Antimicrobial Prophylaxis in Robot-Assisted Laparoscopic Radical Prostatectomy: A Systematic Review
Abstract
:1. Introduction
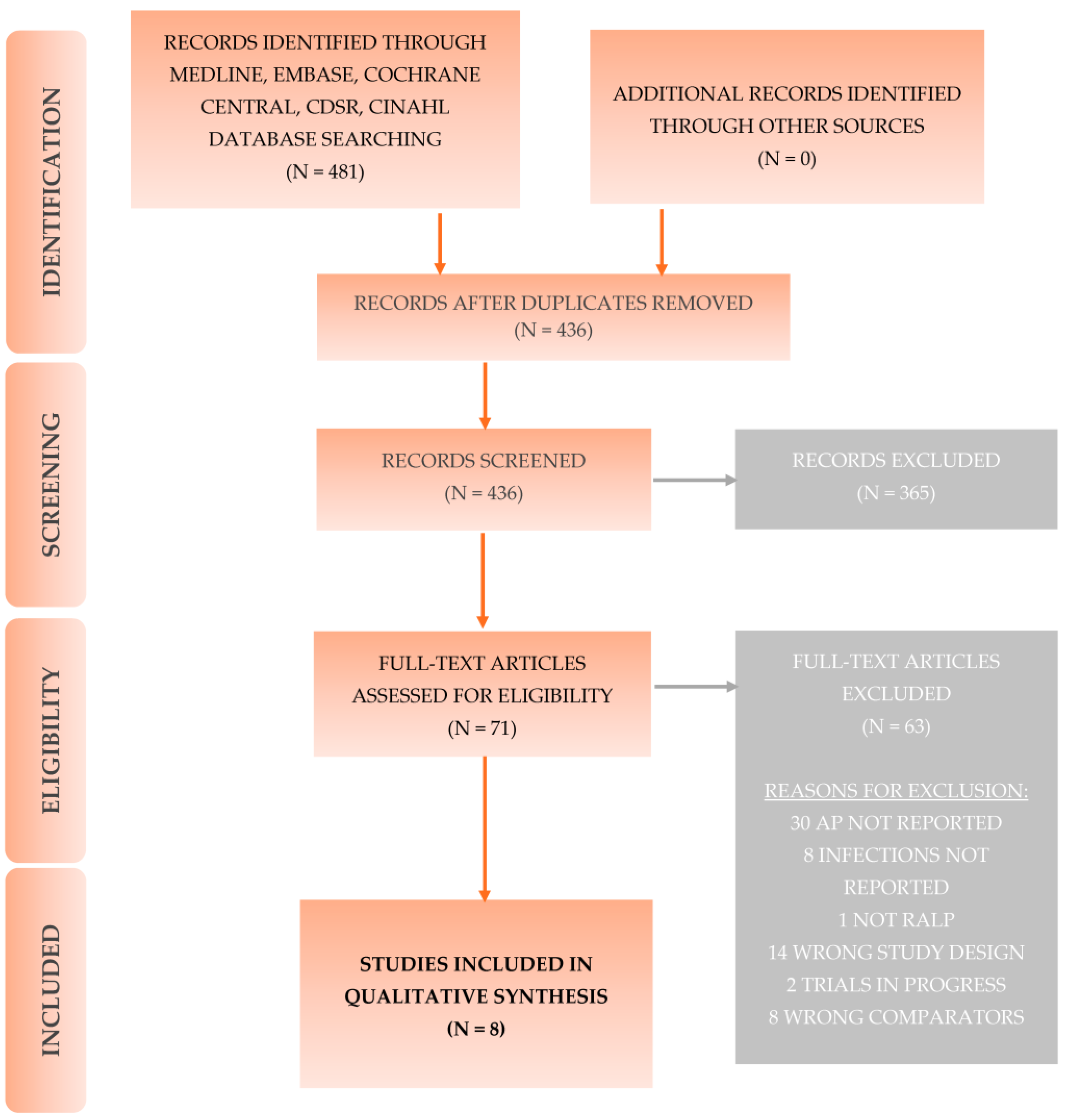
2. Methods
- Definitions Used
- Study Variables
- Literature Search
2.1. Search Strategy
2.2. Study Eligibility
2.3. Selection of Studies
2.4. Data Extraction
2.5. Risk of Bias Assessment
2.6. Assessment of Study Heterogeneity and Data Synthesis
3. Results
- Evidence Base
3.1. Study Design
3.1.1. Comparative Cohort Studies on RALP vs. Open PE
3.1.2. Comparative Cohort Studies on Long-Term AP vs. Single-Dose AP
3.1.3. Case Series with Long-Term AP
3.1.4. Case Series with Short-Term AP
3.1.5. Case Series with Single-Dose AP
3.2. Outcomes
3.2.1. Primary Outcome
3.2.2. Secondary Outcomes
3.2.3. Tertiary Outcomes
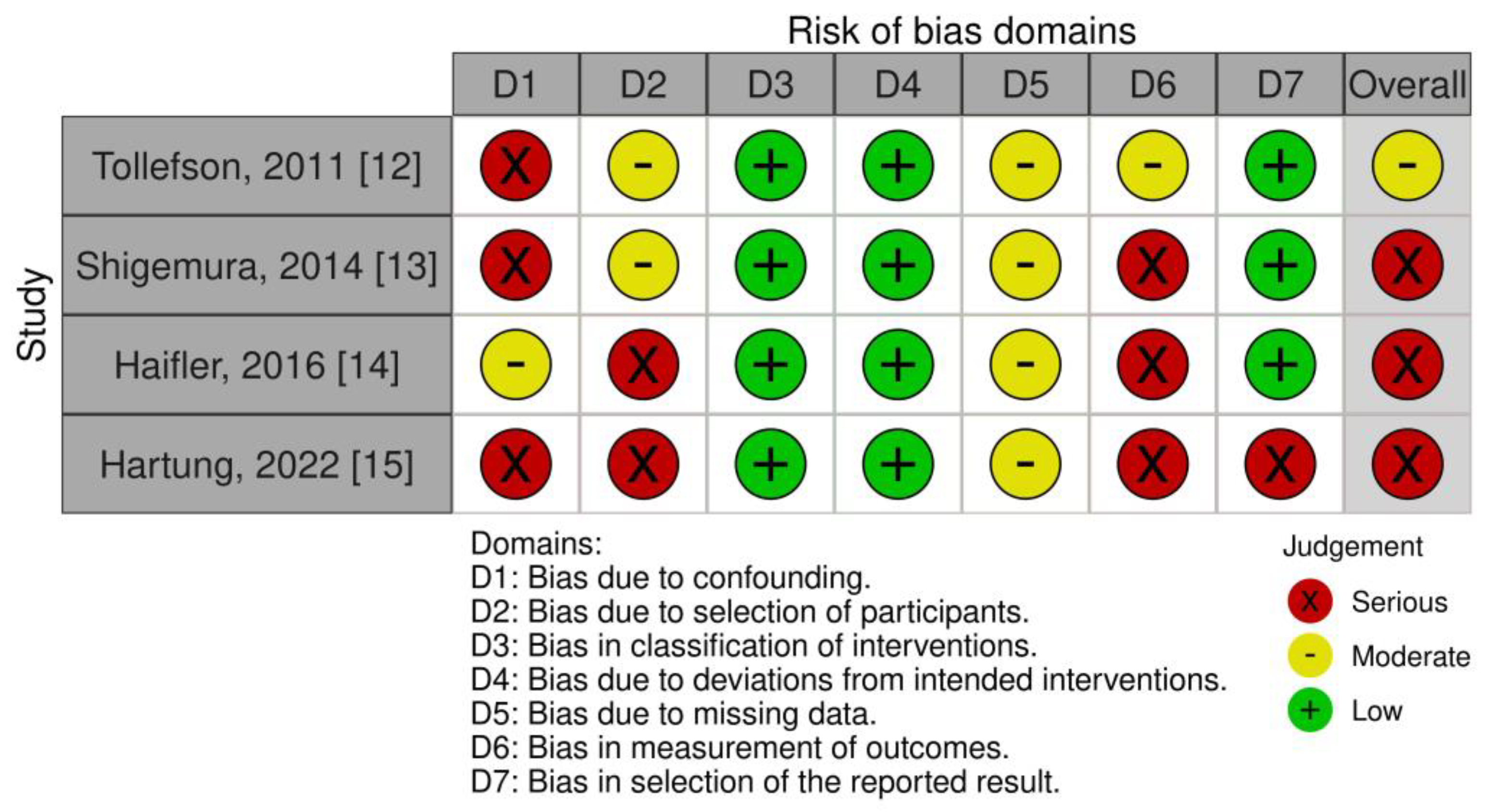
3.3. Risk of Bias Assessment
4. Discussion
4.1. Most Important Findings
4.2. Implications for Clinical Practice
4.3. Understanding the Findings
4.4. Strengths and Weaknesses
4.5. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AP = Antimicrobial prophylaxis/ Antibiotic prophylaxis |
| AUA = American Association of Urology |
| CAUTI = Catheter-associated urinary tract infection |
| CDC = Centers for disease control and prevention |
| CINAHL = Cumulative Index to Nursing and Allied Health Literature |
| Clostridioides difficile = C. difficile |
| CRP = C-reactive protein |
