The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies
Abstract
:1. Introduction
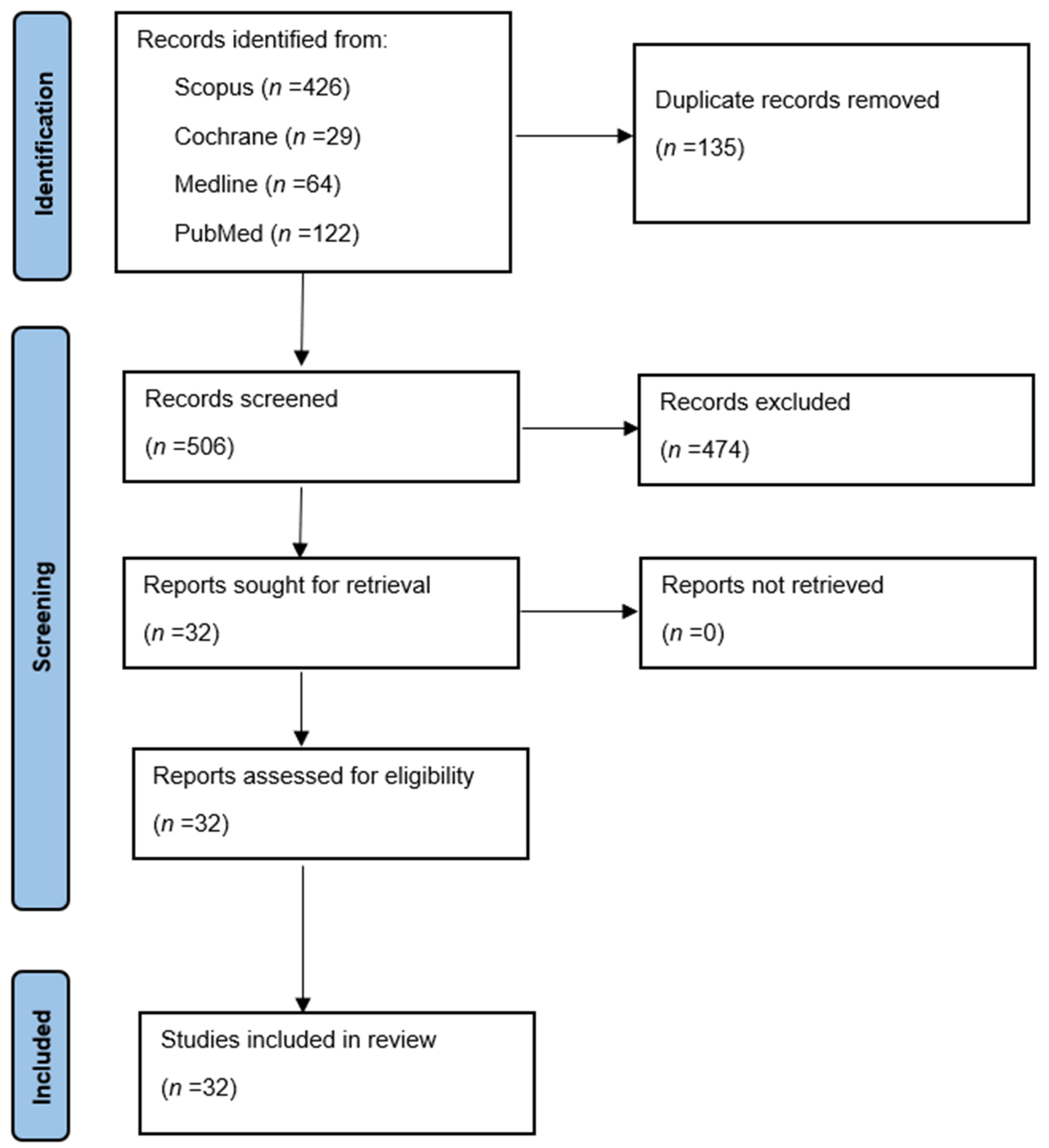
2. Results
2.1. Methodology of Studies
2.2. Microbial Profile
2.2.1. Phyla
2.2.2. Genus
2.2.3. Microbiome Complex
2.2.4. Peri-Implantitis with Periodontitis
2.2.5. Peri-Implantitis with Peri-Implant Mucositis
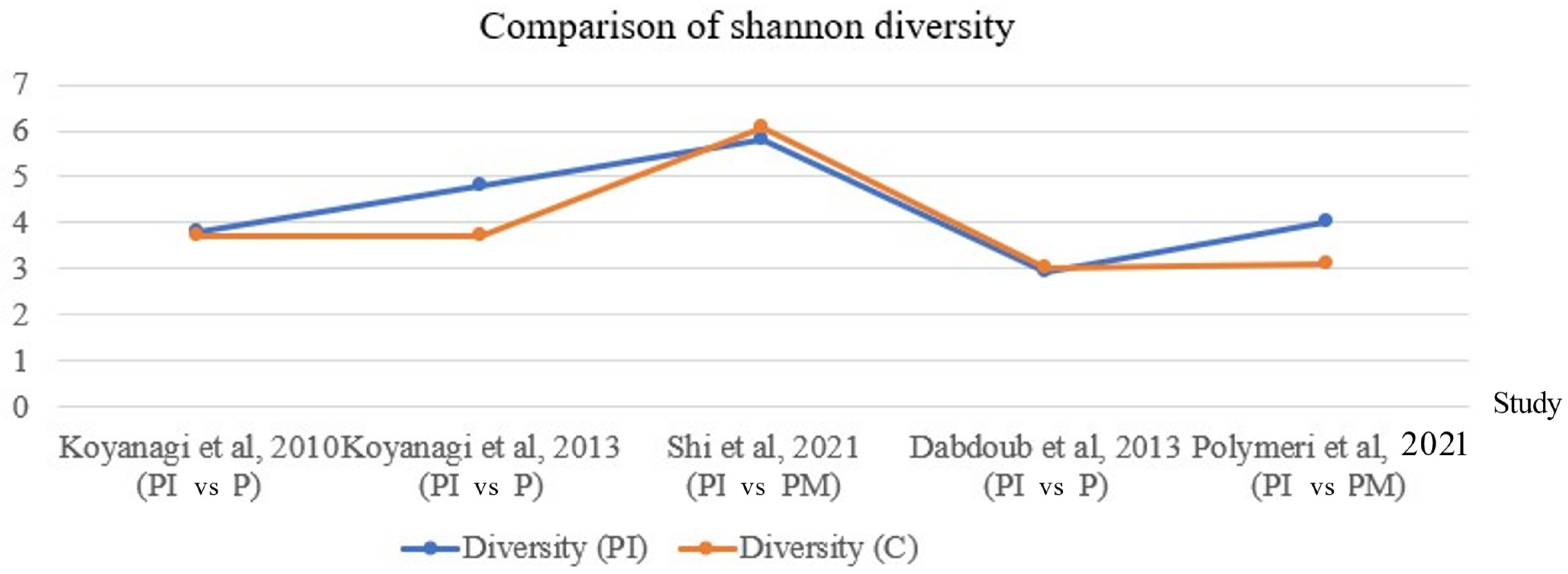
2.3. Heterogeneity of Studies
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buser, D.; Janner, S.F.M.; Wittneben, J.-G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients: 10-Year Survival and Success Rates of SLA Implants. Clin. Implant Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Hultin, K. Periimplant Disease and Prosthetic Risk Indicators: A Literature Review. Implant Dent. 2019, 28, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Canullo, L.; Grusovin, M.G.; de Bruyn, H.; Cosyn, J.; Pera, P. Systematic Review of Some Prosthetic Risk Factors for Periimplantitis. J. Prosthet. Dent. 2015, 114, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-Implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef]
- Rodrigo, D.; Sanz-Sánchez, I.; Figuero, E.; Llodrá, J.C.; Bravo, M.; Caffesse, R.G.; Vallcorba, N.; Guerrero, A.; Herrera, D. Prevalence and Risk Indicators of Peri-Implant Diseases in Spain. J. Clin. Periodontol. 2018, 45, 1510–1520. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.-M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to Fourteen-Year Follow-up of Implant Treatment. Part II: Presence of Peri-Implant Lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar] [CrossRef]
- Fransson, C.; Lekholm, U.; Jemt, T.; Berglundh, T. Prevalence of Subjects with Progressive Bone Loss at Implants: Prevalence of Subjects with Progressive Bone Loss at Implants. Clin. Oral Implant Res. 2005, 16, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; Gonzalo, E.; Villagra, L.J.G.; Miegimolle, B.; Suarez, M.J. What Is the Prevalence of Peri-Implantitis? A Systematic Review and Meta-Analysis. BMC Oral Health 2022, 22, 449. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, V.; Ríos-Carrasco, B.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Bullón, B.; Herrero-Climent, M.; Bullón, P. Incidence of Peri-Implantitis and Relationship with Different Conditions: A Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 4147. [Google Scholar] [CrossRef] [PubMed]
- Hashim, D.; Cionca, N. A Comprehensive Review of Peri-Implantitis Risk Factors. Curr. Oral Health Rep. 2020, 7, 262–273. [Google Scholar] [CrossRef]
- Kumar, P.S. Systemic Risk Factors for the Development of Periimplant Diseases. Implant Dent. 2019, 28, 115–119. [Google Scholar] [CrossRef]
