Impact of Beta-Lactam Target Attainment on Resistance Development in Patients with Gram-Negative Infections
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Data Collection
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention. About Antimicrobial Resistance. 2022. Available online: https://www.cdc.gov/drugresistance/about.html (accessed on 23 March 2023).
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. 1 April 2014. Available online: https://www.who.int/publications/i/item/9789241564748 (accessed on 23 March 2023).
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Antibacterial discovery and development—The failure of success? Nat. Biotechnol. 2006, 24, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- MacVane, S.H. Antimicrobial Resistance in the Intensive Care Unit: A Focus on Gram-Negative Bacterial Infections. J. Intensiv. Care Med. 2016, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef]
- Heffernan, A.J.; Sime, F.B.; Lipman, J.; Roberts, J.A. Individualising Therapy to Minimize Bacterial Multidrug Resistance. Drugs 2018, 78, 621–641. [Google Scholar] [CrossRef]
- Adembri, C.; Novelli, A.; Nobili, S. Some Suggestions from PK/PD Principles to Contain Resistance in the Clinical Setting—Focus on ICU Patients and Gram-Negative Strains. Antibiotics 2020, 9, 676. [Google Scholar] [CrossRef]
- Rybak, M.J. Pharmacodynamics: Relation to antimicrobial resistance. Am. J. Infect. Control 2006, 34, S38–S45; discussion S64–S73. [Google Scholar] [CrossRef]
- Alshaer, M.H.; Maranchick, N.; Bai, C.; Maguigan, K.L.; Shoulders, B.; Felton, T.W.; Mathew, S.K.; Mardini, M.T.; Peloquin, C.A. Using Machine Learning to Define the Impact of Beta-Lactam Early and Cumulative Target Attainment on Outcomes in Intensive Care Unit Patients with Hospital-Acquired and Ventilator-Associated Pneumonia. Antimicrob. Agents Chemother. 2022, 66, e0056322. [Google Scholar] [CrossRef]
- Roberts, J.A.; Paul, S.K.; Akova, M.; Bassetti, M.; De Waele, J.J.; Dimopoulos, G.; Kaukonen, K.-M.; Koulenti, D.; Martin, C.; Montravers, P.; et al. DALI: Defining Antibiotic Levels in Intensive Care Unit Patients: Are Current β-Lactam Antibiotic Doses Sufficient for Critically Ill Patients? Clin. Infect. Dis. 2014, 58, 1072–1083. [Google Scholar] [CrossRef]