| EAU = European Association of Urology |
| EMBASE = Excerpta Medica dataBASE |
| Escherichia coli = E. coli |
| GPIU = Global Prevalence Study on Infections in Urology |
| iLC = Infected lymphocele |
| JBI = Joanna Briggs InstituteKISS= Krankenhaus-Infektions-Surveillance-System |
| LC = Lymphocele |
| LUT = Lower urinary tract |
| MEDLINE = Medical Literature Analysis and Retrieval System Online |
| NRSI = Non-randomized study of intervention |
| RALP = Robot-assisted laparoscopic radical prostatectomy |
| RCT = Randomized controlled trial |
| ROBINS-I = Risk of Bias in Non-randomised Studies of Interventions |
| PC = Prostate cancer |
| PE = Prostatectomy |
| PLND = Pelvic lymph node dissection |
| PRIMSA = Preferred Reporting Items for Systematic Review and Meta-analysis |
| PROSPERO = International Prospective Register of Systematic Reviews |
| SSI = Surgical site infection |
| UTI = Urinary tract infection |
| WBC = White blood cells. |
References
- Montorsi, F.; Wilson, T.G.; Rosen, R.C.; Ahlering, T.E.; Artibani, W.; Carroll, P.R.; Costello, A.; Eastham, J.A.; Ficarra, V.; Guazzoni, G.; et al. Best Practices in Robot-assisted Radical Prostatectomy: Recommendations of the Pasadena Consensus Panel. Eur. Urol. 2012, 62, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance: Global Report on Surveillance 2014. World Health Organization. 2014. Available online: https://www.who.int/publications/i/item/9789241564748 (accessed on 27 April 2023).
