study. Transpl. Infect. Dis. 2019, 21, e13031. [Google Scholar] [CrossRef]
- Srisang, S.; Nasongkla, N. Spray coating of foley urinary catheter by chlorhexidine-loadedpoly(epsilon-caprolactone) nanospheres: Effect of lyoprotectants, characteristics, and antibacterial activity evaluation. Pharm. Dev. Technol. 2019, 24, 402–409. [Google Scholar] [CrossRef]
- Yu, K.; Lo, J.C.; Yan, M.; Yang, X.; Brooks, D.E.; Hancock, R.E.; Lange, D.; Kizhakkedathu, J.N. Anti-adhesive antimicrobial peptide coating prevents catheter associated infection in a mouse urinary infection model. Biomaterials 2017, 116, 69–81. [Google Scholar] [CrossRef]
- Monteiro, C.; Costa, F.; Pirttila, A.M.; Tejesvi, M.V.; Martins, M.C.L. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci. Rep. 2019, 9, 10753. [Google Scholar] [CrossRef]
- Subramanian, S.; Huiszoon, R.C.; Chu, S.; Bentley, W.E.; Ghodssi, R. Microsystems for biofilm characterization and sensing—A review. Biofilm 2020, 2, 100015. [Google Scholar] [CrossRef] [PubMed]
- Brun-Buisson, C.; Abrouk, F.; Legrand, P.; Huet, Y.; Larabi, S.; Rapin, M. Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch. Intern. Med. 1987, 147, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). European Society of Clinical Microbiology and Infectious Diseases.V.2.0.; EUCAST: Paris, France, 2017. [Google Scholar]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011, 47, 2437. [Google Scholar] [CrossRef]
- Hiemstra, H.S.; Duinkerken, G.; Benckhuijsen, W.E.; Amons, R.; de Vries, R.R.P.; Roep, B.O.; Drijfhout, J.W. The identification of CD4+ T cell epitopes with dedicated synthetic peptide libraries. Proc. Natl. Acad. Sci. USA 1997, 94, 10313–10318. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.J.; Tjabringa, G.S.; Wafelman, A.R.; Verrijk, R.; Hiemstra, P.S.; Drijfhout, J.W.; Grote, J.J. Development of novel LL-37 derived antimicrobial peptides with LPS and LTA neutralizing and antimicrobial activities for therapeutic application. Peptides 2006, 27, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Paduszynska, M.A.; Greber, K.E.; Paduszynski, W.; Sawicki, W.; Kamysz, W. Activity of temporin A and short lipopeptides combined with gentamicin against biofilm formed by Staphylococcus aureus and Pseudomonas aeruginosa. Antibiotics 2020, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Compain, F.; Babosan, A.; Brisse, S.; Genel, N.; Audo, J.; Ailloud, F.; Kassis-Chikhani, N.; Arlet, G.; Decré, D. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J. Clin. Microbiol. 2014, 52, 4377–4380. [Google Scholar] [CrossRef]
- Shah, R.K.; Ni, Z.H.; Sun, X.Y.; Wang, G.Q.; Li, F. The Determination and Correlation of Various Virulence Genes, ESBL, Serum Bactericidal Effect and Biofilm Formation of Clinical Isolated Classical Klebsiella pneumoniae and Hypervirulent Klebsiella pneumoniae from Respiratory Tract Infected Patients. Pol. J. Microbiol. 2017, 66, 501–508. [Google Scholar] [CrossRef]
- El Fertas-Aissani, R.; Messai, Y.; Alouache, S.; Bakour, R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol. Biol. 2013, 61, 209–216. [Google Scholar] [CrossRef]
