Rapid Detection of Antimicrobial Resistance Genes in Critically Ill Children Using a Custom TaqMan Array Card
Abstract
:1. Introduction
- Validate a custom AMR-TAC using isolates with known phenotypic and genotypic AMR.
- Identify the prevalence of AMR genes found in critically ill children with suspected LRTIs.
- Identify the correlation between the gastrointestinal and respiratory resistomes in critically ill children with suspected LRTIs.
- Describe the AMR genes found in critically ill children with suspected LRTIs who had AMR identified using conventional antimicrobial susceptibility testing.
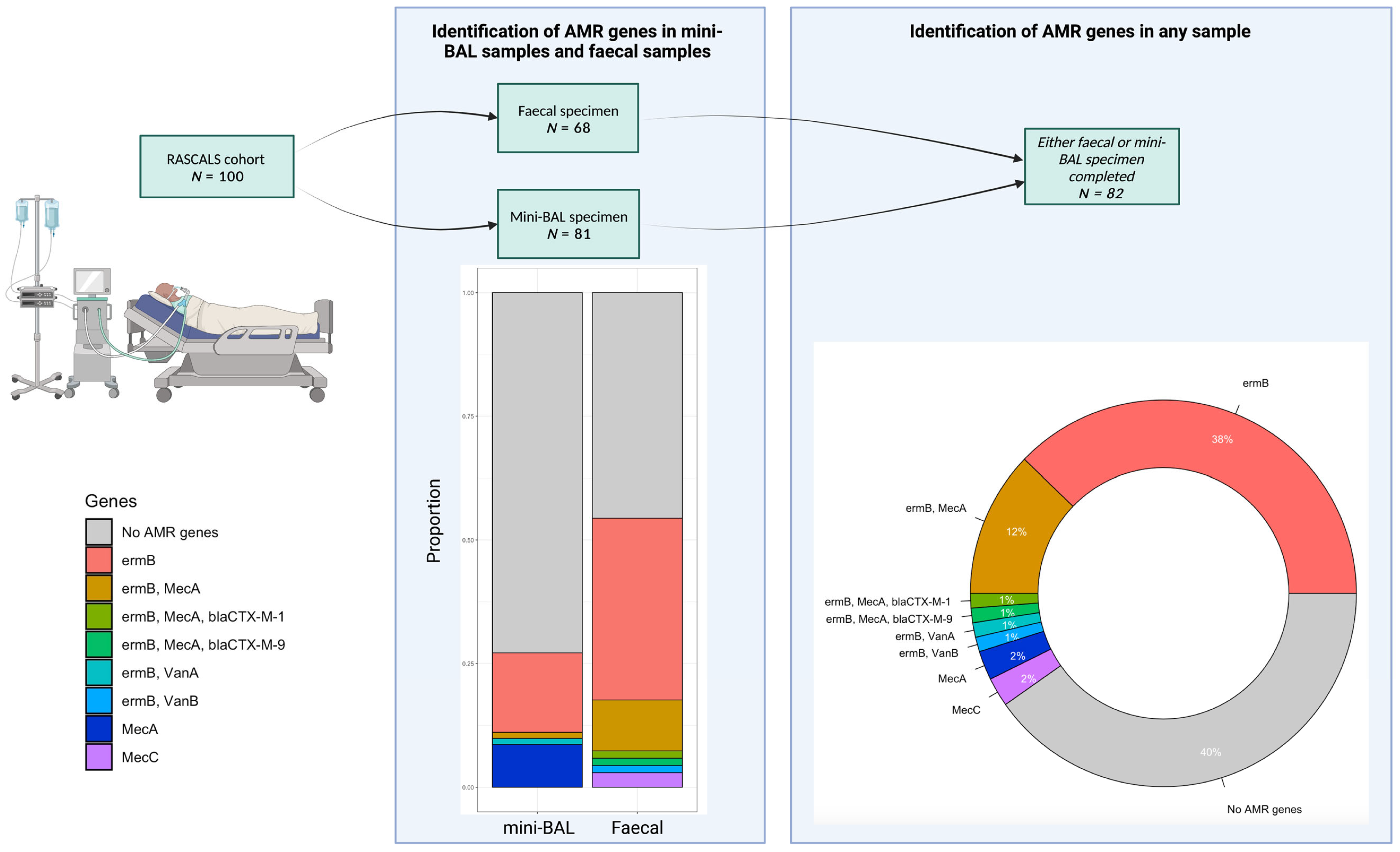
2. Results
2.1. Antimicrobial Resistance Gene TaqMan Array Card Validation
2.2. Demographics
2.3. Respiratory Microbiology Results Compared to an Antimicrobial Resistance Gene TaqMan Array Card
2.4. Antimicrobial Resistance Gene TaqMan Array Card Detections Correlating to Clinical Cases
3. Materials and Methods
3.1. Study Design and Population
3.2. Eligibility Criteria
- The child was aged <18 years old;
- The child was receiving mechanical ventilation at the time of enrolment;
- The child was commencing or already receiving antimicrobial therapy to treat a suspected or confirmed LRTI.
