Temporary Root Canal Obturation with a Calcium Hydroxide-Based Dressing: A Randomized Controlled Clinical Trial
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Treatment Procedure
- First sampling (Baseline)—following the removal of the temporary filling material and calcium hydroxide paste (Figure 2).
- Second sampling (Root Canal Treatment)—after clinical screening and inclusion of appropriate teeth into the study, the included teeth were prepared via chemomechanical root canal preparation up to size 30.09 (ProTaper Gold; Dentsply Sirona GmbH, Bensheim, Germany) under irrigation with sodium hypochlorite (3%; 5 mL total, applied over the duration of the root canal preparation) and ethylenediaminetetraacetic acid (15%; 2 mL).
- Third sampling (Disinfection I)—following additional rinsing with sodium hypochlorite (5 mL) (within the same treatment session as the second sampling).
- Fourth sampling [Medication (7d)]–following the removal of the temporary filling and calcium hydroxide paste and only if the tooth has been free of symptoms for 1 week.
- Fifth sampling (Disinfection II)—following a final rinse with sodium hypochlorite (5 mL) (within the same treatment session as 4th sampling).
3.2. Microbiological Analysis
3.3. Preliminary Study
3.4. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ordinola-Zapata, R.; Noblett, W.C.; Perez-Ron, A.; Ye, Z.; Vera, J. Present status and future directions of intracanal medicaments. Int. Endod. J. 2022, 55 (Suppl. S3), 613–636. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.-S.; Falk, W.; Frankenberger, R.; Braun, A. Impact of Adjunctive Laser Irradiation on the Bacterial Load of Dental Root Canals: A Randomized Controlled Clinical Trial. Antibiotics 2021, 10, 1557. [Google Scholar] [CrossRef] [PubMed]
- Shuping, G.B.; Orstavik, D.; Sigurdsson, A.; Trope, M. Reduction of intracanal bacteria using nickel-titanium rotary instrumentation and various medications. J. Endod. 2000, 26, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, M.; Kontakiotis, E.; Nakou, M. In vitro evaluation of the effectiveness of calcium hydroxide and paramonochlorophenol on anaerobic bacteria from the root canal. Endod. Dent. Traumatol. 1993, 9, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Heithersay, G.S. Calcium hydroxide in the treatment of pulpless teeth with associated pathology. J. Br. Endod. Soc. 1975, 8, 74–93. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Lopes, H.P. Mechanisms of antimicrobial activity of calcium hydroxide: A critical review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef]
- Vera, J.; Siqueira, J.F., Jr.; Ricucci, D.; Loghin, S.; Fernández, N.; Flores, B.; Cruz, A.G. One- versus two-visit endodontic treatment of teeth with apical periodontitis: A histobacteriologic study. J. Endod. 2012, 38, 1040–1052. [Google Scholar] [CrossRef]