- Steiger-Ronay, V.; Merlini, A.; Wiedemeier, D.B.; Schmidlin, P.R.; Attin, T.; Sahrmann, P. Location of Unaccessible Implant Surface Areas during Debridement in Simulated Peri-Implantitis Therapy. BMC Oral Health 2017, 17, 137. [Google Scholar] [CrossRef]
- Apatzidou, D.A. Modern Approaches to Non-Surgical Biofilm Management. In Frontiers of Oral Biology; Kinane, D.F., Mombelli, A., Eds.; KARGER: Basel, Switzerland, 2011; Volume 15, pp. 99–116. ISBN 978-3-8055-9834-7. [Google Scholar]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine Mouthrinse as an Adjunctive Treatment for Gingival Health. Cochrane Database Syst. Rev. 2017, 2021, CD008676. [Google Scholar] [CrossRef]
- Lombardo, G.; Signoriello, A.; Corrocher, G.; Signoretto, C.; Burlacchini, G.; Pardo, A.; Nocini, P.F. A Topical Desiccant Agent in Association with Manual Debridement in the Initial Treatment of Peri-Implant Mucositis: A Clinical and Microbiological Pilot Study. Antibiotics 2019, 8, 82. [Google Scholar] [CrossRef]
- Herrera, D.; Berglundh, T.; Schwarz, F.; Chapple, I.; Jepsen, S.; Sculean, A.; Kebschull, M.; Papapanou, P.N.; Tonetti, M.S.; Sanz, M.; et al. Prevention and Treatment of Peri-Implant Diseases—The EFP S3 Level Clinical Practice Guideline. J. Clin. Periodontol. 2023, 50, 4–76. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.D.; Martins, C.C.; Amaral, S.A.; Vieira, T.R.; Albuquerque, B.N.; Cota, L.O.M.; Esteves Lima, R.P.; Costa, F.O. Periodontitis as a Risk Factor for Peri-Implantitis: Systematic Review and Meta-Analysis of Observational Studies. J. Dent. 2018, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lindahl, C.; Renvert, H.; Persson, G.R. Clinical and Microbiological Analysis of Subjects Treated with Brånemark or AstraTech Implants: A 7-Year Follow-up Study. Clin. Oral Implant Res. 2008, 19, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Fürst, M.M.; Lang, N.P.; Persson, G.R. One-Year Bacterial Colonization Patterns of Staphylococcus Aureus and Other Bacteria at Implants and Adjacent Teeth. Clin. Oral Implant Res. 2008, 19, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martin, I.; Doolittle-Hall, J.; Teles, R.P.; Patel, M.; Belibasakis, G.N.; Hämmerle, C.H.F.; Jung, R.E.; Teles, F.R.F. Exploring the Microbiome of Healthy and Diseased Peri-Implant Sites Using Illumina Sequencing. J. Clin. Periodontol. 2017, 44, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T.; Sakamoto, M.; Takeuchi, Y.; Maruyama, N.; Ohkuma, M.; Izumi, Y. Comprehensive Microbiological Findings in Peri-Implantitis and Periodontitis. J. Clin. Periodontol. 2013, 40, 218–226. [Google Scholar] [CrossRef]