- The patient had a non-survivable illness and was no longer on an active treatment pathway;
- The child was aged <37 weeks corrected gestation.
3.3. Non-Bronchoscopic Bronchoalveolar Lavage Sampling
3.4. Faecal Sampling
3.5. Nucleic Acid Extraction from Non-Bronchoscopic Bronchoalveolar Lavage Samples
3.6. Nucleic Acid Extraction from Faecal Samples
3.7. Nucleic Acid Extraction from Raw Sewage Samples
3.8. Antimicrobial Resistance Gene TaqMan Array Card
- For AMR genes with one target on the TAC, Ct value ≤ 32;
- For AMR genes with ≥2 targets on the TAC, either of the following:
- (a)
- At least one target, Ct ≤ 32;
- (b)
- At least two targets had Ct < 34.
3.9. Conventional Respiratory Pathogen Testing
3.10. Data Collection
3.11. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Mathot, F.; Duke, T.; Daley, A.J.; Butcher, T. Bacteremia and Pneumonia in a Tertiary PICU: An 11-Year Study. Pediatr. Crit. Care Med. 2015, 16, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Akinkugbe, O.; Cooke, F.J.; Pathan, N. Healthcare-Associated Bacterial Infections in the Paediatric ICU. JAC Antimicrob. Resist. 2020, 2, dlaa066. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.; White, D.; Daubney, E.; Curran, M.; Bousfield, R.; Gouliouris, T.; Powell, E.; Palmer, A.; Agrawal, S.; Inwald, D.; et al. Low Diagnostic Yield and Time to Diagnostic Confirmation Results in Prolonged Use of Antimicrobials in Critically Ill Children. Wellcome Open Res. 2021, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Papan, C.; Meyer-Buehn, M.; Laniado, G.; Nicolai, T.; Griese, M.; Huebner, J. Assessment of the Multiplex PCR-Based Assay Unyvero Pneumonia Application for Detection of Bacterial Pathogens and Antibiotic Resistance Genes in Children and Neonates. Infection 2018, 46, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tao, Y.; Tang, M.; Du, B.; Xia, Y.; Mo, X.; Cao, Q. Rapid Detection of Respiratory Organisms with the FilmArray Respiratory Panel in a Large Children’s Hospital in China. BMC Infect. Dis. 2018, 18, 510. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Conway Morris, A.; Curran, M.D.; White, D.; Daubney, E.; Kean, I.R.L.; Navapurkar, V.; Bartholdson Scott, J.; Maes, M.; Bousfield, R.; et al. The Rapid Detection of Respiratory Pathogens in Critically Ill Children. Crit. Care 2023, 27, 11. [Google Scholar] [CrossRef] [PubMed]
- Sukumar, S.; Wang, F.; Simpson, C.A.; Willet, C.E.; Chew, T.; Hughes, T.E.; Bockmann, M.R.; Sadsad, R.; Martin, F.E.; Lydecker, H.W.; et al. Development of the Oral Resistome during the First Decade of Life. Nat. Commun. 2023, 14, 1291. [Google Scholar] [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef]
- Carlet, J. The Gut Is the Epicentre of Antibiotic Resistance. Antimicrob. Resist. Infect. Control 2012, 1, 39. [Google Scholar] [CrossRef]
- Whiteside, S.A.; McGinniss, J.E.; Collman, R.G. The Lung Microbiome: Progress and Promise. J. Clin. Investig. 2021, 131, e150473. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.P.; Kreth, J. The Impact of Horizontal Gene Transfer on the Adaptive Ability of the Human Oral Microbiome. Front. Cell Infect. Microbiol. 2014, 4, 124. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Kean, I.R.L.; Curran, M.D.; Khokhar, F.; White, D.; Daubney, E.; Conway Morris, A.; Navapurkar, V.; Bartholdson Scott, J.; Maes, M.; et al. Rapid Assay for Sick Children with Acute Lung Infection Study (RASCALS): Diagnostic Cohort Study Protocol. BMJ Open 2021, 11, e056197. [Google Scholar] [CrossRef] [PubMed]
