Prevalence of Azole-Resistant Aspergillus Section Fumigati Strains Isolated from Romanian Vineyard Soil Samples
Abstract
:1. Introduction
2. Results
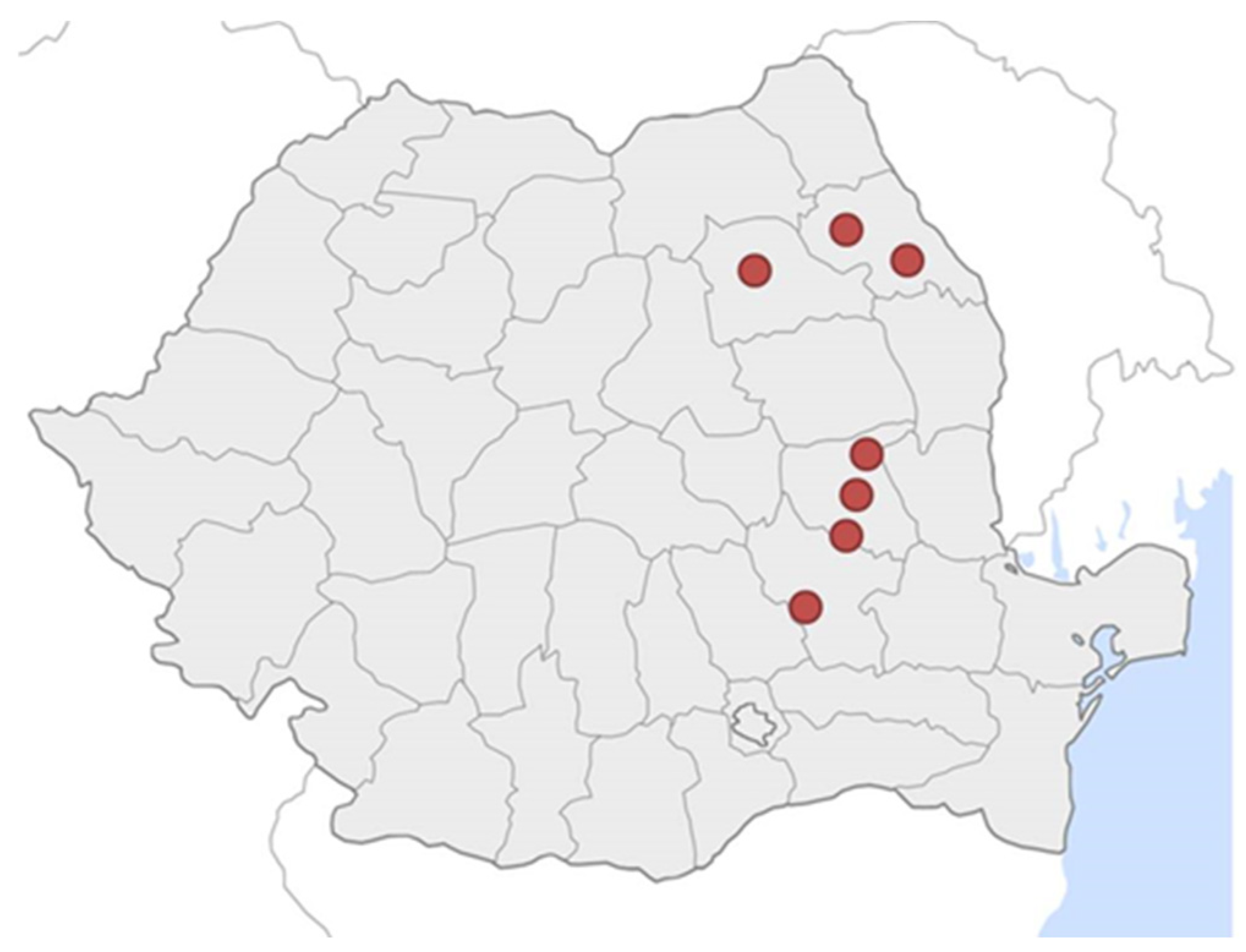
2.1. Vineyards and Treatments Applied to Soil
2.2. Screening of Azole-Resistant Fungi
2.3. Azole Susceptibility Testing of Aspergillus Section Fumigati Isolates
3. Discussion
4. Materials and Methods
4.1. Soil Sample Collection
4.2. Soil Sample Processing
4.3. Molecular Analysis
4.4. Azole Susceptibility Testing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugui, J.A.; Kwon-Chung, K.J.; Juvvadi, P.R.; Latgé, J.P.; Steinbach, W.J. Aspergillus fumigatus and related species. Cold Spring Harb. Perspect. Med. 2014, 5, a019786. [Google Scholar] [CrossRef] [PubMed]
- Latgé, J. The pathobiology of Aspergillus fumigatus. Trend Microbiol. 2001, 9, 382–389. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Risk Assessment on the Impact of Environmental Usage of Triazoles on the Development and Spread of Resistance to Medical Triazoles in Aspergillus Species; ECDC: Stockholm, Sweden, 2013. [Google Scholar]
- Mareș, M.; Moroti-Constantinescu, V.R.; Denning, D.W. The Burden of Fungal Diseases in Romania. J. Fungi 2018, 4, 31. [Google Scholar] [CrossRef]
- Organisation Internationale de la Vigne et du Vin. 2022. Available online: https://www.oiv.int/sites/default/files/documents/eng-state-of-the-world-vine-and-wine-sector-april-2022-v6_0.pdf (accessed on 27 October 2023).
