Could an Optimized Joint Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin-Tazobactam Be a Valuable Innovative Approach for Maximizing the Effectiveness of Monotherapy Even in the Treatment of Critically Ill Patients with Documented Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Bloodstream Infections and/or Ventilator-Associated Pneumonia?
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design and Inclusion Criteria
4.2. Data Collection and Variables Definition
4.3. Piperacillin-Tazobactam Dosing Regimens, Sampling Procedure, and Procedure for Optimizing PK/PD Target Attainment
4.4. Definition of Optimized Joint PK/PD Target Attainment of Piperacillin-Tazobactam
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaukonen, K.-M.; Bailey, M.; Suzuki, S.; Pilcher, D.; Bellomo, R. Mortality Related to Severe Sepsis and Septic Shock among Critically Ill Patients in Australia and New Zealand, 2000–2012. JAMA 2014, 311, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.C.; van der Poll, T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Sakr, Y.; Singer, M.; Martin-Loeches, I.; Machado, F.R.; Marshall, J.C.; Finfer, S.; Pelosi, P.; Brazzi, L.; Aditianingsih, D.; et al. Prevalence and Outcomes of Infection Among Patients in Intensive Care Units in 2017. JAMA 2020, 323, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.J.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K.; International Forum of Acute Care Trialists Assessment of Global Incidence and Mortality of Hospital-Treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef] [PubMed]
- MacVane, S.H. Antimicrobial Resistance in the Intensive Care Unit: A Focus on Gram-Negative Bacterial Infections. J. Intensive Care Med. 2017, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Alberti, C.; Brun-Buisson, C.; Burchardi, H.; Martin, C.; Goodman, S.; Artigas, A.; Sicignano, A.; Palazzo, M.; Moreno, R.; Boulmé, R.; et al. Epidemiology of Sepsis and Infection in ICU Patients from an International Multicentre Cohort Study. Intensive Care Med. 2002, 28, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-Lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas Aeruginosa with Difficult-to-Treat Resistance (DTR-P. Aeruginosa). Clin. Infect. Dis. 2022, 72, ciac268. [Google Scholar] [CrossRef]
- Paul, M.; Carrara, E.; Retamar, P.; Tängdén, T.; Bitterman, R.; Bonomo, R.A.; de Waele, J.; Daikos, G.L.; Akova, M.; Harbarth, S.; et al. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli (Endorsed by European Society of Intensive Care Medicine). Clin. Microbiol. Infect. 2022, 28, 521–547. [Google Scholar] [CrossRef]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused at Creating Algorithms for Targeted Therapy of BSIs, CUTIs, and CIAIs Caused by Enterobacterales in Critically Ill Adult Patients. Infect. Drug Resist. 2021, 14, 2461–2498. [Google Scholar] [CrossRef]
- Gatti, M.; Viaggi, B.; Rossolini, G.M.; Pea, F.; Viale, P. An Evidence-Based Multidisciplinary Approach Focused on Creating Algorithms for Targeted Therapy of Infection-Related Ventilator-Associated Complications (IVACs) Caused by Pseudomonas Aeruginosa and Acinetobacter Baumannii in Critically Ill Adult Patients. Antibiotics 2021, 11, 33. [Google Scholar] [CrossRef]
- Karaiskos, I.; Giamarellou, H. Carbapenem-Sparing Strategies for ESBL Producers: When and How. Antibiotics 2020, 9, 61. [Google Scholar] [CrossRef]
- Tamma, P.D.; Rodriguez-Bano, J. The Use of Noncarbapenem β-Lactams for the Treatment of Extended-Spectrum β-Lactamase Infections. Clin. Infect. Dis. 2017, 64, 972–980. [Google Scholar] [CrossRef]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Tam, V.H.; Chang, K.-T.; Zhou, J.; Ledesma, K.R.; Phe, K.; Gao, S.; Van Bambeke, F.; Sánchez-Díaz, A.M.; Zamorano, L.; Oliver, A.; et al. Determining β-Lactam Exposure Threshold to Suppress Resistance Development in Gram-Negative Bacteria. J. Antimicrob. Chemother. 2017, 72, 1421–1428. [Google Scholar] [CrossRef]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of Bolus Dosing versus Continuous Infusion of Piperacillin and Tazobactam on the Development of Antimicrobial Resistance in Pseudomonas Aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef]