- Kvist, T.; Molander, A.; Dahlén, G.; Reit, C. Microbiological evaluation of one- and two-visit endodontic treatment of teeth with apical periodontitis: A randomized, clinical trial. J. Endod. 2004, 30, 572–576. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Guimarães-Pinto, T.; Rôças, I.N. Effects of chemomechanical preparation with 2.5% sodium hypochlorite and intracanal medication with calcium hydroxide on cultivable bacteria in infected root canals. J. Endod. 2007, 33, 800–805. [Google Scholar] [CrossRef]
- Fabricius, L.; Dahlén, G.; Sundqvist, G.; Happonen, R.-P.; Möller, A.J.R. Influence of residual bacteria on periapical tissue healing after chemomechanical treatment and root filling of experimentally infected monkey teeth. Eur. J. Oral Sci. 2006, 114, 278–285. [Google Scholar] [CrossRef]
- Sundqvist, G.; Figdor, D.; Persson, S.; Sjögren, U. Microbiologic analysis of teeth with failed endodontic treatment and the outcome of conservative re-treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, U.; Figdor, D.; Spångberg, L.; Sundqvist, G. The antimicrobial effect of calcium hydroxide as a short-term intracanal dressing. Int. Endod. J. 1991, 24, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bystrom, A.; Claesson, R.; Sundqvist, G. The antibacterial effect of camphorated paramonochlorophenol, camphorated phenol and calcium hydroxide in the treatment of infected root canals. Endod. Dent. Traumatol. 1985, 1, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Blome, B.; Braun, A.; Sobarzo, V.; Jepsen, S. Molecular identification and quantification of bacteria from endodontic infections using real-time polymerase chain reaction. Oral Microbiol. Immunol. 2008, 23, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sathorn, C.; Parashos, P.; Messer, H. Antibacterial efficacy of calcium hydroxide intracanal dressing: A systematic review and meta-analysis. Int. Endod. J. 2007, 40, 2–10. [Google Scholar] [CrossRef]
- Koçkapan, C. Curriculum Endodontie; Quintessenz Publishing: Berlin, Germany, 2003. [Google Scholar]
- Kawashima, N.; Wadachi, R.; Suda, H.; Yeng, T.; Parashos, P. Root canal medicaments. Int. Dent. J. 2009, 59, 5–11. [Google Scholar]
- Duque, T.M.; Prado, M.; Herrera, D.R.; Gomes, B.P.F.A. Periodontal and endodontic infectious/inflammatory profile in primary periodontal lesions with secondary endodontic involvement after a calcium hydroxide-based intracanal medication. Clin. Oral Investig. 2019, 23, 53–63. [Google Scholar] [CrossRef]
- Safavi, K.E.; Nichols, F.C. Alteration of biological properties of bacterial lipopolysaccharide by calcium hydroxide treatment. J. Endod. 1994, 20, 127–129. [Google Scholar] [CrossRef]
- Chong, B.S.; Pitt Ford, T.R. The role of intracanal medication in root canal treatment. Int. Endod. J. 1992, 25, 97–106. [Google Scholar] [CrossRef]