- Dumitriu (Gabur), G.D.; Teodosiu, C.; Cotea, V. Management of Pesticides from Vineyard to Wines: Focus on Wine Safety and Pesticides Removal by Emerging Technologies. In Grapes and Wine; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Popescu, A.; Tindeche, C.; Marcuta, A.; Marcuta, L.; Hontus, A. Pesticides—A Problem in Romania’s Agriculture? Sci. Pap. Ser. Manag. Econ. Eng. Agric. Rural. Dev. 2021, 21, 4. [Google Scholar]
- Schwartz, S.; Ruhnke, M.; Ribaud, P.; Corey, L.; Driscoll, T.; Cornely, O.A.; Schuler, U.; Lutsar, I.; Troke, P.; Thiel, E. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood 2005, 106, 2641–2645. [Google Scholar] [CrossRef]
- Patterson, T.F.; Kirkpatrick, W.R.; White, M.; Hiemenz, J.W.; Wingard, J.R.; Dupont, B.; Rinaldi, M.G.; Stevens, D.A.; Graybill, J.R. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine 2000, 79, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Testempasis, S.I.; Kamou, N.N.; Papadakis, E.-N.; Menkissoglu-Spiroudi, U.; Karaoglanidis, G.S. Conventional vs. organic vineyards: Black Aspergilli population structure, mycotoxigenic capacity and mycotoxin contamination assessment in wines, using a new Q-TOF MS-MS detection method. Food Control 2022, 136, 108860. [Google Scholar] [CrossRef]
- Palumbo, J.D.; O’Keeffe, T.L.; Fidelibus, M.W. Characterization of Aspergillus section Nigri species populations in vineyard soil using droplet digital PCR. Lett. Appl. Microbiol. 2016, 63, 458–465. [Google Scholar] [CrossRef]
- Bader, O.; Tünnermann, J.; Dudakova, A.; Tangwattanachuleeporn, M.; Weig, M.; Groß, U.; Hoberg, N.; Geibel, S.; Vogel, E.; Büntzel, J.; et al. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob. Agents Chemother. 2015, 59, 4356–4359. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Moreno, C.; Lavergne, R.A.; Hagen, F.; Morio, F.; Meis, J.F.; Le Pape, P. Fungicide-driven alterations in azole-resistant Aspergillus fumigatus are related to vegetable crops in Colombia, South America. Mycologia 2019, 111, 217–224. [Google Scholar] [CrossRef]
- Prigitano, A.; Venier, V.; Cogliati, M.; De Lorenzis, G.; Esposto, M.C.; Tortorano, A.M. Azole-resistant Aspergillus fumigatus in the environment of Northern Italy, May 2011 to June 2012. Eurosurveillance 2014, 19, 20747. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, K.L.; Mellado, E.; Lass-Flörl, C.; Rodriguez-Tudela, J.L.; Johansen, H.K.; Arendrup, M.C. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 2010, 54, 4545–4549. [Google Scholar] [CrossRef] [PubMed]
- Sewell, T.R.; Zhang, Y.; Brackin, A.P.; Shelton, J.M.G.; Rhodes, J.; Fisher, M.C. Elevated prevalence of azole resistant Aspergillus fumigatus in urban versus rural environments in the United Kingdom. Antimicrob. Agents Chemother. 2019, 63, e00548-19. [Google Scholar] [CrossRef] [PubMed]
- Jeanvoine, A.; Rocchi, S.; Reboux, G.; Crini, N.; Crini, G.; Millon, L. Azole-resistant Aspergillus fumigatus in sawmills of eastern France. J. Appl. Microbiol. 2017, 123, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Isler, B.; Simos, P.; Farquhar, D.; George, N.; Golmayo, M.; Heney, C. Aspergillus Species Causing Invasive Fungal Disease in Queensland, Australia. Mycopathologia 2023, 188, 211–219. [Google Scholar] [CrossRef]