- Bootsma, A.M.; Laguna Pes, M.P.; Geerlings, S.E.; Goossens, A. Antibiotic prophylaxis in urologic procedures: A systematic review. Eur. Urol. 2008, 54, 1270–1286. [Google Scholar] [CrossRef] [PubMed]
- Çek, M.; Tandogdu, Z.; Naber, K.; Tenke, P.; Wagenlehner, F.; van Oostrum, E.; Kristensen, B.; Johansen, T.E.B. Antibiotic prophylaxis in urology departments, 2005–2010. Eur. Urol. 2013, 63, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Mangram, A.J.; Horan, T.C.; Pearson, M.L.; Silver, L.C.; Jarvis, W.R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am. J. Infect. Control 1999, 27, 97–132, quiz 133–134; discussion 96. [Google Scholar] [CrossRef] [PubMed]
- EAU Guidelines. Presented at the EAU Annual Congress, Milan, Italy, 10–13 March 2023; ISBN 978-94-92671-19-6.
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane 2019, 2019, ED000142. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- PROSPERO. Search Prospero; University of York: York, UK, 2023; Available online: https://www.crd.york.ac.uk/PROSPERO/ (accessed on 27 November 2023).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- The Joanna Briggs Institute Critical Appraisal Tools. Checklist for Case Series. JBI. Available online: https://jbi.global/critical-appraisal-tools (accessed on 31 July 2023).