- Al-Shaer, M.H.; Rubido, E.; Cherabuddi, K.; Venugopalan, V.; Klinker, K.; Peloquin, C. Early Therapeutic Monitoring of β-Lactams and Associated Therapy Outcomes in Critically Ill Patients. J. Antimicrob. Chemother. 2020, 75, 3644–3651. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Alexander, K.M.; Manigaba, K.; Shoulders, B.R.; Felton, T.W.; Mathew, S.K.; Peloquin, C.A. Beta-Lactam Target Attainment and Associated Outcomes in Patients with Bloodstream Infections. Int. J. Antimicrob. Agents 2023, 61, 106727. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Bai, C.; Maguigan, K.L.; Shoulders, B.; Felton, T.W.; Mathew, S.K.; Mardini, M.T.; Peloquin, C.A. Using Machine Learning to Define the Impact of Beta-Lactam Early and Cumulative Target Attainment on Outcomes in Intensive Care Unit Patients with Hospital-Acquired and Ventilator-Associated Pneumonia. Antimicrob. Agents Chemother. 2022, 66, e0056322. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Chua, N.G.; Loo, L.; Hee, D.K.H.; Lim, T.P.; Ng, T.M.; Hoo, G.S.R.; Soong, J.L.; Ong, J.C.L.; Tang, S.S.L.; Zhou, Y.P.; et al. Therapeutic Drug Monitoring of Meropenem and Piperacillin-Tazobactam in the Singapore Critically Ill Population—A Prospective, Multi-Center, Observational Study (BLAST 1). J. Crit. Care 2022, 68, 107–113. [Google Scholar] [CrossRef] [PubMed]
- De Waele, J.J.; Carrette, S.; Carlier, M.; Stove, V.; Boelens, J.; Claeys, G.; Leroux-Roels, I.; Hoste, E.; Depuydt, P.; Decruyenaere, J.; et al. Therapeutic Drug Monitoring-Based Dose Optimisation of Piperacillin and Meropenem: A Randomised Controlled Trial. Intensive Care Med. 2014, 40, 380–387. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert Clinical Pharmacological Advice May Make an Antimicrobial TDM Program for Emerging Candidates More Clinically Useful in Tailoring Therapy of Critically Ill Patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef]
- Hagel, S.; Bach, F.; Brenner, T.; Bracht, H.; Brinkmann, A.; Annecke, T.; Hohn, A.; Weigand, M.; Michels, G.; Kluge, S.; et al. Effect of Therapeutic Drug Monitoring-Based Dose Optimization of Piperacillin/Tazobactam on Sepsis-Related Organ Dysfunction in Patients with Sepsis: A Randomized Controlled Trial. Intensive Care Med. 2022, 48, 311–321. [Google Scholar] [CrossRef]
- Chiriac, U.; Richter, D.C.; Frey, O.R.; Röhr, A.C.; Helbig, S.; Preisenberger, J.; Hagel, S.; Roberts, J.A.; Weigand, M.A.; Brinkmann, A. Personalized Piperacillin Dosing for the Critically Ill: A Retrospective Analysis of Clinical Experience with Dosing Software and Therapeutic Drug Monitoring to Optimize Antimicrobial Dosing. Antibiotics 2021, 10, 667. [Google Scholar] [CrossRef]
- Richter, D.C.; Frey, O.; Röhr, A.; Roberts, J.A.; Köberer, A.; Fuchs, T.; Papadimas, N.; Heinzel-Gutenbrunner, M.; Brenner, T.; Lichtenstern, C.; et al. Therapeutic Drug Monitoring-Guided Continuous Infusion of Piperacillin/Tazobactam Significantly Improves Pharmacokinetic Target Attainment in Critically Ill Patients: A Retrospective Analysis of Four Years of Clinical Experience. Infection 2019, 47, 1001–1011. [Google Scholar] [CrossRef]
- Dräger, S.; von Rotz, M.; Labhardt, N.D.; Siegemund, M.; Rentsch, K.M.; Osthoff, M.; Franzeck, F.C. Early Target Attainment with Continuous Infusion Meropenem and Piperacillin/Tazobactam and Utilization of Therapeutic Drug Monitoring in Critically Ill Patients: A Retrospective Cohort Study from 2017 to 2020. Open Forum Infect. Dis. 2023, 10, ofad143. [Google Scholar] [CrossRef]
- Berrino, P.M.; Gatti, M.; Rinaldi, M.; Brunocilla, E.; Viale, P.; Pea, F. Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin-tazobactam or Meropenem and Microbiological Outcome among Urologic Patients with Documented Gram-Negative Infections. Antibiotics 2023, 12, 1388. [Google Scholar] [CrossRef]
- Gatti, M.; Rinaldi, M.; Bonazzetti, C.; Gaibani, P.; Giannella, M.; Viale, P.; Pea, F. Could an Optimized Joint Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Ceftazidime-Avibactam Be a Way to Avoid the Need for Combo Therapy in the Targeted Treatment of Deep-Seated DTR Gram-Negative Infections? Antimicrob. Agents Chemother. 2023, 67, e0096923. [Google Scholar] [CrossRef] [PubMed]