- Guilhaumou, R.; Benaboud, S.; Bennis, Y.; Dahyot-Fizelier, C.; Dailly, E.; Gandia, P.; Goutelle, S.; Lefeuvre, S.; Mongardon, N.; Roger, C.; et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients—Guidelines from the French Society of Pharmacology and Therapeutics (Société Française de Pharmacologie et Thérapeutique—SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Société Française d’Anesthésie et Réanimation—SFAR). Crit. Care 2019, 23, 104. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Drusano, G.L.; Louie, A.; MacGowan, A.; Hope, W. Suppression of Emergence of Resistance in Pathogenic Bacteria: Keeping Our Powder Dry, Part 1. Antimicrob. Agents Chemother. 2016, 60, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, P.S.; Paladino, J.A.; Schentag, J.J. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 2008, 31, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Schentag, J.J.; Nix, D.E.; Adelman, M.H. Mathematical Examination of Dual Individualization Principles (I): Relationships between AUC above MIC and Area under the Inhibitory Curve for Cefmenoxime, Ciprofloxacin, and Tobramycin. DICP 1991, 25, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.A. State-of-the-Art Clinical Article: Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men. Clin. Infect. Dis. 1998, 26, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.W.; Goodwin, J.; O’Connor, L.; Sharp, A.; Gregson, L.; Livermore, J.; Howard, S.J.; Neely, M.N.; Hope, W.W. Impact of Bolus Dosing versus Continuous Infusion of Piperacillin and Tazobactam on the Development of Antimicrobial Resistance in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 5811–5819. [Google Scholar] [CrossRef] [PubMed]
- Taccone, F.S.; Laterre, P.-F.; Dugernier, T.; Spapen, H.; Delattre, I.; Witebolle, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.-L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Dijkstra, A.; Hunfeld, N.G.M.; Endeman, H.; Bahmany, S.; Ewoldt, T.M.J.; Muller, A.E.; van Gelder, T.; Gommers, D.; Koch, B.C.P. Failure of target attainment of beta-lactam antibiotics in critically ill patients and associated risk factors: A two-center prospective study (EXPAT). Crit. Care 2020, 24, 558. [Google Scholar] [CrossRef]