- Tollefson, M.K.; Frank, I.; Gettman, M.T. Robotic-assisted radical prostatectomy decreases the incidence and morbidity of surgical site infections. Urology 2011, 78, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, K.; Tanaka, K.; Yamamichi, F.; Muramaki, M.; Arakawa, S.; Miyake, H.; Fujisawa, M. Comparison of postoperative infection between robotic-assisted laparoscopic prostatectomy and open radical prostatectomy. Urol. Int. 2014, 92, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Haifler, M.; Mor, Y.; Dotan, Z.; Ramon, J.; Zilberman, D.E. Prophylactic antibiotic treatment following laparoscopic robot-assisted radical prostatectomy for the prevention of catheter-associated urinary tract infections: Did the AUA guidelines make a difference? J. Robot. Surg. 2016, 11, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Hartung, F.O.; Herrmann, J.; Kowalewski, K.F.; Neuberger, M.; Weiß, C.; Kriegmair, M.C.; Michel, M.S.; Ritter, M.; Rassweiler-Seyfried, M.C. Perioperative Antibiotic Prophylaxis in Radical Prostatectomy: “Single-Shot“ versus Multiday Regimen. Urol. Int. 2022, 107, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mazzola, B.; Roggero, E.; D’Antonio, E.; Mestre, R.P.; Porcu, G.; Stoffel, F.; Renard, J. Current evidence between hospital volume and perioperative outcome: Prospective assessment of robotic radical prostatectomy safety profile in a regional center of medium annual caseload. Can. Urol. Assoc. J. 2021, 15, E153–E159. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Rhee, J.; Sutherland, D.; Benjamin, C.; Engel, J.; Frazier, H. Surgical complications after robot-assisted laparoscopic radical prostatectomy: The initial 1000 cases stratified by the clavien classification system. J. Endourol. 2012, 26, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Coelho, R.F.; Palmer, K.J.; Rocco, B.; Moniz, R.R.; Chauhan, S.; Orvieto, M.A.; Coughlin, G.; Patel, V.R. Early complication rates in a single-surgeon series of 2500 robotic-assisted radical prostatectomies: Report applying a standardized grading system. Eur. Urol. 2010, 57, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Hamada, A.; Hwang, C.; Fleisher, J.; Tuerk, I. Microbiological evaluation of infected pelvic lymphocele after robotic prostatectomy: Potential predictors for culture positivity and selection of the best empirical antimicrobial therapy. Int. Urol. Nephrol. 2017, 49, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- National Reference Center for Surveillance of Nosocomial Infections (NRZ). Available online: https://www.nrz-hygiene.de/welcome (accessed on 31 July 2023).
- Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals. Available online: https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/PPS-HAI-antimicrobial-use-EU-acute-care-hospitals-V5-3.pdf (accessed on 31 July 2023).
- Cek, M.; Tandogdu, Z.; Wagenlehner, F.; Tenke, P.; Naber, K.; Bjerklund-Johansen, T.E. Healthcare-associated urinary tract infections in hospitalized urological patients- a global perspective: Results from the GPIU studies 2003–2010. World J. Urol. 2014, 32, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.S.; Edwards, J.R.; Bamberg, W.; Beldavs, Z.G.; Dumyati, G.; Kainer, M.A.; Lynfield, R.; Maloney, M.; McAllister-Hollod, L.; Nadle, J.; et al. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014, 370, 1198–1208. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Wagenlehner, F.M. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bjerklund Johansen, T.E.; Bonkat, G.; Cai, T.; Tandogdu, Z.; Wagenlehner, F.; Grabe, M. Grey Zones in the Field of Urinary Tract Infections. Eur. Urol. Focus 2016, 2, 460–462. [Google Scholar] [CrossRef] [PubMed]
| 1st Author, Year of Publication | Study Design/ Timespan | Population | Antibiotic Prophylaxis (AP) | Outcome Parameter | Outcome | Catheter Time, Days Mean ± SD | Remarks |
|---|---|---|---|---|---|---|---|
| Tollefson, 2011 [12] | retrospective cohort study (NRSI) (2004–2008) | 1084 RALP vs. 4824 open PE | Short-term: Cephalexin within 1 h before surgical incision. AP was continued for 24 h after surgery. | SSI in RALP SSI in open PE UTI RALP Sepsis/ Bacteremia RALP (within 30 days) | 6/1084 (0.6%) 216/4824 (4.5%) (p < 0.001) 17/1084 (1.6%) (p = 0.28) 1/1084 (0.1%) (p = 1.0) | not reported | SSI in RALP significantly less frequent and less severe compared to open PE |