- Udy, A.A.; Roberts, J.A.; Boots, R.J.; Paterson, D.L.; Lipman, J. Augmented Renal Clearance: Implications for Antibacterial Dosing in the Critically Ill. Clin. Pharmacokinet. 2010, 49, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Abdul-Aziz, M.H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised Antibiotic Dosing for Patients Who Are Critically Ill: Challenges and Potential Solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Lipman, J.; Roberts, J.A. Identifying “at-Risk” Patients for Sub-Optimal Beta-Lactam Exposure in Critically Ill Patients with Severe Infections. Crit. Care 2017, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Joynt, G.M.; Choi, G.Y.S.; Gomersall, C.D.; Lipman, J. How to Optimise Antimicrobial Prescriptions in the Intensive Care Unit: Principles of Individualised Dosing Using Pharmacokinetics and Pharmacodynamics. Int. J. Antimicrob. Agents 2012, 39, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Carrié, C.; Petit, L.; d’Houdain, N.; Sauvage, N.; Cottenceau, V.; Lafitte, M.; Foumenteze, C.; Hisz, Q.; Menu, D.; Legeron, R.; et al. Association between Augmented Renal Clearance, Antibiotic Exposure and Clinical Outcome in Critically Ill Septic Patients Receiving High Doses of β-Lactams Administered by Continuous Infusion: A Prospective Observational Study. Int. J. Antimicrob. Agents 2018, 51, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of Target Attainment of Beta-Lactam Antibiotics in Critically Ill Patients and Associated Risk Factors: A Two-Center Prospective Study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.H.; Abodakpi, H.; Wang, W.; Ledesma, K.R.; Merlau, P.R.; Chan, K.; Altman, R.; Tran, T.T.; Nikolaou, M.; Sofjan, A.K. Optimizing Pharmacokinetics/Pharmacodynamics of β-Lactam/β-Lactamase Inhibitor Combinations against High Inocula of ESBL-Producing Bacteria. J. Antimicrob. Chemother. 2021, 76, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Lenhard, J.R.; Bulman, Z.P. Inoculum Effect of β-Lactam Antibiotics. J. Antimicrob. Chemother. 2019, 74, 2825–2843. [Google Scholar] [CrossRef] [PubMed]
- Abodakpi, H.; Chang, K.-T.; Gao, S.; Sánchez-Díaz, A.M.; Cantón, R.; Tam, V.H. Optimal Piperacillin-Tazobactam Dosing Strategies against Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63, e01906-18. [Google Scholar] [CrossRef]
- Abodakpi, H.; Chang, K.T.; Zhou, J.; Byerly, C.; Tam, V.H. A Novel Framework to Compare the Effectiveness of β-Lactamase Inhibitors against Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. Clin. Microbiol. Infect. 2019, 25, 1154.e9–1154.e14. [Google Scholar] [CrossRef]
- Felton, T.W.; Ogungbenro, K.; Boselli, E.; Hope, W.W.; Rodvold, K.A. Comparison of Piperacillin Exposure in the Lungs of Critically Ill Patients and Healthy Volunteers. J. Antimicrob. Chemother. 2018, 73, 1340–1347. [Google Scholar] [CrossRef]
- Felton, T.W.; McCalman, K.; Malagon, I.; Isalska, B.; Whalley, S.; Goodwin, J.; Bentley, A.M.; Hope, W.W. Pulmonary Penetration of Piperacillin and Tazobactam in Critically Ill Patients. Clin. Pharmacol. Ther. 2014, 96, 438–448. [Google Scholar] [CrossRef]
- Harris, P.N.A.; Tambyah, P.A.; Lye, D.C.; Mo, Y.; Lee, T.H.; Yilmaz, M.; Alenazi, T.H.; Arabi, Y.; Falcone, M.; Bassetti, M.; et al. Effect of Piperacillin-Tazobactam vs Meropenem on 30-Day Mortality for Patients with E coli or Klebsiella pneumoniae Bloodstream Infection and Ceftriaxone Resistance: A Randomized Clinical Trial. JAMA 2018, 320, 984–994. [Google Scholar] [CrossRef]
- Sime, F.B.; Udy, A.A.; Roberts, J.A. Augmented Renal Clearance in Critically Ill Patients: Etiology, Definition and Implications for Beta-Lactam Dose Optimization. Curr. Opin. Pharmacol. 2015, 24, 1–6. [Google Scholar] [CrossRef]
- Carrié, C.; Chadefaux, G.; Sauvage, N.; de Courson, H.; Petit, L.; Nouette-Gaulain, K.; Pereira, B.; Biais, M. Increased β-Lactams Dosing Regimens Improve Clinical Outcome in Critically Ill Patients with Augmented Renal Clearance Treated for a First Episode of Hospital or Ventilator-Acquired Pneumonia: A before and after Study. Crit. Care 2019, 23, 379. [Google Scholar] [CrossRef]