- Qiagen. EZ1 Virus Handbook; Qiagen: Hilden, Germany, 2010. [Google Scholar]
- Sridhar, S.; Forrest, S.; Kean, I.; Young, J.; Bartholdson Scott, J.; Maes, M.; Pereira-Dias, J.; Parmar, S.; Routledge, M.; Sparkes, D.; et al. A Blueprint for the Implementation of a Validated Approach for the Detection of SARS-CoV2 in Clinical Samples in Academic Facilities. Wellcome Open Res. 2020, 5, 110. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.K.; Conway Morris, A.; Curran, M.D.; Parmar, S.; Sule, O.; Enoch, D.A.; Aliyu, S.H.; Zhang, H.; Jalal, H.; Navapurkar, V.; et al. Evaluating the Use of a 22-Pathogen TaqMan Array Card for Rapid Diagnosis of Respiratory Pathogens in Intensive Care. J. Med. Microbiol. 2020, 69, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Pholwat, S.; Liu, J.; Taniuchi, M.; Chinli, R.; Pongpan, T.; Thaipisutikul, I.; Ratanakorn, P.; Platts-Mills, J.A.; Fleece, M.; Stroup, S.; et al. Genotypic Antimicrobial Resistance Assays for Use on E. Coli Isolates and Stool Specimens. PLoS ONE 2019, 14, e0216747. [Google Scholar] [CrossRef] [PubMed]
- Bortolaia, V.; Kaas, R.S.; Ruppe, E.; Roberts, M.C.; Schwarz, S.; Cattoir, V.; Philippon, A.; Allesoe, R.L.; Rebelo, A.R.; Florensa, A.F.; et al. ResFinder 4.0 for Predictions of Phenotypes from Genotypes. J. Antimicrob. Chemother. 2020, 75, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Public Health England. UK Standards for Microbiology Investigations: Investigation of Bronchoalveolar Lavage, Sputum and Associated Specimens. Bacteriology 2019, B57, 1–38. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Disk Diffusion Method; EUCAST: Växjö, Sweden, 2023. [Google Scholar]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. The REDCap Consortium: Building an International Community of Software Platform Partners. J. Biomed. Inf. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Straney, L.; Clements, A.; Parslow, R.C.; Pearson, G.; Shann, F.; Alexander, J.; Slater, A. Paediatric Index of Mortality 3: An Updated Model for Predicting Mortality in Pediatric Intensive Care. Pediatr. Crit. Care Med. 2013, 14, 673–681. [Google Scholar] [CrossRef]
- Yehya, N.; Harhay, M.O.; Curley, M.A.Q.; Schoenfeld, D.A.; Reeder, R.W. Reappraisal of Ventilator-Free Days in Critical Care Research. Am. J. Respir. Crit. Care Med. 2019, 200, 828–836. [Google Scholar] [CrossRef] [PubMed]
- R Studio Team. RStudio: Integrated Development for R; R Studio v7.1, R Version 4.2.0; PBC: Boston, MA, USA, 2022. [Google Scholar]
- Altman, D. Practical Statistics for Medical Research, 1st ed.; Chapman and Hall: Oxford, UK, 1991. [Google Scholar]