- Doern, G.V.; Brecher, S.M. The Clinical Predictive Value (or Lack Thereof) of the Results of In Vitro Antimicrobial Susceptibility Tests. J. Clin. Microbiol. 2011, 49, S11–S14. [Google Scholar] [CrossRef]
- Venugopalan, V.; Hamza, M.; Santevecchi, B.; DeSear, K.; Cherabuddi, K.; Peloquin, C.A.; Alshaer, M.H. Implementation of a β-lactam therapeutic drug monitoring program: Experience from a large academic medical center. Am. J. Health Pharm. 2022, 79, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Gattu, S.; Marchaim, D.; Bhargava, A.; Palla, M.; Alshabani, K.; Gudur, U.M.; Pulluru, H.; Bathina, P.; Sundaragiri, P.R.; et al. Epidemiology and Risk Factors for Isolation of Escherichia coli Producing CTX-M-Type Extended-Spectrum β-Lactamase in a Large U.S. Medical Center. Antimicrob. Agents Chemother. 2013, 57, 4010–4018. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Rodríguez-Baño, J.; Arslan, H.; Pitout, J.D.D.; Quentin, C.; Calbo, E.S.; Azap, K.; Arpin, C.; Pascual, A.; Livermore, D.M.; et al. A Multinational Survey of Risk Factors for Infection with Extended-Spectrum β-Lactamase–Producing Enterobacteriaceae in Nonhospitalized Patients. Clin. Infect. Dis. 2009, 49, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Maina, J.W.; Onyambu, F.G.; Kibet, P.S.; Musyoki, A.M. Multidrug-resistant Gram-negative bacterial infections and associated factors in a Kenyan intensive care unit: A cross-sectional study. Ann. Clin. Microbiol. Antimicrob. 2023, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Al Hamdan, A.S.; Alghamdi, A.; Alyousif, G.F.; Hamza, F.; Shafey, M.M.; AlAmri, A.M.; Sunki, A.A. Evaluating the Prevalence and the Risk Factors of Gram-Negative Multi-Drug Resistant Bacteria in Eastern Saudi Arabia. Infect. Drug Resist. 2022, 15, 475–490. [Google Scholar] [CrossRef]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin. Infect. Dis. 2023, 2023, ciad428. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wei, H.; Zhao, Y.; Shang, L.; Di, L.; Lyu, C.; Liu, J. Analysis of multidrug-resistant bacteria in 3223 patients with hospital-acquired infections (HAI) from a tertiary general hospital in China. Bosn. J. Basic Med. Sci. 