- Vatankhah, M.; Khosravi, K.; Zargar, N.; Shirvani, A.; Nekoofar, M.H.; Dianat, O. Antibacterial efficacy of antibiotic pastes versus calcium hydroxide intracanal dressing: A systematic review and meta-analysis of ex vivo studies. J. Conserv. Dent. 2022, 25, 463–480. [Google Scholar] [CrossRef]
- Böcher, S.; Wenzler, J.-S.; Falk, W.; Braun, A. Comparison of different laser-based photochemical systems for periodontal treatment. Photodiagnosis Photodyn. Ther. 2019, 27, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ratih, D.N.; Mulyawati, E.; Fajrianti, H. Antibacterial efficacy, calcium ion release, and pH using calcium hydroxide with three vehicles. J. Conserv. Dent. 2022, 25, 515–520. [Google Scholar] [CrossRef]
- Lana, P.E.P.; Scelza, M.F.Z.; Silva, L.E.; Mattos-Guaraldi, A.L.d.; Hirata Júnior, R. Antimicrobial activity of calcium hydroxide pastes on Enterococcus faecalis cultivated in root canal systems. Braz. Dent. J. 2009, 20, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Wenzler, J.-S.; Krause, F.; Böcher, S.; Falk, W.; Birkenmaier, A.; Conrads, G.; Braun, A. Antimicrobial Impact of Different Air-Polishing Powders in a Subgingival Biofilm Model. Antibiotics 2021, 10, 1464. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Brändle, N.; Zehnder, M.; Weiger, R.; Waltimo, T. Impact of growth conditions on susceptibility of five microbial species to alkaline stress. J. Endod. 2008, 34, 579–582. [Google Scholar] [CrossRef]
- Distel, J.W.; Hatton, J.F.; Gillespie, M.J. Biofilm formation in medicated root canals. J. Endod. 2002, 28, 689–693. [Google Scholar] [CrossRef]
- Peters, O.A. Current challenges and concepts in the preparation of root canal systems: A review. J. Endod. 2004, 30, 559–567. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Kastrinakis, E.; Lambrianidis, T.; Verhaagen, B.; Versluis, M.; van der Sluis, L.W.M. Formation and removal of apical vapor lock during syringe irrigation: A combined experimental and Computational Fluid Dynamics approach. Int. Endod. J. 2014, 47, 191–201. [Google Scholar] [CrossRef]
- Pesse, A.V.; Warrier, G.R.; Dhir, V.K. An experimental study of the gas entrapment process in closed-end microchannels. Int. J. Heat Mass Transf. 2005, 48, 5150–5165. [Google Scholar] [CrossRef]
- Puleio, F.; Lizio, A.; Coppini, V.; Lo Giudice, R.; Lo Giudice, G. CBCT-Based Assessment of Vapor Lock Effects on Endodontic Disinfection. Appl. Sci. 2023, 13, 9542. [Google Scholar] [CrossRef]
- Kurihara, H.; Kobayashi, Y.; Francisco, I.A.; Isoshima, O.; Nagai, A.; Murayama, Y. A microbiological and immunological study of endodontic-periodontic lesions. J. Endod. 1995, 21, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Reit, C.; Molander, A.; Dahlén, G. The diagnostic accuracy of microbiologic root canal sampling and the influence of antimicrobial dressings. Endod. Dent. Traumatol. 1999, 15, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M. Endodontic infection caused by localized aggressive periodontitis: A case report and bacteriologic evaluation. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001, 92, 440–445. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rôças, I.N.; Lopes, H.P.; Magalhães, F.A.C.; de Uzeda, M. Elimination of Candida albicans infection of the radicular dentin by intracanal medications. J. Endod. 2003, 29, 501–504. [Google Scholar] [CrossRef]