- Harimaya, A.; Yamazaki, N.; Himi, T.; Yokota, S.; Sato, K.; Fujii, N. High Prevalence of Erythromycin Resistance and Macrolide-Resistance Genes, MefA and ErmB, in Streptococcus Pneumoniae Isolates from the Upper Respiratory Tracts of Children in the Sapporo District, Japan. J. Infect. Chemother. 2007, 13, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Kresken, M.; Henrichfreise, B.; Bagel, S.; Brauers, J.; Wiedemann, B. High Prevalence of the ErmB Gene among Erythromycin-Resistant Streptococcus Pneumoniae Isolates in Germany during the Winter of 2000–2001 and In Vitro Activity of Telithromycin. Antimicrob. Agents Chemother. 2004, 48, 3193–3195. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Courvalin, P. Bacterial Resistance to Macrolide, Lincosamide, and Streptogramin Antibiotics by Target Modification. Antimicrob. Agents Chemother. 1991, 35, 1267–1272. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, F.; Ghajavand, H.; Havaei, R.; Jami, M.-S.; Khademi, F.; Heydari, L.; Shahin, M.; Havaei, S. Distribution of Erm Genes among Staphylococcus Aureus Isolates with Inducible Resistance to Clindamycin in Isfahan, Iran. Adv. Biomed. Res. 2016, 5, 62. [Google Scholar] [CrossRef]
- Taylor, S.L.; Leong, L.E.X.; Mobegi, F.M.; Choo, J.M.; Burr, L.D.; Wesselingh, S.; Rogers, G.B. Understanding the Impact of Antibiotic Therapies on the Respiratory Tract Resistome: A Novel Pooled-Template Metagenomic Sequencing Strategy. Multidiscip. Respir. Med. 2018, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Clark, J.; Coote, N.; Fletcher, P.; Harnden, A.; McKean, M.; Thomson, A. British Thoracic Society Guidelines for the Management of Community Acquired Pneumonia in Children: Update 2011. Thorax 2011, 66 (Suppl. S2), ii1–ii23. [Google Scholar] [CrossRef]
- Gupta, V.; Yu, K.C.; Schranz, J.; Gelone, S.P. A Multicenter Evaluation of the US Prevalence and Regional Variation in Macrolide-Resistant S. Pneumoniae in Ambulatory and Hospitalized Adult Patients in the United States. Open Forum Infect. Dis. 2021, 8, ofab063. [Google Scholar] [CrossRef]
- Zhang, A.-N.; Gaston, J.M.; Dai, C.L.; Zhao, S.; Poyet, M.; Groussin, M.; Yin, X.; Li, L.-G.; van Loosdrecht, M.C.M.; Topp, E.; et al. An Omics-Based Framework for Assessing the Health Risk of Antimicrobial Resistance Genes. Nat. Commun. 2021, 12, 4765. [Google Scholar] [CrossRef]
- Li, D.X.; Sick-Samuels, A.C.; Suwantarat, N.; Same, R.G.; Simner, P.J.; Tamma, P.D. Risk Factors for Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae Carriage Upon Pediatric Intensive Care Unit Admission. Infect. Control Hosp. Epidemiol. 2018, 39, 116–118. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Sands, K.; Thomson, K.; Portal, E.; Mathias, J.; Milton, R.; Gillespie, D.; Dyer, C.; Akpulu, C.; Boostrom, I.; et al. Antibiotic Resistance Genes in the Gut Microbiota of Mothers and Linked Neonates with or without Sepsis from Low- and Middle-Income Countries. Nat. Microbiol. 2022, 7, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, R.M.; Coker, M.O.; Dade, E.F.; Palys, T.J.; Morrison, H.G.; Ross, B.D.; Baker, E.R.; Karagas, M.R.; Madan, J.C.; Hoen, A.G. The Infant Gut Resistome Is Associated with E. Coli and Early-Life Exposures. BMC Microbiol. 2021, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Chau, K.K.; Barker, L.; Budgell, E.P.; Vihta, K.D.; Sims, N.; Kasprzyk-Hordern, B.; Harriss, E.; Crook, D.W.; Read, D.S.; Walker, A.S.; et al. Systematic Review of Wastewater Surveillance of Antimicrobial Resistance in Human Populations. Environ. Int. 2022, 162, 107171. [Google Scholar] [CrossRef] [PubMed]