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Balkhair, A.; Al-Farsi, Y.M.; Al-Muharrmi, Z.; Al-Rashdi, R.; Al-Jabri, M.; Neilson, F.; Al-Adawi, S.S.; El-Beeli, M.; Al-Adawi, S. Epidemiology of Multi-Drug Resistant Organisms in a Teaching Hospital in Oman: A One-Year Hospital-Based Study. Sci. World J. 2014, 2014, 157102. [Google Scholar] [CrossRef]
- Wong, G.; Briscoe, S.; Adnan, S.; McWhinney, B.; Ungerer, J.; Lipman, J.; Roberts, J.A. Protein Binding of β-Lactam Antibiotics in Critically Ill Patients: Can We Successfully Predict Unbound Concentrations? Antimicrob. Agents Chemother. 2013, 57, 6165–6170. [Google Scholar] [CrossRef]
- Craig, W.A. The Pharmacology of Meropenem, A New Carbapenem Antibiotic. Clin. Infect. Dis. 1997, 24, S266–S275. [Google Scholar] [CrossRef]
- Adnan, S.; Paterson, D.L.; Lipman, J.; Kumar, S.; Li, J.; Rudd, M.; Roberts, J.A. Pharmacokinetics of Beta-Lactam Antibiotics in Patients with Intra-Abdominal Disease: A Structured Review. Surg. Infect. 2012, 13, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, M.H.; Goutelle, S.; Santevecchi, B.A.; Shoulders, B.R.; Venugopalan, V.; Cherabuddi, K.; Liu, J.; Kiel, P.J.; Roberts, J.A.; Sime, F.B.; et al. Cefepime Precision Dosing Tool: From Standard to Precise Dose Using Nonparametric Population Pharmacokinetics. Antimicrob. Agents Chemother. 2022, 66, e0204621. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.K.; Mathew, B.S.; Neely, M.N.; Naik, G.S.; Prabha, R.; Jacob, G.G.; Subramani, K.; Fleming, D.H. A Nonparametric Pharmacokinetic Approach to Determine the Optimal Dosing Regimen for 30-Minute and 3-Hour Meropenem Infusions in Critically Ill Patients. Ther. Drug Monit. 2016, 38, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Felton, T.W.; Roberts, J.A.; Lodise, T.P.; Van Guilder, M.; Boselli, E.; Neely, M.N.; Hope, W.W. Individualization of Piperacillin Dosing for Critically Ill Patients: Dosing Software to Optimize Antimicrobial Therapy. Antimicrob. Agents Chemother. 2014, 58, 4094–4102. [Google Scholar] [CrossRef]

| Clinical Characteristics | Median (IQR) or n (%) |
|---|---|
| Age, years | 58 (42–69) |
| Sex, Male | 151 (59) |