- Carrié, C.; Legeron, R.; Petit, L.; Ollivier, J.; Cottenceau, V.; d’Houdain, N.; Boyer, P.; Lafitte, M.; Xuereb, F.; Sztark, F.; et al. Higher than Standard Dosing Regimen Are Needed to Achieve Optimal Antibiotic Exposure in Critically Ill Patients with Augmented Renal Clearance Receiving Piperacillin-Tazobactam Administered by Continuous Infusion. J. Crit. Care 2018, 48, 66–71. [Google Scholar] [CrossRef]
- Udy, A.A.; De Waele, J.J.; Lipman, J. Augmented Renal Clearance and Therapeutic Monitoring of β-Lactams. Int. J. Antimicrob. Agents 2015, 45, 331–333. [Google Scholar] [CrossRef]
- Udy, A.A.; Dulhunty, J.M.; Roberts, J.A.; Davis, J.S.; Webb, S.A.R.; Bellomo, R.; Gomersall, C.; Shirwadkar, C.; Eastwood, G.M.; Myburgh, J.; et al. Association between Augmented Renal Clearance and Clinical Outcomes in Patients Receiving β-Lactam Antibiotic Therapy by Continuous or Intermittent Infusion: A Nested Cohort Study of the BLING-II Randomised, Placebo-Controlled, Clinical Trial. Int. J. Antimicrob. Agents 2017, 49, 624–630. [Google Scholar] [CrossRef]
- Udy, A.A.; Varghese, J.M.; Altukroni, M.; Briscoe, S.; McWhinney, B.C.; Ungerer, J.P.; Lipman, J.; Roberts, J.A. Subtherapeutic Initial β-Lactam Concentrations in Select Critically Ill Patients: Association between Augmented Renal Clearance and Low Trough Drug Concentrations. Chest 2012, 142, 30–39. [Google Scholar] [CrossRef]
- Huttner, A.; Von Dach, E.; Renzoni, A.; Huttner, B.D.; Affaticati, M.; Pagani, L.; Daali, Y.; Pugin, J.; Karmime, A.; Fathi, M.; et al. Augmented Renal Clearance, Low β-Lactam Concentrations and Clinical Outcomes in the Critically Ill: An Observational Prospective Cohort Study. Int. J. Antimicrob. Agents 2015, 45, 385–392. [Google Scholar] [CrossRef]
- Taccone, F.S.; Bogossian, E.G.; Tironi, R.M.; Antonucci, E.; Hites, M.; Knoop, C.; Etienne, I.; Jacobs, F.; Creteur, J. Early β-Lactam Concentrations and Infectious Complications after Lung Transplantation. Am. J. Transplant. 2021, 21, 2489–2497. [Google Scholar] [CrossRef]
- Van Der Heggen, T.; Dhont, E.; Willems, J.; Herck, I.; Delanghe, J.R.; Stove, V.; Verstraete, A.G.; Vanhaesebrouck, S.; De Paepe, P.; De Cock, P.A.J.G. Suboptimal Beta-Lactam Therapy in Critically Ill Children: Risk Factors and Outcome. Pediatr. Crit. Care Med. 2022, 23, e309–e318. [Google Scholar] [CrossRef]
- Imani, S.; Buscher, H.; Day, R.; Gentili, S.; Jones, G.R.D.; Marriott, D.; Norris, R.; Sandaradura, I. An Evaluation of Risk Factors to Predict Target Concentration Non-Attainment in Critically Ill Patients Prior to Empiric β-Lactam Therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 2171–2175. [Google Scholar] [CrossRef]
- Pai Mangalore, R.; Ashok, A.; Lee, S.J.; Romero, L.; Peel, T.N.; Udy, A.A.; Peleg, A.Y. Beta-Lactam Antibiotic Therapeutic Drug Monitoring in Critically Ill Patients: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2022, 75, 1848–1860. [Google Scholar] [CrossRef]
- Sanz-Codina, M.; Bozkir, H.Ö.; Jorda, A.; Zeitlinger, M. Individualized Antimicrobial Dose Optimization: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Microbiol. Infect. 2023, 29, 845–857. [Google Scholar] [CrossRef]
- Cook, A.M.; Hatton-Kolpek, J. Augmented Renal Clearance. Pharmacotherapy 2019, 39, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN Surveillance Definition of Health Care-Associated Infection and Criteria for Specific Types of Infections in the Acute Care Setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Chastre, J.; Fagon, J.-Y. Ventilator-Associated Pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Binnicker, M.J.; Campbell, S.; Carroll, K.C.; Chapin, K.C.; Gilligan, P.H.; Gonzalez, M.D.; Jerris, R.C.; Kehl, S.C.; Patel, R.; et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin. Infect. Dis. 2018, 67, e1–e94. [Google Scholar] [CrossRef]
- EUCAST—European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters Version 12.0; Valid from 2022-01-01; EUCAST: Vaxjo, Sweden, 2023. [Google Scholar]
- EUCAST—European Committee on Antimicrobial Susceptibility Testing. Piperacillin-Tazobactam Breakpoints for Enterobacterales. In Proceedings of the General Consultation, 17 July–15 September 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/2020/Pip-Taz_Enterobacterales_General_consultation_final_20200710.pdf (accessed on 1 December 2023).