- Wu, M.-K.; Dummer, P.M.H.; Wesselink, P.R. Consequences of and strategies to deal with residual post-treatment root canal infection. Int. Endod. J. 2006, 39, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.G.; Rabello, D.G.D.; Corazza, B.J.M.; Gomes, A.P.M.; Silva, E.G.; Martinho, F.C. Comparison of the effectiveness of single- and multiple-sessions disinfection protocols against endotoxins in root canal infections: Systematic review and meta-analysis. Sci. Rep. 2021, 11, 1226. [Google Scholar] [CrossRef]
- Sharma, G.; Ahmed, H.M.A.; Zilm, P.S.; Rossi-Fedele, G. Antimicrobial properties of calcium hydroxide dressing when used for long-term application: A systematic review. Aust. Endod. J. 2018, 44, 60–65. [Google Scholar] [CrossRef]
- Gonçalves, L.S.; Rodrigues, R.C.V.; Andrade Junior, C.V.; Soares, R.G.; Vettore, M.V. The Effect of Sodium Hypochlorite and Chlorhexidine as Irrigant Solutions for Root Canal Disinfection: A Systematic Review of Clinical Trials. J. Endod. 2016, 42, 527–532. [Google Scholar] [CrossRef]
- Neelakantan, P.; Herrera, D.R.; Pecorari, V.G.A.; Gomes, B.P.F.A. Endotoxin levels after chemomechanical preparation of root canals with sodium hypochlorite or chlorhexidine: A systematic review of clinical trials and meta-analysis. Int. Endod. J. 2019, 52, 19–27. [Google Scholar] [CrossRef]
- Ruksakiet, K.; Hanák, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-Ngoen, T.; Garami, A.; Mikó, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041.e7. [Google Scholar] [CrossRef] [PubMed]
- Balto, K.A. Calcium hydroxide has limited effectiveness in eliminating bacteria from human root canal. Evid. Based Dent. 2007, 8, 15–16. [Google Scholar] [CrossRef]
- Vianna, M.E.; Horz, H.-P.; Conrads, G.; Zaia, A.A.; Souza-Filho, F.J.; Gomes, B.P.F.A. Effect of root canal procedures on endotoxins and endodontic pathogens. Oral Microbiol. Immunol. 2007, 22, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Feliz Matos, L.; Rodriguez, I.D.L.S.; Gonzalez, M.L.R.; Pereyra, D.; Monzon Velez, E.R. Coronal microleakage of 3 temporary filling materials used for endodontic treatment: An in vitro study. Gen. Dent. 2013, 61, 52–55. [Google Scholar]
- Wuersching, S.N.; Moser, L.; Obermeier, K.T.; Kollmuss, M. Microleakage of Restorative Materials Used for Temporization of Endodontic Access Cavities. J. Clin. Med. 2023, 12, 4762. [Google Scholar] [CrossRef] [PubMed]
- Babu, N.S.V.; Bhanushali, P.V.; Bhanushali, N.V.; Patel, P. Comparative analysis of microleakage of temporary filling materials used for multivisit endodontic treatment sessions in primary teeth: An in vitro study. Eur. Arch. Paediatr. Dent. 2019, 20, 565–570. [Google Scholar] [CrossRef]