| Weight, kg | 73.6 (60.9–94.3) |
| Serum creatinine, mg/dL | 0.81 (0.57–1.27) |
| BMI, kg/m2 | 26 (21.4–33.4) |
| Renal replacement therapy | 44 (17) |
| Liver Disease | 78 (30) |
| COPD | 55 (21) |
| Diabetes | 113 (44) |
| Heart failure | 105 (41) |
| Mechanical Ventilation | 66 (26) |
| Patient in ICU at beta-lactam initiation | 203 (79) |
| Length of stay, days ICU Hospital | 14 (2–29) 25 (16–47) |
| Mortality | 122 (48) |
| Gram-negative isolates, n | 628 |
| Common isolated bacteria, n (% developing resistance) Pseudomonas aeruginosa Escherichia coli Klebsiella pneumoniae Enterobacter cloacae Serratia marcescens Proteus mirabilis Acinetobacter baumannii Klebsiella aerogenes | 134 (35.6) 30 (6.7) 26 (11.5) 20 (0) 19 (5.2) 14 (0) 14 (14.2) 10 (10) |
| Multi-drug resistant isolates # | 62 (9.8) |
| All Culture Sources Lung Blood Wound Urinary Tract Abscess/body fluid Other | 233 230 80 36 32 17 |
| Culture Sources (Same Final and Initial Source) Lung Blood Wound Urinary Tract Abscess/body fluid Other | 204 214 46 32 16 6 |
| Beta-lactam Received ^ Cefepime Meropenem Piperacillin/tazobactam | 179 (65) 54 (20) 41 (15) |
| Number of samples Cefepime Meropenem Piperacillin/tazobactam | 316 91 73 |
| Beta-lactam therapy duration, days | 8 (5–14) |
| Time between cultures, days | 7 (4–15) |
| Time between start of beta-lactam therapy and TDM, days | 3 (2–8) |
| Concomitant Antibiotics -Aminoglycoside -Fluoroquinolone -Polymyxin | 124 (48) 57 (22) 30 (12) |
| Resistance (Any Increase in MIC) | Resistance (≥2× MIC Increase) | |||
|---|---|---|---|---|
| Covariates | OR | p-Value | OR | p-Value |
| Age (per 1 year) | 0.99 | 0.08 | 0.99 | 0.35 |
| BMI (per 1 kg/m2) | 1.00 | 0.73 | 1.00 | 0.82 |
| RRT during admission | 2.24 | 0.03 | 2.50 | 0.01 |
| Days on antibiotic therapy (per 1 day) | 1.01 | 0.06 | 1.01 | 0.06 |
| Days between cultures (per 1 day) | 1.06 | <0.0001 | 1.06 | <0.0001 |
| Mechanical Ventilation | 2.27 | 0.007 | 2.61 | 0.002 |
| Hospital LOS (per 1 day) | 1.01 | <0.0001 | 1.01 | <0.0001 |
| ICU LOS (per 1 day) | 1.02 | <0.0001 | 1.02 | <0.0001 |
| Diabetes | 1.14 | 0.67 | 0.94 | 0.88 |
| Liver Disease | 1.38 | 0.28 | 1.30 | 0.43 |
| COPD | 1.38 | 0.39 | 1.38 | 0.38 |