- Shields, R.K.; Nguyen, M.H.; Chen, L.; Press, E.G.; Kreiswirth, B.N.; Clancy, C.J. Pneumonia and Renal Replacement Therapy Are Risk Factors for Ceftazidime-Avibactam Treatment Failures and Resistance among Patients with Carbapenem-Resistant Enterobacteriaceae Infections. Antimicrob. Agents Chemother. 2018, 62, 5. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Giacobbe, D.R.; Castaldo, N.; Cattelan, A.; Mussini, C.; Luzzati, R.; Rosa, F.G.D.; Del Puente, F.; Mastroianni, C.M.; Cascio, A.; et al. Clinical Experience with Ceftazidime-Avibactam for the Treatment of Infections Due to Multidrug-Resistant Gram-Negative Bacteria Other than Carbapenem-Resistant Enterobacterales. Antibiotics 2020, 9, 71. [Google Scholar] [CrossRef]
- Loeuille, G.; D’Huart, E.; Vigneron, J.; Nisse, Y.-E.; Beiler, B.; Polo, C.; Ayari, G.; Sacrez, M.; Demoré, B.; Charmillon, A. Stability Studies of 16 Antibiotics for Continuous Infusion in Intensive Care Units and for Performing Outpatient Parenteral Antimicrobial Therapy. Antibiotics 2022, 11, 458. [Google Scholar] [CrossRef]
- Sörgel, F.; Kinzig, M. The Chemistry, Pharmacokinetics and Tissue Distribution of Piperacillin/Tazobactam. J. Antimicrob. Chemother. 1993, 31 (Suppl. A), 39–60. [Google Scholar] [CrossRef]
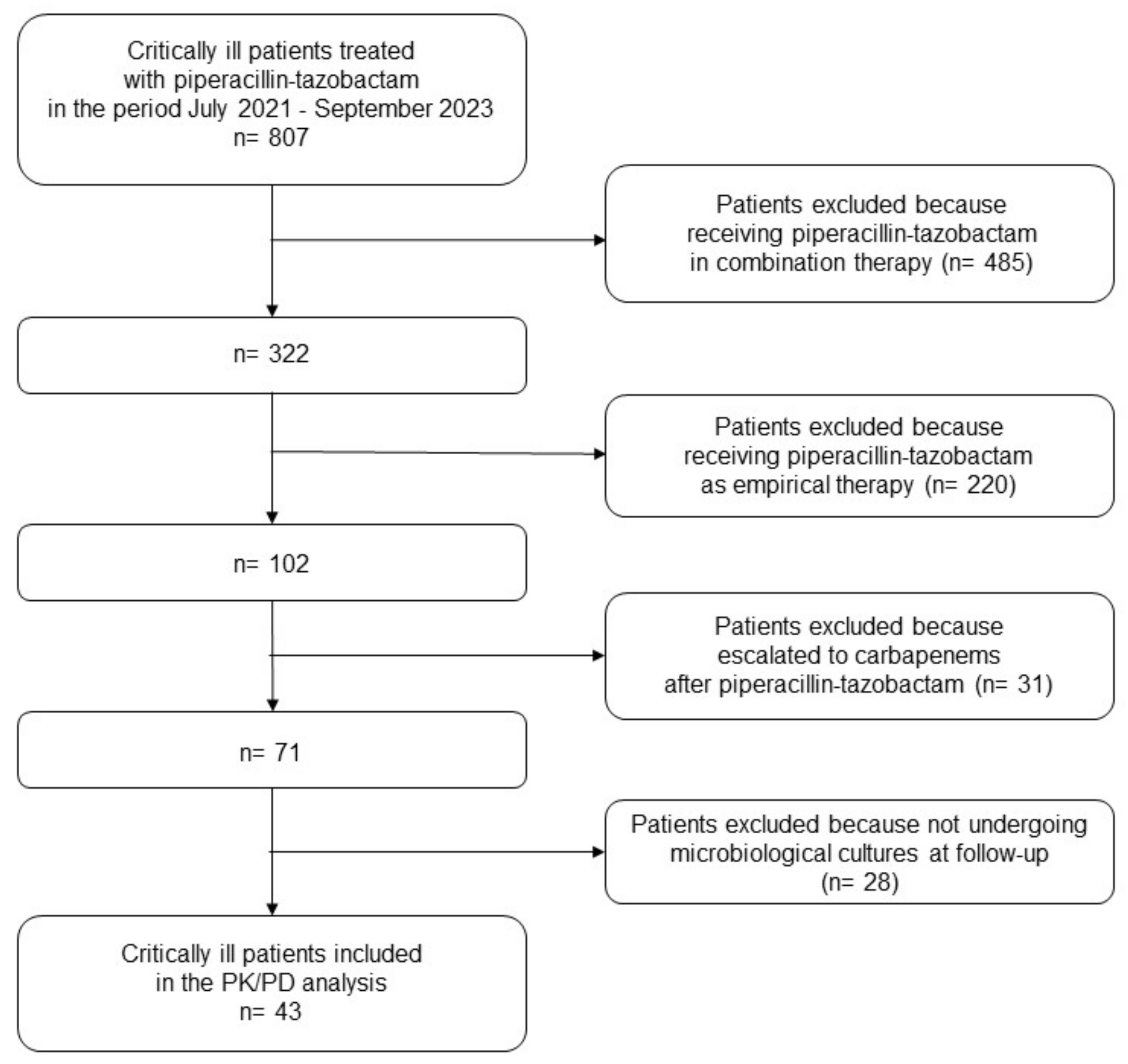
| Demographics and Clinical Variables | ICU Patients (N = 43) |
|---|---|
| Patient demographics | |
| Age (years) (median (IQR)) | 69 (57–74) |
| Gender (male/female) (n (%)) | 25/18 (58.1/41.9) |
| Body weight (Kg) (median (IQR)) | 80 (65–90) |
| Body mass index (Kg/m2) (median (IQR)) | 26.1 (23.1–29.4) |
| Underlying diseases (n (%)) | |
| Post-anoxic coma after resuscitated cardiac arrest | 5 (11.6) |
| Bowel occlusion/perforation | 5 (11.6) |
| Acute pulmonary edema | 4 (9.3) |
| Solid cancer | 4 (9.3) |
| Drug intoxication | 4 (9.3) |
| Acute pancreatitis | 2 (4.7) |
| OLT | 2 (4.7) |
| ARDS in COVID-19 | 2 (4.7) |
| Inflammatory bowel disease | 2 (4.7) |
| Other a | 13 (30.1) |
| Severity of clinical conditions | |
| Baseline SOFA score (median (IQR)) | 8 (4–11) |
| Mechanical ventilation (n (%)) | 35 (81.4) |
| Vasopressors (n (%)) | 27 (62.8) |
| Baseline CLCR (mL/min/1.73 m2) (median (IQR)) | 88.0 (57.3–102.0) |
| Continuous renal replacement therapy (n (%)) | 11 (25.6) |
| Augmented renal clearance (n (%)) | 3 (7.0) |
| Site of infection (n (%)) | |
| BSI | 24 (55.8) |
| VAP | 16 (37.2) |
| VAP + BSI | 3 (7.0) |
| Gram-negative clinical isolates b (n (%)) | |
| Escherichia coli | 18 (37.5) |
| Pseudomonas aeruginosa | 14 (29.0) |