- Cruz-Córdova, A.; Esteban-Kenel, V.; Espinosa-Mazariego, K.; Ochoa, S.A.; Espinosa, S.M.; de la Garza Elhain, A.; Rendón, E.F.; Villegas, E.O.L.; Xicohtencatl-Cortes, J. Pathogenic determinants of clinical Klebsiella pneumoniae strains associated with their persistence in the hospital environment. Bol. Med. Hosp. Infant. Mex. 2014, 71, 1. [Google Scholar]
- Yun, K.W.; Kim, H.Y.; Park, H.K.; Kim, W.; Lim, I.S. Virulence factors of uropathogenic Escherichia coli of urinary tract infections and asymptomatic bacteriuria in children. J. Microbiol. Immunol. Infect. 2014, 47, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Singh, S. PCR based detection of adhesive curli gene “crl” and ‘csgA’ in avian pathogenic Escherichia coli. Indian J. Anim. Res. 2007, 41, 226–229. [Google Scholar]
- Moulin-Schouleur, M.; Répérant, M.; Laurent, S.; Brée, A.; Mignon-Grasteau, S.; Germon, P.; Rasschaert, D.; Schouler, C. Extraintestinal pathogenic Escherichia coli strains of avian and human origin: Link between phylogenetic relationships and common virulence patterns. J. Clin. Microbiol. 2007, 45, 3366–3376. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wu, Y.Q.; Chen, L.P.; Gao, X.; Huang, H.N.; Qiu, F.L.; Wu, D.C. Biofilm-Related Genes: Analyses in Multi-Antibiotic Resistant Acinetobacter Baumannii Isolates from Mainland China. Med. Sci. Monit. 2016, 22, 1801–1807. [Google Scholar] [CrossRef]
- Fallah, A.; Rezaee, M.A.; Hasani, A.; Barhaghi, M.H.S.; Kafil, H.S. Frequency of bap and cpaA virulence genes in drug resistant clinical isolates of Acinetobacter baumannii and their role in biofilm formation. Iran. J. Basic. Med. Sci. 2017, 20, 849–855. [Google Scholar] [CrossRef]
| Identity | Origin | * Resistance Profile | ** Biofilm Mass (OD Values) | *** Classification [52] | **** Virulence Profile |
|---|---|---|---|---|---|
| Klebsiella pneumoniae KP1 | URC | AMCR; FEPR; TETR; GENR | 0.292 ± 0.041 ⸸⸸ | Strongly adherent | mrkD+; fimH+; ycfM+; ecpA+ |
| Klebsiella pneumoniae KP2 | URC | AMCR; CTXR; CAZR; FEPR; TETR; GENI | 0.254 ± 0.022 ⸸⸸ | Strongly adherent | mrkD+ fimH+; ycfM+; ecpA+ |
| Klebsiella pneumoniae KP3 | URC | AMCR; CTXR; CAZR; FEPR; TETR; GENR | 0.121 ± 0.031 ⸸⸸ | Strongly adherent | mrkD+; fimH+; ycfM+; ecpA+ |
| Klebsiella pneumoniae KP4 | URC | AMCR; CTXR; CAZR; FEPR; TETR | 0.187 ± 0.011 ⸸⸸ | Strongly adherent | mrkD+; fimH+; ycfM+; ecpA+ |
| Klebsiella pneumoniae KP5 | URC | AMCR; CTXR; CAZR; FEPR; TETR; CIPI; GENI | 0.074 ± 0.031 ⸸ | Moderately adherent | mrkD+; fimH+; ycfM+; ecpA+ |
| Acinetobacter baumannii AB1 | IVC | CTXR; CAZR; FEPR; IPMR; TETR; CIPR; CO-TRIR | 0.242 ± 0.020 ⸸⸸ | Strongly adherent | csuE+; ompA+; bap+ |
| Acinetobacter baumannii AB2 | IVC | CTXR; CAZR; FEPR; IPMR; TETR; CIPR; CO-TRIR | 0.088 ± 0.013 ⸸⸸ | Moderately adherent | csuE+; ompA+; bap+ |
| Acinetobacter baumannii AB3 | IVC | CTXR; CAZR; FEPR; IPMR; TETR; CIPR; CO-TRIR | 0.057 ± 0.025 ⸸ | Weakly adherent | csuE+; ompA+; bap+ |
| Acinetobacter lwoffii AL1 | IVC | FEPR; IPMR; TETR; CIPR; CO-TRIR | 0.349 ± 0.048 ⸸⸸ | Strongly adherent | csuE₋; ompA+; bap₋ |
| Acinetobacter baumannii AB4 | IVC | CTXR; CAZR; FEPR; IPMR; TETR; CIPR; CO-TRIR | 0.059 ± 0.159 ⸸ | Weakly adherent | csuE+; ompA+; bap₋ |