- Shaw, L.P.; Chau, K.K.; Kavanagh, J.; AbuOun, M.; Stubberfield, E.; Gweon, H.S.; Barker, L.; Rodger, G.; Bowes, M.J.; Hubbard, A.T.M.; et al. Niche and Local Geography Shape the Pangenome of Wastewater- and Livestock-Associated Enterobacteriaceae. Sci. Adv. 2021, 7, eabe3868. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, N.J.; Russell, C.D.; McHugh, M.P.; Mark, H.; Conway Morris, A.; Laurenson, I.F.; Hill, A.T.; Templeton, K.E. Comprehensive Molecular Testing for Respiratory Pathogens in Community-Acquired Pneumonia. Clin. Infect. Dis. 2016, 62, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Personne, Y.; Ozongwu, C.; Platt, G.; Basurto-Lozada, P.; Shamin, M.; Gant, V.A.; Zumla, A.; Enne, V.I. ‘Sample-in, Answer-out’? Evaluation and Comprehensive Analysis of the Unyvero P50 Pneumonia Assay. Diagn. Microbiol. Infect. Dis. 2016, 86, 5–10. [Google Scholar] [CrossRef]
- Jitmuang, A.; Puttinad, S.; Hemvimol, S.; Pansasiri, S.; Horthongkham, N. A Multiplex Pneumonia Panel for Diagnosis of Hospital-Acquired and Ventilator-Associated Pneumonia in the Era of Emerging Antimicrobial Resistance. Front. Cell Infect. Microbiol. 2022, 12, 977320. [Google Scholar] [CrossRef]
- Gastli, N.; Loubinoux, J.; Daragon, M.; Lavigne, J.-P.; Saint-Sardos, P.; Pailhoriès, H.; Lemarié, C.; Benmansour, H.; d’Humières, C.; Broutin, L.; et al. Multicentric Evaluation of BioFire FilmArray Pneumonia Panel for Rapid Bacteriological Documentation of Pneumonia. Clin. Microbiol. Infect. 2021, 27, 1308–1314. [Google Scholar] [CrossRef]
- Yoo, I.Y.; Huh, K.; Shim, H.J.; Yun, S.A.; Chung, Y.N.; Kang, O.K.; Huh, H.J.; Lee, N.Y. Evaluation of the BioFire FilmArray Pneumonia Panel for Rapid Detection of Respiratory Bacterial Pathogens and Antibiotic Resistance Genes in Sputum and Endotracheal Aspirate Specimens. Int. J. Infect. Dis. 2020, 95, 326–331. [Google Scholar] [CrossRef]
- Mitton, B.; Rule, R.; Said, M. Laboratory Evaluation of the BioFire FilmArray Pneumonia plus Panel Compared to Conventional Methods for the Identification of Bacteria in Lower Respiratory Tract Specimens: A Prospective Cross-Sectional Study from South Africa. Diagn. Microbiol. Infect. Dis. 2021, 99, 115236. [Google Scholar] [CrossRef]
- Lee, S.H.; Ruan, S.-Y.; Pan, S.-C.; Lee, T.-F.; Chien, J.-Y.; Hsueh, P.-R. Performance of a Multiplex PCR Pneumonia Panel for the Identification of Respiratory Pathogens and the Main Determinants of Resistance from the Lower Respiratory Tract Specimens of Adult Patients in Intensive Care Units. J. Microbiol. Immunol. Infect. 2019, 52, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Darie, A.M.; Khanna, N.; Jahn, K.; Osthoff, M.; Bassetti, S.; Osthoff, M.; Schumann, D.M.; Albrich, W.C.; Hirsch, H.; Brutsche, M.; et al. Fast Multiplex Bacterial PCR of Bronchoalveolar Lavage for Antibiotic Stewardship in Hospitalised Patients with Pneumonia at Risk of Gram-Negative Bacterial Infection (Flagship II): A Multicentre, Randomised Controlled Trial. Lancet Respir. Med. 2022, 10, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Murphy, C.N.; Fowler, R.; Balada-Llasat, J.M.; Carroll, A.; Stone, H.; Akerele, O.; Buchan, B.; Windham, S.; Hopp, A.; Ronen, S.; et al. Multicenter Evaluation of the BioFire FilmArray Pneumonia/Pneumonia Plus Panel for Detection and Quantification of Agents of Lower Respiratory Tract Infection. J. Clin. Microbiol. 2020, 58, e00128-20. [Google Scholar] [CrossRef] [PubMed]