| Klebsiella pneumoniae | 6 (12.5) |
| Klebsiella aerogenes | 2 (4.2) |
| Proteus mirabilis | 2 (4.2) |
| Proteus vulgaris | 2 (4.2) |
| Serratia marcescens | 1 (2.1) |
| Citrobacter koseri | 1 (2.1) |
| Citrobacter braakii | 1 (2.1) |
| Klebsiella oxytoca | 1 (2.1) |
| Piperacillin-tazobactam treatment | |
| Daily dose (mg) (median (IQR)) | 18 g/day (13.5–18 g/day) |
| Treatment duration (days) (median (IQR)) | 9 (7–12) |
| Piperacillin fCss (mg/L) (median (IQR)) | 54.6 (41.0–91.2) |
| Tazobactam fCss (mg/L) (median (IQR)) | 7.2 (4.6–11.6) |
| Piperacillin fCss/MIC ratio (median (IQR)) | 7.6 (4.8–13.0) |
| Tazobactam fCss/CT ratio )median (IQR)] | 1.8 (1.2–2.9) |
| PK/PD target attainment | |
| Overall optimal joint PK/PD target (n (%)) | 36 (83.7) |
| Overall quasi-optimal joint PK/PD target (n (%)) | 6 (14.0) |
| Overall suboptimal joint PK/PD target (n (%)) | 1 (2.3) |
| ECPA program | |
| Overall TDM-based ECPAs | 93 |
| N. of TDM-based ECPA per treatment course (median (IQR)) | 2 (1–2.5) |
| N. of dosage confirmations at first TDM assessment (n (%)) | 15 (34.9) |
| N. of dosage increases at first TDM assessment (n (%)) | 1 (2.3) |
| N. of dosage decreases at first TDM assessment (n (%)) | 27 (62.8) |
| Overall n. of dosage confirmations (n (%)) | 49 (52.7) |
| Overall n. of dosage increases (n (%)) | 39 (41.9) |
| Overall n. of dosage decreases (n (%)) | 5 (5.4) |
| Outcome | |
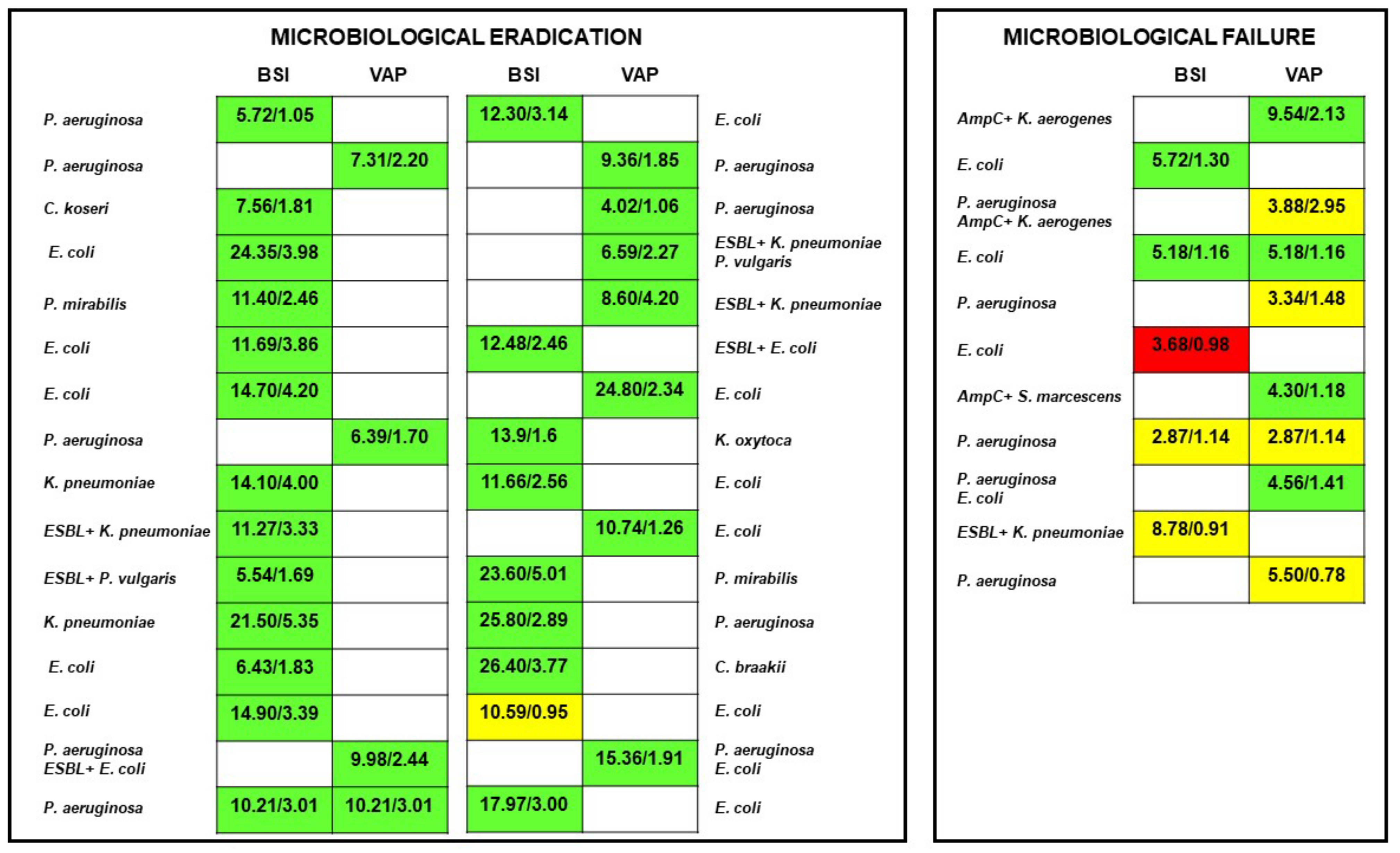
| Microbiological eradication (n (%)) | 32 (74.4) |
| Resistance occurrence (n (%)) | 3 (7.0) |
| Clinical cure (n (%)) | 29 (67.4) |
| 90-days MDR colonization (n (%)) | 4 (9.3) |
| Delta 48-h SOFA (median (IQR)) | 0 (0–2) |
| Delta 7-days SOFA (median (IQR)) | 2 (0–4.5) |
| ICU mortality (n (%)) | 4 (9.3) |
| 30-day mortality (n (%)) | 6 (14.0) |
| Variables | Optimal Joint PK/PD Target Attainment (N = 36) | Quasi-Optimal/Suboptimal Joint PK/PD Target Attainment (N = 7) | Univariate Analysis p Value | Multivariate Analysis (OR; 95%CI) | Multivariate Analysis p Value |
|---|---|---|---|---|---|
| Patient demographics | |||||
| Age (years) (median (IQR)) | 68.5 (56.75–73.5) | 69 (63.5–73) | 0.79 | ||
| Gender (male/female) (n (%)) | 19/17 (52.8/47.2) | 6/1 (85.7/14.3) | 0.21 | ||
| Body weight (Kg) (median (IQR)) | 75 (65–90) | 81 (77.5–102.5) | 0.18 | ||
| Body mass index (Kg/m2) (median (IQR)) | 26.0 (22.8–28.5) | 31.3 (26.3–32.5) | 0.11 | ||
| Severity of clinical conditions | |||||
| Baseline SOFA score (median (IQR)) | 8.5 (5.75–11) | 4 (3–11) | 0.38 | ||
| Mechanical ventilation (n (%)) | 28 (77.8) | 7 (100.0) | 0.31 | ||