| Acinetobacter baumannii AB5 | IVC | CTXR; CAZR; FEPR; IPMR; TETR; CIPR; CO-TRIR | 0.021 ± 0.013 | Non-adherent | csuE+; ompA+; bap+ |
| Escherichia coli EC1 | IVC | TETR; NAR | 0.03 ± 0.013 | Non-adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC2 | URC | TETR; GENI; NAR | 0.376 ± 0.022 ⸸⸸ | Strongly adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC3 | IVC | Sensitive to all antibiotics | 0.028 ± 0.01 | Non-adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC4 | URC | NAR | 0.101 ± 0.019 ⸸⸸ | Moderately adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC5 | URC | CIPI; NAR | 0.259 ± 0.007 ⸸⸸ | Strongly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC6 | IVC | NAR | 0.261 ± 0.032 ⸸⸸ | Strongly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC7 | IVC | NAR | 0249 ± 0.032 ⸸⸸ | Strongly adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC8 | IVC | TETI; NAR | 0.292 ± 0.024 ⸸⸸ | Strongly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC9 | IVC | TETR; NAR | 0.222 ± 0.012 ⸸⸸ | Strongly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC10 | IVC | AMCR; NAR | 0.284 ± 0.018 ⸸⸸ | Strongly adherent | fimH+; hlyF-; csgA+ |
| Escherichia coli EC11 | IVC | AMCR; CO-TRIR | 0.027 ± 0.011 | Non-adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC12 | URC | NAR | 0.068 ± 0.022 ⸸ | Moderately adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC13 | IVC | CIPI | 0.243 ± 0.015 ⸸⸸ | Strongly adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC14 | IVC | AMCR; CO-TRIR | 0.051 ± 0.007 ⸸ | Weakly adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC15 | IVC | AMCR; TETR | 0.044 ± 0.019 ns | Weakly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC16 | IVC | TETR; NAR | 0.094 ± 0.008 ⸸⸸ | Moderately adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC17 | IVC | TETR; NAR | 0.141 ± 0.017 ⸸⸸ | Strongly adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC18 | IVC | CIPI; NAR | 0.096 ± 0.011 ⸸⸸ | Moderately adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC19 | IVC | TETR; NAR | 0.047 ± 0.034 ns | Weakly adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC20 | IVC | CIPI; NAR | 0.131 ± 0.032 ⸸⸸ | Strongly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC21 | IVC | TETR; NAR | 0.04 ± 0.023 ns | Weakly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC22 | IVC | AMCR; NAR | 0.122 ± 0.003 ⸸⸸ | Strongly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC23 | IVC | CIPI; NAR | 0.105 ± 0.007 ⸸⸸ | Moderately adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC24 | IVC | TETR; NAR | 0.101 ± 0.029 ⸸⸸ | Moderately adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC25 | IVC | TETR; NAR | 0.042 ± 0.015 ns | Weakly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC26 | IVC | AMCR; NAR | 0.039 ± 0.006 ns | Weakly adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC27 | IVC | TETR; NAR | 0.024 ± 0.003 | Non-adherent | fimH+; hlyF+; csgA+ |
| Escherichia coli EC28 | IVC | AMCR; CO-TRIR | 0.023 ± 0.011 | Non-adherent | fimH+; hlyF₋; csgA+ |
| Escherichia coli EC29 | IVC | Sensitive to all antibiotics | 0.052 ± 0.008 ⸸ | Weakly adherent | fimH+; hlyF₋; csgA₋ |