- Badali, H.; Cañete-Gibas, C.; McCarthy, D.; Patterson, H.; Sanders, C.; David, M.P.; Mele, J.; Fan, H.; Wiederhold, N.P. Species Distribution and Antifungal Susceptibilities of Aspergillus Section Fumigati Isolates in Clinical Samples from the United States. J. Clin. Microbiol. 2022, 60, e0028022. [Google Scholar] [CrossRef]
- Balajee, S.A.; Kano, R.; Baddley, J.W.; Moser, S.A.; Marr, K.A.; Alexander, B.D.; Andes, D.; Kontoyiannis, D.P.; Perrone, G.; Peterson, S.; et al. Molecular identification of Aspergillus species collected for the Transplant-Associated Infection Surveillance Network. J. Clin. Microbiol. 2009, 47, 3138–3141. [Google Scholar] [CrossRef]
- Alastruey-Izquierdo, A.; Mellado, E.; Peláez, T.; Pemán, J.; Zapico, S.; Alvarez, M.; Rodríguez-Tudela, J.L.; Cuenca-Estrella, M.; FILPOP Study Group. Population-based survey of filamentous fungi and antifungal resistance in Spain (FILPOP study). Antimicrob. Agents Chemother. 2013, 57, 3380–3387. [Google Scholar] [CrossRef]
- Cighir, A.; Mare, A.D.; Cighir, T.; Coșeriu, R.L.; Vintilă, C.; Man, A. Filamentous Fungi Infections: Yet Another Victim of COVID-19? Life 2023, 13, 546. [Google Scholar] [CrossRef]
- Bogdan, I.; Reddyreddy, A.R.; Nelluri, A.; Maganti, R.K.; Bratosin, F.; Fericean, R.M.; Dumitru, C.; Barata, P.I.; Tapalaga, G.; Marincu, I. Fungal Infections Identified with Multiplex PCR in Severe COVID-19 Patients during Six Pandemic Waves. Medicina 2023, 59, 1253. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Aranha Caetano, L.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2022, 32, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Siopi, M.; Rivero-Menendez, O.; Gkotsis, G.; Panara, A.; Thomaidis, N.S.; Alastruey-Izquierdo, A.; Pournaras, S.; Meletiadis, J. Nationwide surveillance of azole-resistant Aspergillus fumigatus environmental isolates in Greece: Detection of pan-azole resistance associated with the TR46/Y121F/T289A cyp51A mutation. J. Antimicrob. Chemother. 2020, 75, 3181–3188. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Guarro, J.; Gené, J.; Figueras, M. Atlas of Clinical Fungi; Centraalbureau voor Schimmelcultures (CBS): Hilversum, The Netherlands, 2000. [Google Scholar]
- Johnson, D.A.; Hamm, P.B.; Miller, J.S.; State, W. Fungicide Application for Management of Potato Late Blight in the Columbia Basin. 2014. Available online: http://public.wsu.edu/~djohnsn/index_files/Paper4.pdf (accessed on 29 November 2023).
- Baibakova, E.V.; Nefedjeva, E.E.; Suska-Malawska, M.; Wilk, M.; Sevriukova, G.A.; Zheltobriukhov, V.F. Modern Fungicides: Mechanisms of Action, Fungal Resistance and Phytotoxic Effects. Annu. Res. Rev. Biol. 2019, 32, 1–16. [Google Scholar] [CrossRef]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Prigitano, A.; Esposto, M.C.; Romanò, L.; Auxilia, F.; Tortorano, A.M. Azole-resistant Aspergillus fumigatus in the Italian environment. J. Glob. Antimicrob. Resist. 2019, 16, 220–224. [Google Scholar] [CrossRef]
- ATCC. Preservation and Recovery of Filamentous Fungi. Available online: https://www.atcc.org/resources/technical-documents/preservation-and-recovery-of-filamentous-fungi (accessed on 5 October 2019).