- Kumar, P.S.; Mason, M.R.; Brooker, M.R.; O’Brien, K. Pyrosequencing Reveals Unique Microbial Signatures Associated with Healthy and Failing Dental Implants. J. Clin. Periodontol. 2012, 39, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Apatzidou, D.; Lappin, D.F.; Hamilton, G.; Papadopoulos, C.A.; Konstantinidis, A.; Riggio, M.P. Microbiome of Peri-Implantitis Affected and Healthy Dental Sites in Patients with a History of Chronic Periodontitis. Arch. Oral Biol. 2017, 83, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Rakic, M.; Grusovin, M.G.; Canullo, L. The Microbiologic Profile Associated with Peri-Implantitis in Humans: A Systematic Review. Int. J. Oral Maxillofac. Implant. 2016, 31, 359–368. [Google Scholar] [CrossRef]
- Dabdoub, S.M.; Tsigarida, A.A.; Kumar, P.S. Patient-Specific Analysis of Periodontal and Peri-Implant Microbiomes. J. Dent. Res. 2013, 92, 168S–175S. [Google Scholar] [CrossRef] [PubMed]
- Heuer, W.; Kettenring, A.; Stumpp, S.N.; Eberhard, J.; Gellermann, E.; Winkel, A.; Stiesch, M. Metagenomic Analysis of the Peri-Implant and Periodontal Microflora in Patients with Clinical Signs of Gingivitis or Mucositis. Clin. Oral Investig. 2012, 16, 843–850. [Google Scholar] [CrossRef]
- Mombelli, A.; Décaillet, F. The Characteristics of Biofilms in Peri-Implant Disease. J. Clin. Periodontol. 2011, 38, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; van Oosten, M.A.; Schurch, E.; Land, N.P. The Microbiota Associated with Successful or Failing Osseointegrated Titanium Implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Qin, D. Next-Generation Sequencing and Its Clinical Application. Cancer Biol. Med. 2019, 16, 4–10. [Google Scholar] [CrossRef]
- Chen, P.; Sun, W.; He, Y. Comparison of the Next-Generation Sequencing (NGS) Technology with Culture Methods in the Diagnosis of Bacterial and Fungal Infections. J. Thorac. Dis. 2020, 12, 4924–4929. [Google Scholar] [CrossRef]
- Torchia, M.T.; Austin, D.C.; Kunkel, S.T.; Dwyer, K.W.; Moschetti, W.E. Next-Generation Sequencing vs Culture-Based Methods for Diagnosing Periprosthetic Joint Infection After Total Knee Arthroplasty: A Cost-Effectiveness Analysis. J. Arthroplast. 2019, 34, 1333–1341. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ahn, D.-H.; Yu, Y.; Han, H.; Kim, S.Y.; Joo, J.-Y.; Chung, J.; Na, H.S.; Lee, J.-Y. Microbial Profiling of Peri-Implantitis Compared to the Periodontal Microbiota in Health and Disease Using 16S rRNA Sequencing. J. Periodontal Implant Sci. 2023, 53, 69–84. [Google Scholar] [CrossRef]
- Song, L.; Jiang, J.; Li, J.; Zhou, C.; Chen, Y.; Lu, H.; He, F. The Characteristics of Microbiome and Cytokines in Healthy Implants and Peri-Implantitis of the Same Individuals. J. Clin. Med. 2022, 11, 5817. [Google Scholar] [CrossRef]