| Heart Failure | 1.18 | 0.56 | 1.36 | 0.30 |
| SOFA Score (per 1 point) | 1.05 | 0.12 | 1.08 | 0.02 |
| Resistance (Any Increase in MIC) | Resistance (≥2× MIC Increase) | |||
|---|---|---|---|---|
| PK/PD Parameter | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value |
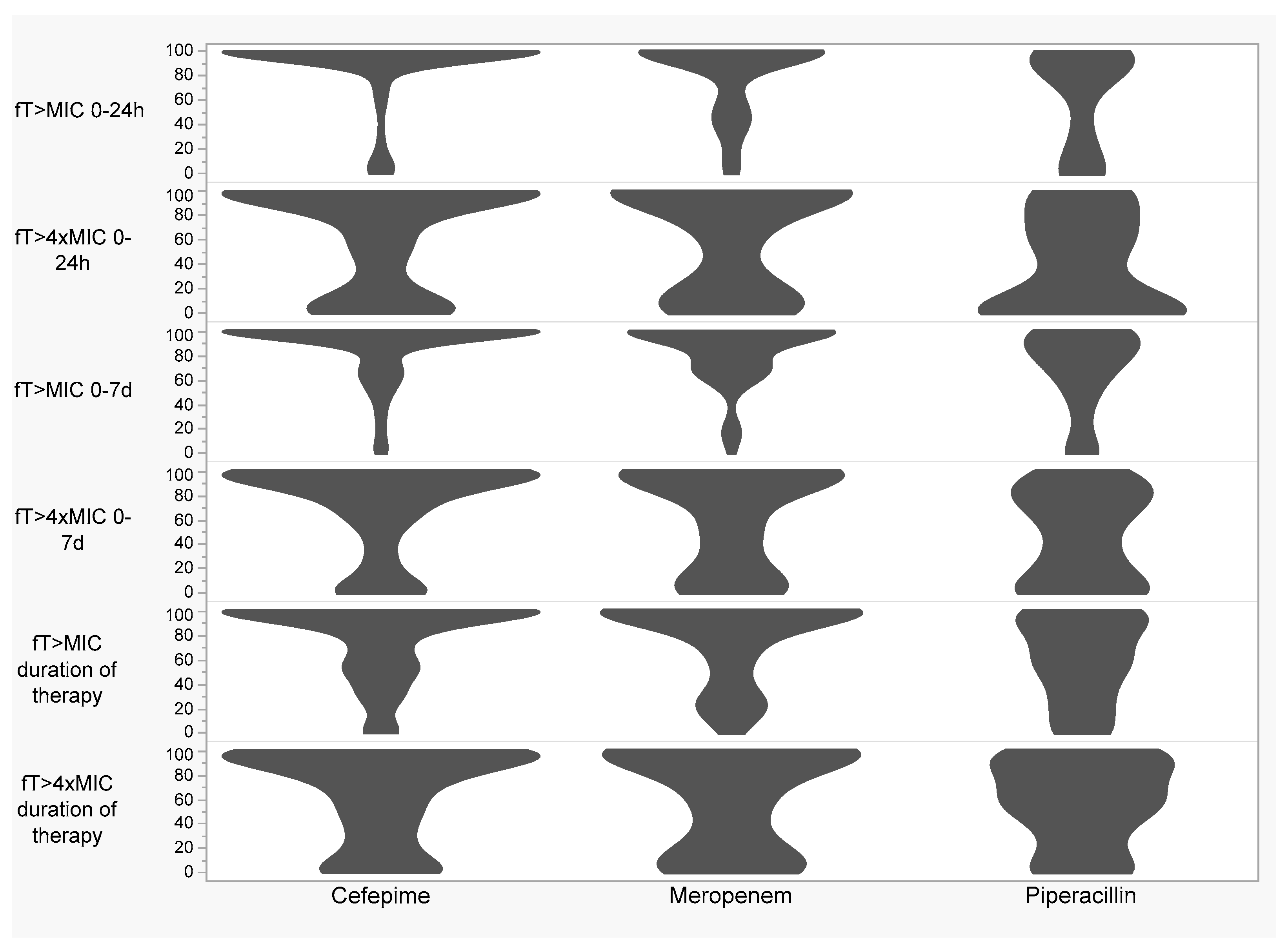
| % ƒT > MIC 0–24 h (per 10%) | 0.96 (0.88–1.07) | 0.50 | 1.06 (0.95–1.20) | 0.34 |
| % ƒT > 4× MIC 0–24 h (per 10%) | 0.98 (0.91–1.06) | 0.64 | 1.02 (0.94–1.10) | 0.71 |
| % ƒT > MIC 0–7 d (per 10%) | 1.06 (0.95–1.21) | 0.31 | 1.08 (0.94–1.24) | 0.27 |
| % ƒT > 4× MIC 0–7 d (per 10%) | 1.03 (0.94–1.12) | 0.51 | 1.08 (0.99–1.19) | 0.08 |
| % ƒT > MIC duration of therapy (per 10%) | 0.98 (0.89–1.08) | 0.67 | 1.03 (0.92–1.14) | 0.63 |
| % ƒT > 4× MIC duration of therapy (per 10%) | 0.99 (0.92–1.09) | 0.95 | 1.03 (0.94–1.13) | 0.53 |
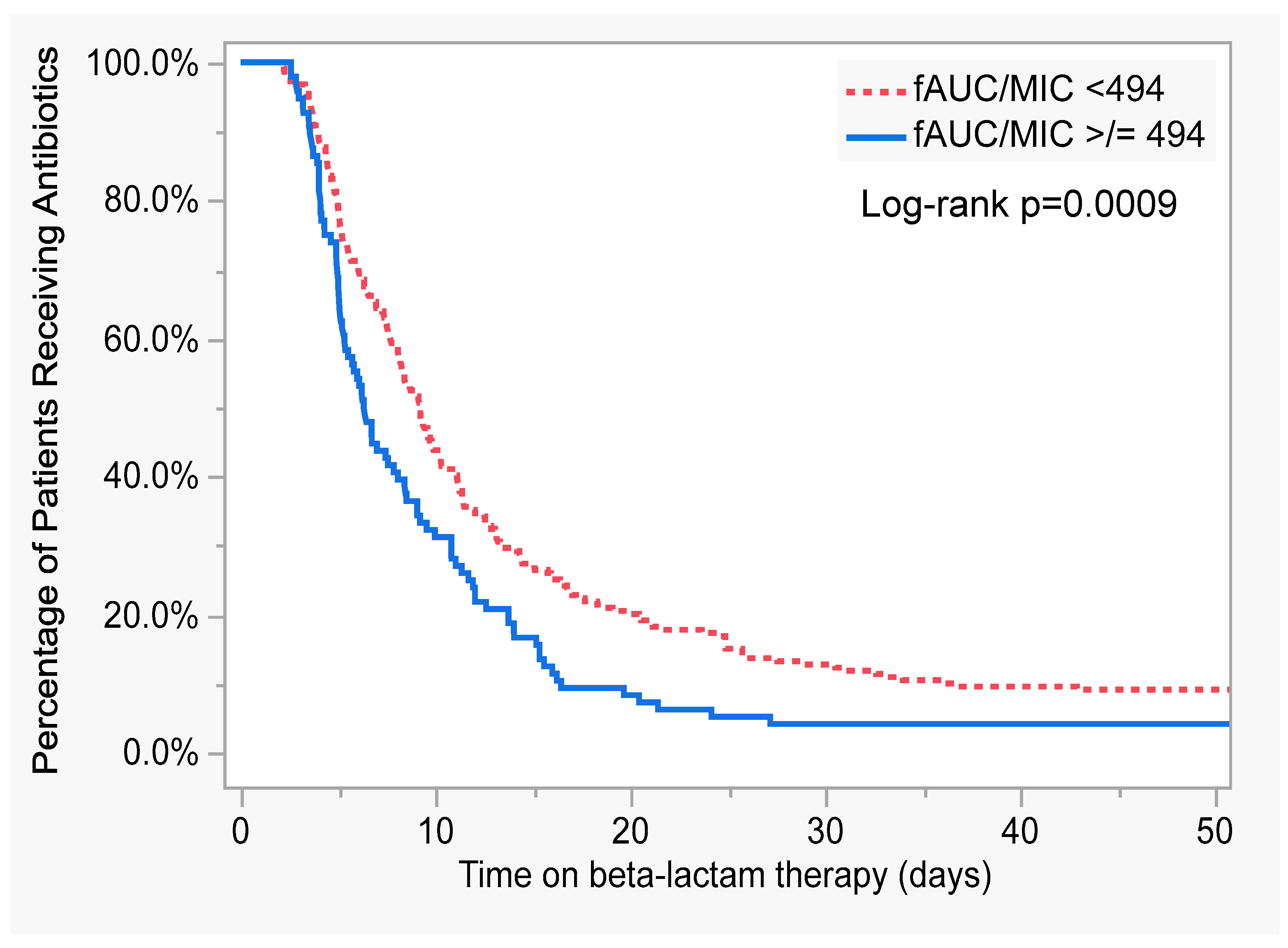
| Mean daily ƒAUC/MIC (per increments of 10) | 0.99 (0.98–1.00) | 0.08 | 1.00 (0.99–1.001) | 0.17 |
| Mean daily ƒAUC/MIC of 494 achieved (yes) | 0.25 (0.11–0.61) | 0.002 | 0.27 (0.11–0.67) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maranchick, N.F.; Webber, J.; Alshaer, M.H.; Felton, T.W.; Peloquin, C.A. Impact of Beta-Lactam Target Attainment on Resistance Development in Patients with Gram-Negative Infections. Antibiotics 2023, 12, 1696. https://doi.org/10.3390/antibiotics12121696
Maranchick NF, Webber J, Alshaer MH, Felton TW, Peloquin CA. Impact of Beta-Lactam Target Attainment on Resistance Development in Patients with Gram-Negative Infections. Antibiotics. 2023; 12(12):1696. https://doi.org/10.3390/antibiotics12121696
Chicago/Turabian StyleMaranchick, Nicole F., Jessica Webber, Mohammad H. Alshaer, Timothy W. Felton, and Charles A. Peloquin. 2023. "Impact of Beta-Lactam Target Attainment on Resistance Development in Patients with Gram-Negative Infections" Antibiotics 12, no. 12: 1696. https://doi.org/10.3390/antibiotics12121696
APA StyleMaranchick, N. F., Webber, J., Alshaer, M. H., Felton, T. W., & Peloquin, C. A. (2023). Impact of Beta-Lactam Target Attainment on Resistance Development in Patients with Gram-Negative Infections. Antibiotics, 12(12), 1696. https://doi.org/10.3390/antibiotics12121696