- The European Committee on Antimicrobial Susceptibility Testing. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.3, E.Def 9.3 and E.Def 11.0 Procedures. Version 2, 2020 and E.Def 7.4, E.Def 9.4 and E.Def 11.0 Procedures. Version 4.0, 2023. Available online: http://www.eucast.org (accessed on 29 November 2023).

| Order of Treatments | Product/Manufacturer | Type | Azole Component | Dosage | Mode of Action | Target Diseases and Pests |
|---|---|---|---|---|---|---|
| Vineyard 2 | ||||||
| 1 | Calcium polysulfide solution | Fungicide, Insecticide, Acaricide | No | 12 L/ha | Contact | Powdery mildew, Moths, Mites |
| 2 | Profiler (Bayer, Leverkusen, Germany) | Fungicide | No | 2.5 kg/ha | Systemic | Downy mildew |
| Topas (Syngenta, Basel, Switzerland) | Fungicide | Penconazole | 0.25 L/ha | Contact | Powdery mildew | |
| Envidor (Bayer, Leverkusen, Germany) | Acaricide | No | 0.4 L/ha | Contact | Erineum mites | |
| 3 | Forum Gold (BASF, Ludwigshafen, Germany) | Fungicide | No | 1.56 kg/ha | Systemic contact | Downy mildew |
| Vivando (BASF, Ludwigshafen, Germany) | Fungicide | No | 0.2 L/ha | Systemic contact | Powdery mildew | |
| Envidor | Acaricide | No | 0.4 L/ha | Contact | Erineum mites | |
| 4 | Forum Gold | Fungicide | No | 1.56 kg/ha | Systemic contact | Downy mildew |
| Vivando | Fungicide | No | 0.2 L/ha | Systemic contact | Powdery mildew | |
| 5 | Folpan 80 WG (Adama, Ashdod, Israel) | Fungicide | No | 1.5 kg/ha | Contact | Downy mildew, Gray mold |
| Kumulus WDG (BASF, Ludwigshafen, Germany) | Fungicide | No | 3.0 kg/ha | Contact | Powdery mildew | |
| 6 | Ridomil (Syngenta, Basel, Switzerland) | Fungicide | No | 2.5 kg/ha | Systemic | Downy mildew |
| Kumulus WDG | Fungicide | No | 3.0 kg/ha | Contact | Powdery mildew | |
| Cantus® (BASF, Ludwigshafen, Germany) | Fungicide | No | 1.2 kg/ha | Systemic | Gray mold | |
| 7 | Ridomil | Fungicide | No | 2.5 kg/ha | Systemic | Downy mildew |
| Kumulus WDG | Fungicide | No | 3.0 kg/ha | Contact | Powdery mildew | |
| Cantus® | Fungicide | No | 1.2 kg/ha | Systemic | Gray mold | |
| 8 | Bouillie bordelaise | Fungicide | No | 5.0 kg/ha | Contact | Downy mildew |
| Vineyard 3 | ||||||
| 1 | Novozir MN 80 (Belchim, Bucuresti, Romania) | Fungicide | No | 2.0 kg/ha | Contact | Downy mildew |
| Polisulfură de calciu WDG | Fungicide | No | 3.0 kg/ha | Contact | Powdery mildew | |
| 2 | Manconova (Trustchem Co. Ltd., Hangzhou, China) | Fungicide | No | 2.5 kg/ha | Contact | Downy mildew |
| Kumulus WDG | Fungicide | No | 3.0 kg/ha | Contact | Powdery mildew | |
| Envidor | Acaricide | No | 0.4 L/ha | Contact | Erineum mites | |
| 3 | Forum Gold | Fungicide | No | 1.56 kg/ha | Systemic contact | Downy mildew |
| KarathaneTM Gold 350 EC (CortevaTM Agriscience, Indianapolis, Indiana, United States) | Fungicide | No | 0.5 L/ha | Systemic | Powdery mildew | |
| 4 | Curzate F (CortevaTM Agriscience, Indianapolis, Indiana, United States) | Fungicide | No | 2.5 L/ha | Systemic | Downy mildew |
| Luna Experience 400 SC (Bayer, Leverkusen, Germany) | Fungicide | Tebuconazole | 0.5 L/ha | Systemic | Powdery mildew | |