| Strains | Antibiotic Resistances | LC 99.9% of SAAP-148 (µM) | LC 99.9% of Halicin (µM) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | 24 h | 4 h | 24 h | ||||||||||||||||
| AMC | CTX | CAZ | FEP | FOX | IPM | TET | CIP | GEN | NA | CO-TRI | PBS | 50% Plasma or Urine | PBS | 50% Plasma or Urine | PBS | 50% Plasma or Urine | PBS | 50% Plasma or Urine | |
| E. coli EC2 | 0.8 | 3.2 | 0.8 | 12.8 | 25.6 (12.8–25.6) | 102.4 (51.2–102.4) | 6.4 | 25.6 (12.8–25.6) | |||||||||||
| A. baumannii AB1 | / | 0.8 | 1.6 | 0.8 | 3.2 | 25.6 | >102.4 | 25.6 | >102.4 | ||||||||||
| K. pneumoniae KP1 | 0.8 (0.8–1.6) | 6.4 | 1.6 | 12.8 | 51.2 (25.6–51.2) | 25.6 | 51.2 | 51.2 | |||||||||||
| K. pneumoniae KP2 | 1.6 (0.8–1.6) | 6.4 (3.2–6.4) | 1.6 | 12.8 | 25.6 (12.8–25.6) | 51.2 (25.6–51.2) | 25.6 | 102.4 (51.2–102.4) | |||||||||||
| Strain | MBEC SAAP-148 (µM) Alone | MBEC SAAP-148 (µM) in Combination | MBEC Halicin (µM) Alone | MBEC Halicin (µM) in Combination | ΣFBEC |
|---|---|---|---|---|---|
| E. coli EC2 | 102.4 | 3.2 | 25.6 | 6.4 | 0.28 (Synergistic effect) |
| 3.2 | 12.8 | 0.53 (Additive effect) | |||
| 6.4 | 12.8 | 0.56 (Additive effect) | |||
| 12.8 | 6.4 | 0.38 (Synergistic effect) | |||
| 12.8 | 12.8 | 0.63 (Additive effect) | |||
| 25.6 | 6.4 | 0.5 (Synergistic effect) | |||
| 25.6 | 12.8 | 0.75 (Additive effect) | |||
| A. baumannii AB1 | 102.4 | No effect | 102.4 | No effect | No effect |
| K. pneumoniae KP1 | 51.2 | 12.8 | 102.4 | 12.8 | 0.38 (Synergistic effect) |
| 12.8 | 25.6 | 0.5 (Synergistic effect) | |||
| K. pneumoniae KP2 | 51.2 | 3.2 | 102.4 | 102.4 | 1.06 (Indifferent effect) |
| 12.8 | 12.8 | 0.38 (Synergistic effect) | |||
| 12.8 | 25.6 | 0.5 (Synergistic effect) | |||
| 12.8 | 51.2 | 0.75 (Additive effect) | |||
| 12.8 | 102.4 | 1.25 (Indifferent effect) | |||
| 25.6 | 51.2 | 1 (Additive effect) | |||
| 25.6 | 102.4 | 1.5 (Indifferent effect) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouhrour, N.; van der Reijden, T.J.K.; Voet, M.M.; Schonkeren-Ravensbergen, B.; Cordfunke, R.A.; Drijfhout, J.W.; Bendali, F.; Nibbering, P.H. Novel Antibacterial Agents SAAP-148 and Halicin Combat Gram-Negative Bacteria Colonizing Catheters. Antibiotics 2023, 12, 1743. https://doi.org/10.3390/antibiotics12121743
Bouhrour N, van der Reijden TJK, Voet MM, Schonkeren-Ravensbergen B, Cordfunke RA, Drijfhout JW, Bendali F, Nibbering PH. Novel Antibacterial Agents SAAP-148 and Halicin Combat Gram-Negative Bacteria Colonizing Catheters. Antibiotics. 2023; 12(12):1743. https://doi.org/10.3390/antibiotics12121743
Chicago/Turabian StyleBouhrour, Nesrine, Tanny J. K. van der Reijden, Michella M. Voet, Bep Schonkeren-Ravensbergen, Robert A. Cordfunke, Jan Wouter Drijfhout, Farida Bendali, and Peter H. Nibbering. 2023. "Novel Antibacterial Agents SAAP-148 and Halicin Combat Gram-Negative Bacteria Colonizing Catheters" Antibiotics 12, no. 12: 1743. https://doi.org/10.3390/antibiotics12121743
APA StyleBouhrour, N., van der Reijden, T. J. K., Voet, M. M., Schonkeren-Ravensbergen, B., Cordfunke, R. A., Drijfhout, J. W., Bendali, F., & Nibbering, P. H. (2023). Novel Antibacterial Agents SAAP-148 and Halicin Combat Gram-Negative Bacteria Colonizing Catheters. Antibiotics, 12(12), 1743. https://doi.org/10.3390/antibiotics12121743