| Shigemura 2013, Japan [13] | retrospective cohort study (NRSI) (2008–2012) | 89 RALP (2010–2012) vs. 105 open PE (2008–2012) | Long-term: 3rd generation Cephalosporin or Ampicillin/Sulbactam in RALP. AP started 30 min prior to surgery and continued up to median 3 days. | SSI in RALP SSI in open PE (within 30 days) | 1/89 (1.12%) 6/105 (4.77%) (p = 0.0876) | not reported | Group wanted to prove benefits of RALP regarding infectious complications as compared to open PE |
| Haifler 2016, USA/Israel [14] | retrospective cohort study (NRSI) (2010–2015) | 229 RALP Prolonged AP: 60 RALP Single Shot SP: 169 RALP | Before 11/2011: Long-term AP: 2nd gen. Cephalosporin+ Aminoglycoside within 60 min. of incision followed by oral Fluoroquinolone until removal of catheter. After 11/2011: Single-dose AP: 2nd gen. Cephalosporin+ Aminoglycoside within 30 min. of incision. | CAUTI Prolonged AP Single Shot AP (within 30 days) | 5/60 (8.3%) 15/169 (8.9%) (p = 0.89) | not reported | SS does not increase CAUTI rate compared to prolonged AP. “Within” 30–60 min of incision does not explain if the AP dose was administered pre- or intraoperatively. |
| Hartung 2022, Germany [15] | retrospective cohort study (NRSI) (2014–2015) | Total 376 Group 1: 216 (75% RALP and 25% open PE) Group 2: 160 (82.50% RALP and 17.50% open PE) | Long-term AP: Fluoroquinolone i.v. within 60 min. before incision, oral continued until removal of catheter. Single-dose AP: Ciprofloxacin or Cefuroxime within 60 min. before incision. | Postoperative wound infections and urinary tract infections per group (within 30 days) | Group 1: 21/216 (9.72%) Group 2: 19/160 (11.88%) (p = 0.5) | 8.25 ± 6.44 8.25 ± 6.3 (p = 0.83) | Cohorts are mixed (RALP and open PE) |
| Ferrari 2020, Switzerland [16] | prospective case series (2011–2019) | 317 RALP - 281/317 (88.6%) with PLND | Long-term AP: 3rd gen. i.v. Cephalosporin 30 min. before incision continued by oral Quinolone until postoperative day 7. | Total infectious complications Wound infections Lower urinary tract infection Respiratory tract infection Hyperpyrexia of unknown origin Infected Lymphocele Balanoposthitis (within 90 days) | n = 21/317 (6.6%) n = 2/317 (0.6%) n = 8/317 (2.5%) n = 2/317 (0.6%) n = 5/317 (1.6%) n = 3/317 (0.9%) n = 1 (0.3%) | 6 | “UTI” and “Genital/LUT infections”, not further specified |
| Ahmed 2012, USA [17] | retrospective case series (2004–2009) | 1000 RALP | Short-term AP: Single preoperative i.v. dose followed by two postoperative doses (antibiotic agent not specified). | Total infectious complications Urinary tract infection C. difficile enterocolitis Upper respiratory infection (within 30 days) | 6/1000 (0.6%) 2/1000 (0.2%) 3/1000 (0.3%) 1/1000 (0.1%) | 7–8 | Reports various complications in details but not about a single symptomatic lymphocele |
| Coelho 2010, Brazil [18] | retrospective case series (2002–2009) | 2500 RALP | Single-dose AP: 1st gen. i.v. cephalosporin preoperatively. | Wound infection UTI after catheter removal acute epididymitis (within 30 days) | 14/2500 (0.56%) 4/2500 (0.16%) 1/2500 (0.04%) | median 5 | Complication rate might be underreported, only one very experienced surgeon |
| Hamada 2017, USA [19] | retrospective single arm study | 865 RALPs + PLND (between 2008–2014) | Single-dose AP: 2 g cefazolin or 600 mg clindamycin (penicillin allergy) within 1 h of incision time. | Frequency of wound infection Infected lymphocele (LC) (follow-up longer than 30 days: Median time to diagnosis was 6.8 ± 4.8 weeks) | 4/865 (0.46%) 26/865 (3%) | not reported | - Urinary tract infection not habitat for infected lymphocele. - Within 1 h of incision does not specify if AP dose was administered pre- or intraoperatively. |
| 1st Author, Year | Definition of Infectious Complication | Period of Infective Complication Rate | Author Comments |
|---|---|---|---|
| Tolleffson, 2011 [12] | Superficial and deep SSI: CDC criteria “Postoperative UTIs: Patients experiencing cystitis thought to be secondary to bacteriuria” “Sepsis or bacteremia” | within 30 days postoperatively | Use CDC criteria. Excluded patients without follow-up of at least 30 days. |