| 5 | Verita (Bayer, Leverkusen, Germany) | Fungicide | No | 2.5 kg/ha | Systemic | Downy mildew |
| Falcon 460 EC (Bayer, Leverkusen, Germany) | Fungicide | No | 0.3 L/ha | Systemic | Powdery mildew | |
| Sulfomat (Mifalchim, Onești, Romania) | Fungicide | No | 4 kg/ha | Contact | Powdery mildew | |
| Pyrus 400 SC (Agriphar, Ougree, Belarus) | Fungicide | No | 1.5 L/ha | Systemic | Gray mold | |
| 6 | Valis M (Belchim, Bucuresti, Romania) | Fungicide | No | 2.0 kg/ha | Systemic contact | Downy mildew, Gray mold |
| Folpan | Fungicide | No | 1.5 kg/ha | Contact | Downy mildew, Gray mold | |
| Kumulus WDG | Fungicide | No | 3.0 kg/ha | Contact | Powdery mildew | |
| Bumper 250 EC (Adama, Ashdod, Israel) | Fungicide | Propiconazole | 0.2 L/ha | Systemic | Powdery mildew | |
| 7 | Pergado F (Syngenta, Basel, Switzerland) | Fungicide | No | 2.5 kg/ha | Systemic contact | Downy mildew |
| Vivando | Fungicide | No | 0.2 L/ha | Systemic contact | Powdery mildew | |
| KarathaneTM Gold 350 EC | Fungicide | No | 0.5 L/ha | Systemic | Powdery mildew | |
| 8 | Melody Compact (Bayer, Leverkusen, Germany) | Fungicide | No | 1.5 kg/ha | Systemic contact | Downy mildew |
| Sulfomat | Fungicide | No | 4 kg/ha | Contact | Powdery mildew | |
| Kumulus WDG | Fungicide | No | 3.0 kg/ha | Contact | Powdery mildew | |
| Pyrinex (Adama, Ashdod, Israel) | Insecticide | No | 2.2 L/ha | Contact ingestion | Moths | |
| Vineyard 1, 4–6—Information not provided | ||||||
| Vineyard 7 | ||||||
| 6–8/year | Switch 62.5 WG (Syngenta, Basel, Switzerland) | Fungicide | No | 1 kg/ha | Systemic contact | Gray mold, Aspergillus spp. |
| NPK 16-16-16 | Fertilizer | No | ||||
| Other | Downy mildew, Powdery mildew | |||||
| Vineyard | No. of Samples Collected per Vineyard | Itraconazole-Resistant Fungi (No. of Positive Samples; Percentage) | Voriconazole-Resistant Fungi (No. of Positive Samples; Percentage) |
|---|---|---|---|
| 1 | 20 | Aspergillus section Nigri (5; 25%) | Aspergillus section Nigri (2; 10%) |
| Aspergillus section Usti (1; 5%) | Aspergillus section Usti (1; 5%) | ||
| Penicillium spp. (7; 35%) | - | ||
| Mucorales (2; 10%) | Mucorales (8; 40%) | ||
| 2 | 55 | Aspergillus section Nigri (13; 23.64%) | Aspergillus section Nigri (3; 5.45%) |
| Aspergillus section Flavi (2; 3.64%) | - | ||
| Aspergillus section Usti (3; 5.45%) | - | ||
| Aspergillus section Fumigati (6; 10.9%) | Aspergillus section Fumigati (6; 10.9%) | ||
| Mucorales (8; 14.55%) | Mucorales (15; 27.27%) | ||
| 3 | 50 | Aspergillus section Nigri (11; 22%) | Aspergillus section Nigri (7; 14%) |
| Aspergillus section Flavi (4; 8%) | Aspergillus section Flavi (2; 4%) | ||
| Aspergillus section Usti (3; 6%) | - | ||
| Aspergillus section Fumigati (2; 4%) | Aspergillus section Fumigati (2; 4%) | ||
| Aspergillus section Terrei (2; 4%) | Aspergillus section Terrei (2; 4%) | ||
| Penicillium spp. (6; 12%) | - | ||
| Mucorales (7; 14%) | Mucorales (16; 32%) | ||
| 4 | 40 | Aspergillus section Nigri (11; 27.5%) | Aspergillus section Nigri (4; 10%) |
| Aspergillus section Usti (3; 7.5%) | Aspergillus section Usti (3; 7.5%) | ||
| Aspergillus section Fumigati (2; 5%) | Aspergillus section Fumigati (2; 5%) | ||