| Vasopressors (n (%)) | 25 (69.4) | 2 (28.6) | 0.08 | ||
| Continuous renal replacement therapy (n (%)) | 10 (27.8) | 1 (14.3) | 0.66 | ||
| Augmented renal clearance (n (%)) | 1 (2.8) | 2 (27.8) | 0.06 | ||
| Site of infection (n (%)) | |||||
| BSI | 21 (58.3) | 3 (42.9) | 0.68 | ||
| VAP | 13 (36.1) | 3 (42.9) | 0.99 | ||
| VAP + BSI | 2 (5.6) | 1 (14.2) | 0.42 | ||
| Outcome | |||||
| Microbiological eradication (n (%)]) | 31 (86.1) | 1 (14.3) | <0.001 | 0.03 (0.003–0.27) | 0.002 |
| Resistance occurrence (n (%)) | 2 (5.6) | 1 (14.3) | 0.42 | ||
| Clinical cure (n (%)) | 28 (77.8) | 1 (14.3) | 0.003 | - | |
| 90-day MDR colonization | 3 (8.3) | 1 (14.3) | 0.52 | ||
| Delta 48-h SOFA score (median (IQR)) | 0 (0–2) | 2 (0–3) | 0.37 | ||
| Delta day 7 SOFA score (median (IQR)) | 2.5 (0–5) | 1 (0–3) | 0.64 | ||
| ICU mortality (n (%)) | 4 (11.1) | 0 (0.0) | 0.99 | ||
| 30-day mortality (n (%)) | 6 (16.7) | 0 (0.0) | 0.57 |
| Variables | Microbiological Eradication (N = 32) | Microbiological Failure (N = 11) | Univariate Analysis p Value | Multivariate Analysis (OR; 95%CI) | Multivariate Analysis p Value |
|---|---|---|---|---|---|
| Patient demographics | |||||
| Age (years) (median (IQR)) | 68.5 (56.75–76.25) | 69 (59–70.5) | 0.79 | ||
| Gender (male/female) (n (%)) | 18/14 (56.3/43.7) | 7/4 (63.6/36.4) | 0.74 | ||
| Body weight (Kg) (median (IQR)) | 75 (64.25–90) | 81 (72.5–92.5) | 0.32 | ||
| Body mass index (Kg/m2) (median (IQR)) | 25.7 (22.4–28.9) | 27.8 (26.0–30.3) | 0.16 | ||
| Severity of clinical conditions | |||||
| Baseline SOFA score (median (IQR)) | 8 (5.75–11) | 9 (3–11) | 0.54 | ||
| Mechanical ventilation (n (%)) | 24 (75.0) | 11 (100.0) | 0.09 | ||
| Vasopressors (n (%)) | 21 (65.6) | 6 (54.5) | 0.72 | ||
| Continuous renal replacement therapy (n (%)) | 9 (28.1) | 2 (18.2) | 0.70 | ||
| Augmented renal clearance (n (%)) | 0 (0.0) | 3 (27.3) | 0.01 | - | |
| Site of infection (n (%)) | |||||
| VAP or bacteremic VAP | 11 (34.4) | 8 (72.7) | 0.04 | - | |
| Gram-negative clinical isolates (n (%)) | |||||
| Escherichia coli | 15 (42.8) | 3 (23.0) | 0.32 | ||
| Pseudomonas aeruginosa | 9 (25.7) | 5 (38.5) | 0.48 | ||
| Klebsiella pneumoniae | 4 (11.4) | 2 (15.4) | 0.66 | ||
| Klebsiella aerogenes | 0 (0.0) | 2 (15.4) | 0.07 | ||
| Proteus mirabilis | 2 (5.7) | 0 (0.0) | 0.99 | ||
| Proteus vulgaris | 2 (5.7) | 0 (0.0) | 0.99 | ||
| Serratia marcescens | 0 (0.0) | 1 (7.7) | 0.27 | ||
| Citrobacter koseri | 1 (2.9) | 0 (0.0) | 0.99 | ||
| Citrobacter braakii | 1 (2.9) | 0 (0.0) | 0.99 | ||
| Klebsiella oxytoca | 1 (2.9) | 0 (0.0) | 0.99 | ||