- Serpa, P.H.; Deng, X.; Abdelghany, M.; Crawford, E.; Malcolm, K.; Caldera, S.; Fung, M.; McGeever, A.; Kalantar, K.L.; Lyden, A.; et al. Metagenomic Prediction of Antimicrobial Resistance in Critically Ill Patients with Lower Respiratory Tract Infections. Genome Med. 2022, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson-Palme, J.; Larsson, D.G.J.; Kristiansson, E. Using Metagenomics to Investigate Human and Environmental Resistomes. J. Antimicrob. Chemother. 2017, 72, 2690–2703. [Google Scholar] [CrossRef] [PubMed]
- De Lencastre, H.; de Jonge, B.L.M.; Matthews, P.R.; Tomasz, A. Molecular Aspects of Methicillin Resistance in Staphylococcus Aureus. J. Antimicrob. Chemother. 1994, 33, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, Z. What We Can Do? The Risk Factors for Multi-Drug Resistant Infection in Pediatric Intensive Care Unit (PICU): A Case-Control Study. Ital. J. Pediatr. 2020, 46, 17. [Google Scholar] [CrossRef]

| Antimicrobial Class | Resistance Target | BioFire Pneumonia Panel N = 7 | Unyvero Pneumonia Cartridge N = 17 | Custom AMR-TAC N = 25 |
|---|---|---|---|---|
| β-lactamase | AmpC (FOX) | √ | ||
| blaCTX-M | √ | √ | √ | |
| blaCTX-M-1 | √ | |||
| blaCTX-M-2 | √ | |||
| blaCTX-M-8/25 | √ | |||
| blaCTX-M-9 | √ | |||
| blaOXA-1 | √ | |||
| mecA | √ | √ | ||
| mecC | √ | √ | ||
| mecA/mecC/MREJ | √ | |||
| blaPER-1 | √ | |||
| blaSHV | √ | |||
| blaTEM | √ | |||
| blaVEB | √ | |||
| blaVIM | √ | √ | √ | |
| Carbapenemase | blaNDM | √ | √ | √ |
| blaNDM-1 | √ | |||
| blaGES | √ | |||
| blaIMP | √ | √ | √ | |
| blaIMP-1 | √ | |||
| blaIMP-2 | √ | |||
| blaKPC | √ | √ | √ | |
| blaOXA-23 | √ | |||
| blaOXA-24/40 | √ | |||
| blaOXA-48- like | √ | |||
| blaOXA-48 | √ | √ | ||
| blaOXA-58 | √ | |||
| Fluoroquinolone | gyrA83 | √ | ||
| gyrA87 | √ | |||
| qnrA | √ | |||
| qnrS | √ | |||
| Glycopeptide | vanA | √ | ||
| vanB | √ | |||
| Macrolide | ermB | √ | √ | |
| Sulphonamide | sul1 | √ |
| Variable | Total | Suspected CAP | Suspected HAP/VAP | p-Value |
|---|---|---|---|---|
| N = 82 | N = 66 | N = 16 | ||
| Demographics | ||||
| Age (years)—median (IQR) | 1.2 (0.3–4.9) | 1.0 (0.2–4.2) | 2.8 (0.6–9.8) | 0.124 a |
| Sex (male)—n (%) | 52 (63) | 39 (59) | 13 (82) | 0.148 b |
| Weight (kilograms)—median (IQR) | 10.2 (5.3–18.0) | 9.5 (5.0–17.3) | 12.8 (6.6–26.7) | 0.139 a |
| Significant comorbidity—n (%) | 29 (35) | 21 (32) | 8 (50) | 0.172 b |
| PIM3 score—median (IQR) | 2.2 (0.5–4.6) | 0.8 (0.5–4.6) | 3.6 (2.1–5.0) | 0.041 a |
| Primary diagnostic category—n (%) | ||||
| Respiratory | 52 (63) | 47 (71) | 5 (31) | 0.003 b |
| Neurological | 9 (11) | 8 (12) | 1 (6) | 0.094 b |
| Cardiovascular | 4 (5) | 2 (3) | 2 (13) | 0.115 b |
| Trauma | 4 (5) | 3 (5) | 1 (6) | 0.776 b |
| Post-operative care | 6 (7) | 1 (2) | 5 (31) | <0.001 b |
| Other | 7 (9) | 5 (8) | 2 (13) | 0.527 b |
| Risk factors for AMR—n (%) | ||||
| Home respiratory support (any) | 6 (7) | 6 (9) | 0 | 0.210 b |
| Tracheostomy | 1 (1) | 0 | 1 (6) | 0.041 b |
| NG feeding/gastrostomy | 10 (12) | 8 (12) | 2 (13) | 0.967 b |
| Hospital admission within the last three months | 40 (49) | 35 (53) | 5 (31) | 0.118 b |
| PICU admission within the last three months | 15 (18) | 11 (17) | 4 (25) | 0.036 b |