| Penicillium spp. (6; 15%) | - | ||
| Mucorales (3; 7.5%) | Mucorales (15; 37.5%) | ||
| 5 | 40 | Aspergillus section Nigri (14; 35%) | Aspergillus section Nigri (5; 12.5%) |
| Aspergillus section Flavi (2; 5%) | - | ||
| Aspergillus section Usti (2; 5%) | Aspergillus section Usti (2; 5%) | ||
| Aspergillus section Fumigati (6; 15%) | Aspergillus section Fumigati (6; 15%) | ||
| Penicillium spp. (5; 12.5%) | - | ||
| Mucorales (7; 17.5%) | Mucorales (15; 37.5%) | ||
| 6 | 40 | Aspergillus section Nigri (3; 7.5%) | - |
| Penicillium spp. (9; 22.5%) | - | ||
| Mucorales (1; 2.5%) | Mucorales (15; 37.5%) | ||
| 7 | 20 | Aspergillus section Nigri (8; 40%) | Aspergillus section Nigri (3; 15%) |
| Aspergillus section Usti (1; 5%) | Aspergillus section Usti (1; 5%) | ||
| Penicillium spp. (6; 30%) | - | ||
| Mucorales (1; 5%) | Mucorales (8; 40%) |
| Vineyard | Species | Itraconazole | Voriconazole | Posaconazole |
|---|---|---|---|---|
| 2 | Aspergillus udagawae | 8 | >8 | 4 |
| 2 | Aspergillus udagawae | >8 | >8 | >4 |
| 2 | Aspergillus udagawae | 8 | >8 | >4 |
| 2 | Aspergillus udagawae | 4 | 8 | 4 |
| 2 | Aspergillus udagawae | 8 | 4 | 2 |
| 2 | Aspergillus udagawae | 2 | 4 | 0.5 |
| 3 | Aspergillus udagawae | 2 | 1 | 0.5 |
| 3 | Aspergillus udagawae | >8 | >8 | 2 |
| 4 | Aspergillus udagawae | 2 | 4 | 0.5 |
| 4 | Aspergillus udagawae | 4 | 8 | 4 |
| 5 | Aspergillus udagawae | 8 | 4 | 4 |
| 5 | Aspergillus udagawae | 2 | 4 | 0.5 |
| 5 | Aspergillus lentulus | 8 | 4 | 4 |
| 5 | Aspergillus udagawae | 4 | 4 | >4 |
| 5 | Aspergillus udagawae | 8 | 4 | >4 |
| 5 | Aspergillus udagawae | >8 | >8 | >4 |
| Calmodulin | CL1 | 20 pb | GAR TWC AAG GAG GCC TTC TC GARTWCAAGGAGGCCTTCTC |
| CL2 | 21 pb | TTT TTG CAT CAT GAG TTG GAC TTTTTGCATCATGAGTTGGAC | |
| Tubulin | Bt2A | 24 pb | GGT AAC CAA ATC GGT GCT GCT TTC GGTAACCAAATCGGTGCTGCTTTC |
| Bt2B | 24 pb | ACC CTC AGT GTA GTG ACC CTT GGC ACCCTCAGTGTAGTGACCCTTGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colosi, H.A.; Baciu, A.M.; Costache, C.; Opris, R.V.; Popp, R.A.; Sabou, M.; Colosi, I.A. Prevalence of Azole-Resistant Aspergillus Section Fumigati Strains Isolated from Romanian Vineyard Soil Samples. Antibiotics 2023, 12, 1695. https://doi.org/10.3390/antibiotics12121695
Colosi HA, Baciu AM, Costache C, Opris RV, Popp RA, Sabou M, Colosi IA. Prevalence of Azole-Resistant Aspergillus Section Fumigati Strains Isolated from Romanian Vineyard Soil Samples. Antibiotics. 2023; 12(12):1695. https://doi.org/10.3390/antibiotics12121695
Chicago/Turabian StyleColosi, Horațiu Alexandru, Alina Mihaela Baciu, Carmen Costache, Razvan Vlad Opris, Radu Anghel Popp, Marcela Sabou, and Ioana Alina Colosi. 2023. "Prevalence of Azole-Resistant Aspergillus Section Fumigati Strains Isolated from Romanian Vineyard Soil Samples" Antibiotics 12, no. 12: 1695. https://doi.org/10.3390/antibiotics12121695
APA StyleColosi, H. A., Baciu, A. M., Costache, C., Opris, R. V., Popp, R. A., Sabou, M., & Colosi, I. A. (2023). Prevalence of Azole-Resistant Aspergillus Section Fumigati Strains Isolated from Romanian Vineyard Soil Samples. Antibiotics, 12(12), 1695. https://doi.org/10.3390/antibiotics12121695