| Shigemura, 2013 [13] | Superficial, deep and organ/space SSI: CDC criteria Measurement of inflammatory laboratory parameters: WBC and CRP | within 30 days postoperatively | Use CDC criteria but do not report about a systematic follow-up after hospital discharge. |
| Haifler, 2016 [14] | “CAUTI: Symptomatic cystitis or orchiepididymitis within 30 days following RALP with or without positive urinary culture (i.e., over 10^5 CFU)” | within 30 days postoperatively | According to CDC a UTI is only catheter associated if the device is still in place or has been removed in the past 48 h. |
| Hartung, 2022 [15] | “UTI and wound infection” KISS* surveillance program [16] (German surveillance tool utilizing CDC definitions) | within 30 days postoperatively | Use CDC but did not apply them accordingly (no systematic follow-up carried out). |
| Ferrari, 2020 [16] | “UTI” “Wound infections” “Hyperpyrexia of unknown origin”, “Lymphocele infection” “Balanoposthitis” | up to 90 days postoperatively | Did not use CDC. Do not report about systematic follow-up at day 30. |
| Ahmed, 2012 [17] | “Infectious complications”, “UTI” “C. difficile enterocolitis” “Upper respiratory infection” | within 30 days postoperatively | Did not use CDC. Hospital records reviewed for complications within 30 days. |
| Coelho, 2010 [18] | “Wound infection” “UTI after catheter removal” “Acute epididymitis” | within 30 days postoperatively | Did not use CDC. Patients were contacted or examined 6 weeks postoperatively. |
| Hamada, 2017 [19] | “Infected Lymphocele” “Wound infection” | longer than 30 days | Only patients with symptomatic LC included. |
| JBI Checklist Questions (4) | Ferrari, 2021 [16] | Ahmed, 2012 [17] | Coelho, 2010 [18] | Hamada, 2017 [19] |
|---|---|---|---|---|
| Were there clear criteria for inclusion in the case series? | Yes | Yes | Yes | Yes |
| Was the condition measured in a standard, reliable way for all participants included in the case series? | Unclear | Unclear | Yes | Unclear |
| Were valid methods used for identification of the condition for all participants included in the case series? | Yes | Yes | Yes | Yes |
| Did the case series have consecutive inclusion of participants? | Yes | Yes | Yes | Unclear |
| Did the case series have complete inclusion of participants? | Yes | Yes | Yes | Unclear |
| Was there clear reporting of the demographics of the participants in the study? | Yes | Yes | Yes | Yes |
| Was there clear reporting of clinical information of the participants? | Yes | Yes | Yes | Yes |
| Were the outcomes or follow-up results of cases clearly reported? | No | No | No | Unclear |
| Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | Yes | No | No | Yes |
| Was statistical analysis appropriate? | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falkensammer, E.; Erenler, E.; Johansen, T.E.B.; Tzelves, L.; Schneidewind, L.; Yuan, Y.; Cai, T.; Koves, B.; Tandogdu, Z. Antimicrobial Prophylaxis in Robot-Assisted Laparoscopic Radical Prostatectomy: A Systematic Review. Antibiotics 2023, 12, 1744. https://doi.org/10.3390/antibiotics12121744
Falkensammer E, Erenler E, Johansen TEB, Tzelves L, Schneidewind L, Yuan Y, Cai T, Koves B, Tandogdu Z. Antimicrobial Prophylaxis in Robot-Assisted Laparoscopic Radical Prostatectomy: A Systematic Review. Antibiotics. 2023; 12(12):1744. https://doi.org/10.3390/antibiotics12121744
Chicago/Turabian StyleFalkensammer, Eva, Ece Erenler, Truls E. Bjerklund Johansen, Lazaros Tzelves, Laila Schneidewind, Yuhong Yuan, Tommaso Cai, Bela Koves, and Zafer Tandogdu. 2023. "Antimicrobial Prophylaxis in Robot-Assisted Laparoscopic Radical Prostatectomy: A Systematic Review" Antibiotics 12, no. 12: 1744. https://doi.org/10.3390/antibiotics12121744
APA StyleFalkensammer, E., Erenler, E., Johansen, T. E. B., Tzelves, L., Schneidewind, L., Yuan, Y., Cai, T., Koves, B., & Tandogdu, Z. (2023). Antimicrobial Prophylaxis in Robot-Assisted Laparoscopic Radical Prostatectomy: A Systematic Review. Antibiotics, 12(12), 1744. https://doi.org/10.3390/antibiotics12121744