| Previous mechanical ventilation | 30 (37) | 23 (35) | 7 (44) | 0.507 b |
| Regular steroids | 4 (5) | 4 (6) | 0 | 0.313 b |
| Neutropaenia | 1 (1) | 1 (2) | 0 | 0.620 b |
| Malignancy | 2 (2) | 2 (3) | 0 | 0.481 b |
| Asplenia | 1 (1) | 1 (2) | 0 | 0.620 b |
| Known AMR—n (%) | ||||
| CPE | 1 (1) | 1 (2) | 0 | 0.620 b |
| ESBL | 2 (2) | 0 | 2 (13) | 0.004 b |
| None | 79 (96) | 65 (98) | 14 (88) | 0.036 b |
| Days free of treatment at 28 days—mean (SD) | ||||
| Antimicrobial therapy | 20.0 (7.4) | 20.1 (7.3) | 16.1 (8.2) | 0.056 c |
| Mechanical ventilation | 19.3 (6.6) | 19.8 (6.6) | 15.8 (7.6) | 0.064 c |
| Inotropes | 26.5 (4.3) | 26.4 (4.7) | 26.9 (1.5) | 0.442 c |
| PICU admission | 17.4 (7.5) | 18.1 (7.7) | 13.3 (6.8) | 0.020 c |
| Survival to hospital discharge—n (%) | 78 (95) | 64 (97) | 14 (88) | 0.115 b |
| Study ID | Lower Respiratory Culture Result | Phenotypic Resistance | AMR-TAC Result, Ct Value(s) | |
|---|---|---|---|---|
| Respiratory | Faecal | |||
| C008 | Pseudomonas aeruginosa | Ciprofloxacin | Nil | N/A |
| C046 | Staphylococcus aureus | Fusidic acid | Nil | N/A |
| C048 | Morganella morganii | Amoxicillin/clavulanate Ampicillin/amoxicillin | ermB 28/28 | Nil |
| C067 | Stenotrophomonas maltophilia | Amoxicillin/clavulanate Ampicillin/amoxicillin | Nil | ermB 33/33 |
| Study ID | Organisms Identified on TAC (Ct Value(s)) | Microbiological Culture Results | AMR-TAC Results (Ct) | |
|---|---|---|---|---|
| Respiratory | Faecal | |||
| C060 | Nil * | ETA: E. faecium Ampicillin/amoxicillin (R) Teicoplanin (R) Vancomycin (R) Peritoneal fluid: E. faecium Ampicillin/amoxicillin (R) Daptomycin (R) 2 mg/L Teicoplanin (R) Vancomycin (R) | ermB 25/25 vanA 27 | N/A |
| C094 | Streptococcus spp.: 29/27 | ESBL screening: positive (multiple time points) | Nil | ermB 22/22 blaCTX-M-1 26/26 blaCTX-M 30 mecA 27/29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, J.A.; Curran, M.D.; Gouliouris, T.; Conway Morris, A.; Bousfield, R.; Navapurkar, V.; Kean, I.R.L.; Daubney, E.; White, D.; Baker, S.; et al. Rapid Detection of Antimicrobial Resistance Genes in Critically Ill Children Using a Custom TaqMan Array Card. Antibiotics 2023, 12, 1701. https://doi.org/10.3390/antibiotics12121701
Clark JA, Curran MD, Gouliouris T, Conway Morris A, Bousfield R, Navapurkar V, Kean IRL, Daubney E, White D, Baker S, et al. Rapid Detection of Antimicrobial Resistance Genes in Critically Ill Children Using a Custom TaqMan Array Card. Antibiotics. 2023; 12(12):1701. https://doi.org/10.3390/antibiotics12121701
Chicago/Turabian StyleClark, John A., Martin D. Curran, Theodore Gouliouris, Andrew Conway Morris, Rachel Bousfield, Vilas Navapurkar, Iain R. L. Kean, Esther Daubney, Deborah White, Stephen Baker, and et al. 2023. "Rapid Detection of Antimicrobial Resistance Genes in Critically Ill Children Using a Custom TaqMan Array Card" Antibiotics 12, no. 12: 1701. https://doi.org/10.3390/antibiotics12121701
APA StyleClark, J. A., Curran, M. D., Gouliouris, T., Conway Morris, A., Bousfield, R., Navapurkar, V., Kean, I. R. L., Daubney, E., White, D., Baker, S., & Pathan, N. (2023). Rapid Detection of Antimicrobial Resistance Genes in Critically Ill Children Using a Custom TaqMan Array Card. Antibiotics, 12(12), 1701. https://doi.org/10.3390/antibiotics12121701