- Pallos, D.; Sousa, V.; Feres, M.; Retamal-Valdes, B.; Chen, T.; Curtis, M.; Boaventura, R.M.; Tanaka, M.H.; Salomão, G.V.D.S.; Zanella, L.; et al. Salivary Microbial Dysbiosis Is Associated With Peri-Implantitis: A Case-Control Study in a Brazilian Population. Front. Cell. Infect. Microbiol. 2022, 11, 696432. [Google Scholar] [CrossRef]
- Barbagallo, G.; Santagati, M.; Guni, A.; Torrisi, P.; Spitale, A.; Stefani, S.; Ferlito, S.; Nibali, L. Microbiome Differences in Periodontal, Peri-Implant, and Healthy Sites: A Cross-Sectional Pilot Study. Clin. Oral Investig. 2022, 26, 2771–2781. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Tong, Z.; Zhang, Y.; Si, M.; He, F. Microbial Profiles of Peri-implant Mucositis and Peri-implantitis: Submucosal Microbial Dysbiosis Correlates with Disease Severity. Clin. Oral Implant. Res. 2022, 33, 172–183. [Google Scholar] [CrossRef]
- Polymeri, A.; Horst, J.; Buijs, M.J.; Zaura, E.; Wismeijer, D.; Crielaard, W.; Loos, B.G.; Laine, M.L.; Brandt, B.W. Submucosal Microbiome of Peri-implant Sites: A Cross-sectional Study. J. Clin. Periodontol. 2021, 48, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Korsch, M.; Marten, S.-M.; Stoll, D.; Prechtl, C.; Dötsch, A. Microbiological Findings in Early and Late Implant Loss: An Observational Clinical Case-Controlled Study. BMC Oral Health 2021, 21, 112. [Google Scholar] [CrossRef]
- Komatsu, K.; Shiba, T.; Takeuchi, Y.; Watanabe, T.; Koyanagi, T.; Nemoto, T.; Shimogishi, M.; Shibasaki, M.; Katagiri, S.; Kasugai, S.; et al. Discriminating Microbial Community Structure Between Peri-Implantitis and Periodontitis With Integrated Metagenomic, Metatranscriptomic, and Network Analysis. Front. Cell. Infect. Microbiol. 2020, 10, 596490. [Google Scholar] [CrossRef] [PubMed]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong Oral Plaque Microbiome Signatures for Dental Implant Diseases Identified by Strain-Resolution Metagenomics. Npj Biofilms Microbiomes 2020, 6, 47. [Google Scholar] [CrossRef]
- Aleksandrowicz, P.; Brzezińska-Błaszczyk, E.; Dudko, A.; Agier, J. Archaea Occurrence in the Subgingival Biofilm in Patients with Peri-Implantitis and Periodontitis. Int. J. Periodontics Restor. Dent. 2020, 40, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chan, Y.; Zhuang, L.; Lai, H.; Lang, N.P.; Keung Leung, W.; Watt, R.M. Intra-oral Single-site Comparisons of Periodontal and Peri-implant Microbiota in Health and Disease. Clin. Oral Implant. Res. 2019, 30, 760–776. [Google Scholar] [CrossRef] [PubMed]