| Susceptibility pattern | |||||
| Full-susceptible | 26 (82.9) | 7 (69.2) | 0.25 | ||
| ESBL-producing Enterobacterales | 6 (17.1) | 1 (7.7) | 0.66 | ||
| AmpC-producing Enterobacterales | 0 (0.0) | 3 (23.1) | 0.01 | - | |
| Piperacillin-tazobactam treatment and joint PK/PD target attainment | |||||
| Quasi-optimal/suboptimal joint PK/PD target attainment | 1 (2.9) | 6 (54.5) | <0.001 | 37.2 (3.66–377.86) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Rinaldi, M.; Tonetti, T.; Siniscalchi, A.; Viale, P.; Pea, F. Could an Optimized Joint Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin-Tazobactam Be a Valuable Innovative Approach for Maximizing the Effectiveness of Monotherapy Even in the Treatment of Critically Ill Patients with Documented Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Bloodstream Infections and/or Ventilator-Associated Pneumonia? Antibiotics 2023, 12, 1736. https://doi.org/10.3390/antibiotics12121736
Gatti M, Rinaldi M, Tonetti T, Siniscalchi A, Viale P, Pea F. Could an Optimized Joint Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin-Tazobactam Be a Valuable Innovative Approach for Maximizing the Effectiveness of Monotherapy Even in the Treatment of Critically Ill Patients with Documented Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Bloodstream Infections and/or Ventilator-Associated Pneumonia? Antibiotics. 2023; 12(12):1736. https://doi.org/10.3390/antibiotics12121736
Chicago/Turabian StyleGatti, Milo, Matteo Rinaldi, Tommaso Tonetti, Antonio Siniscalchi, Pierluigi Viale, and Federico Pea. 2023. "Could an Optimized Joint Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin-Tazobactam Be a Valuable Innovative Approach for Maximizing the Effectiveness of Monotherapy Even in the Treatment of Critically Ill Patients with Documented Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Bloodstream Infections and/or Ventilator-Associated Pneumonia?" Antibiotics 12, no. 12: 1736. https://doi.org/10.3390/antibiotics12121736
APA StyleGatti, M., Rinaldi, M., Tonetti, T., Siniscalchi, A., Viale, P., & Pea, F. (2023). Could an Optimized Joint Pharmacokinetic/Pharmacodynamic Target Attainment of Continuous Infusion Piperacillin-Tazobactam Be a Valuable Innovative Approach for Maximizing the Effectiveness of Monotherapy Even in the Treatment of Critically Ill Patients with Documented Extended-Spectrum Beta-Lactamase-Producing Enterobacterales Bloodstream Infections and/or Ventilator-Associated Pneumonia? Antibiotics, 12(12), 1736. https://doi.org/10.3390/antibiotics12121736