- Brundin, M.; Figdor, D.; Roth, C.; Davies, J.K.; Sundqvist, G.; Sjögren, U. Persistence of dead-cell bacterial DNA in ex vivo root canals and influence of nucleases on DNA decay in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 789–794. [Google Scholar] [CrossRef]
- Rôças, I.N.; Siqueira, J.F., Jr. Identification of bacteria enduring endodontic treatment procedures by a combined reverse transcriptase-polymerase chain reaction and reverse-capture checkerboard approach. J. Endod. 2010, 36, 45–52. [Google Scholar] [CrossRef]
- Meire, M.A.; van der Waal, S.V. A critical analysis of research methods and experimental models to study intracanal medicaments. Int. Endod. J. 2022, 55 (Suppl. S2), 330–345. [Google Scholar] [CrossRef]
- Arnold, M.; Arnold, W.; Attin, T.; Barbakow, F.; Benz, C.; Braun, A.; Bürkle, V.; Clauder, T.; Daubländer, M.; Edelhoff, D.; et al. Endodontologie, 2., Überarbeitete und Erweiterte Auflage; Georg Thieme Verlag KG: Stuttgart, Germany, 2008; ISBN 9783131902122. [Google Scholar]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
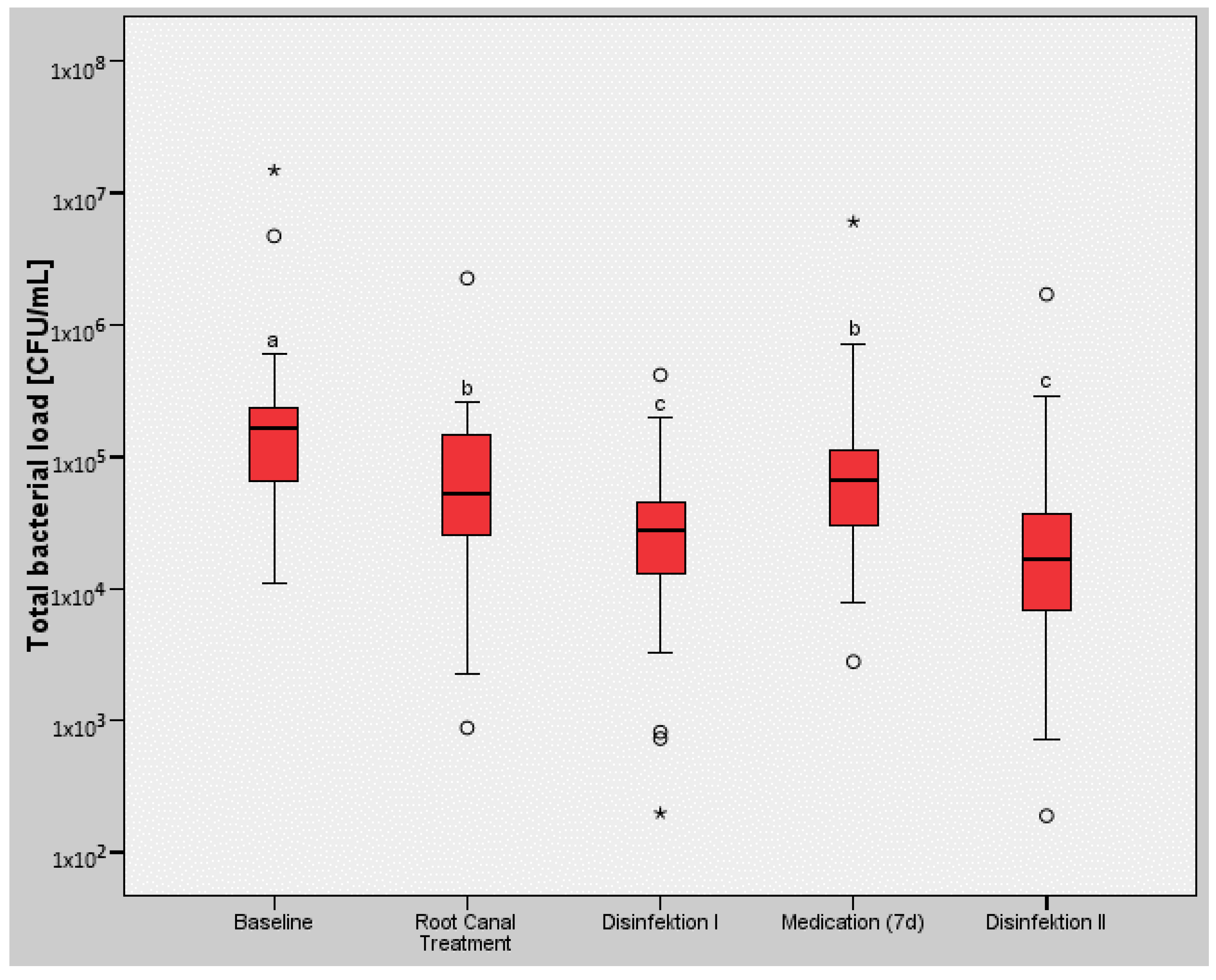
| Baseline | Root Canal Treatment | Disinfection I | Medication (7d) | Disinfection II | |
|---|---|---|---|---|---|
| Mean | 9.05 × 105 | 1.65 × 105 | 5.12 × 104 | 3.34 × 105 | 1.02 × 105 |
| Standard Deviation | 2.99 × 106 | 4.33 × 105 | 8.53 × 104 | 1.18 × 106 | 3.35 × 105 |
| Median | 1.63 × 105 | 5.26 × 104 | 2.78 × 104 | 6.76 × 104 | 1.68 × 104 |
| Minimum | 1.11 × 104 | 8.81 × 102 | 1.98 × 102 | 2.80 × 103 | 1.89 × 102 |
| Maximum | 1.49 × 107 | 2.26 × 106 | 4.18 × 105 | 6.07 × 106 | 1.71 × 106 |
| Interquartile Range | 1.60 × 105 | 1.14 × 105 | 3.05 × 104 | 7.78 × 104 | 2.99 × 104 |
| n | 26 | 26 | 26 | 26 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wenzler, J.-S.; Falk, W.; Frankenberger, R.; Braun, A. Temporary Root Canal Obturation with a Calcium Hydroxide-Based Dressing: A Randomized Controlled Clinical Trial. Antibiotics 2023, 12, 1663. https://doi.org/10.3390/antibiotics12121663
Wenzler J-S, Falk W, Frankenberger R, Braun A. Temporary Root Canal Obturation with a Calcium Hydroxide-Based Dressing: A Randomized Controlled Clinical Trial. Antibiotics. 2023; 12(12):1663. https://doi.org/10.3390/antibiotics12121663
Chicago/Turabian StyleWenzler, Johannes-Simon, Wolfgang Falk, Roland Frankenberger, and Andreas Braun. 2023. "Temporary Root Canal Obturation with a Calcium Hydroxide-Based Dressing: A Randomized Controlled Clinical Trial" Antibiotics 12, no. 12: 1663. https://doi.org/10.3390/antibiotics12121663
APA StyleWenzler, J.-S., Falk, W., Frankenberger, R., & Braun, A. (2023). Temporary Root Canal Obturation with a Calcium Hydroxide-Based Dressing: A Randomized Controlled Clinical Trial. Antibiotics, 12(12), 1663. https://doi.org/10.3390/antibiotics12121663