- Kröger, A.; Hülsmann, C.; Fickl, S.; Spinell, T.; Hüttig, F.; Kaufmann, F.; Heimbach, A.; Hoffmann, P.; Enkling, N.; Renvert, S.; et al. The Severity of Human Peri-implantitis Lesions Correlates with the Level of Submucosal Microbial Dysbiosis. J. Clin. Periodontol. 2018, 45, 1498–1509. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, J.; Sun, X.; Li, X.; Zhou, Y. Diversity Analysis of Subgingival Microbial Bacteria in Peri-Implantitis in Uygur Population. Medicine 2018, 97, e9774. [Google Scholar] [CrossRef] [PubMed]
- Daubert, D.; Pozhitkov, A.; McLean, J.; Kotsakis, G. Titanium as a Modifier of the Peri-Implant Microbiome Structure. Clin. Implant. Dent. Relat. Res. 2018, 20, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Muzafferiy, F.; Anderson, A.C.; Wölber, J.P.; Ratka-Krüger, P.; Fretwurst, T.; Nelson, K.; Vach, K.; Hellwig, E. Shift of Microbial Composition of Peri-Implantitis-Associated Oral Biofilm as Revealed by 16S rRNA Gene Cloning. J. Med. Microbiol. 2018, 67, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Nibali, L.; Spratt, D.; Dopico, J.; Mardas, N.; Petrie, A.; Donos, N. Peri-Implant and Periodontal Microbiome Diversity in Aggressive Periodontitis Patients: A Pilot Study. Clin. Oral Implant. Res. 2017, 28, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-L.; Chan, Y.; Zhuang, L.-F.; Lai, H.-C.; Lang, N.P.; Lacap-Bugler, D.C.; Leung, W.K.; Watt, R.M. Distributions of Synergistetes in Clinically-Healthy and Diseased Periodontal and Peri-Implant Niches. Microb. Pathog. 2016, 94, 90–103. [Google Scholar] [CrossRef]
- Shiba, T.; Watanabe, T.; Kachi, H.; Koyanagi, T.; Maruyama, N.; Murase, K.; Takeuchi, Y.; Maruyama, F.; Izumi, Y.; Nakagawa, I. Distinct Interacting Core Taxa in Co-Occurrence Networks Enable Discrimination of Polymicrobial Oral Diseases with Similar Symptoms. Sci. Rep. 2016, 6, 30997. [Google Scholar] [CrossRef] [PubMed]
- Tsigarida, A.A.; Dabdoub, S.M.; Nagaraja, H.N.; Kumar, P.S. The Influence of Smoking on the Peri-Implant Microbiome. J. Dent. Res. 2015, 94, 1202–1217. [Google Scholar] [CrossRef]
- Jakobi, M.; Stumpp, S.; Stiesch, M.; Eberhard, J.; Heuer, W. The Peri-Implant and Periodontal Microbiota in Patients with and without Clinical Signs of Inflammation. Dent. J. 2015, 3, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, L.; Wang, Z.; Li, L.; Zhang, J.; Zhang, Q.; Chen, T.; Lin, J.; Chen, F. Subgingival Microbiome in Patients with Healthy and Ailing Dental Implants. Sci. Rep. 2015, 5, 10948. [Google Scholar] [CrossRef] [PubMed]
- Schaumann, S.; Staufenbiel, I.; Scherer, R.; Schilhabel, M.; Winkel, A.; Stumpp, S.N.; Eberhard, J.; Stiesch, M. Pyrosequencing of Supra- and Subgingival Biofilms from Inflamed Peri-Implant and Periodontal Sites. BMC Oral Health 2014, 14, 157. [Google Scholar] [CrossRef]
- Maruyama, N.; Maruyama, F.; Takeuchi, Y.; Aikawa, C.; Izumi, Y.; Nakagawa, I. Intraindividual Variation in Core Microbiota in Peri-Implantitis and Periodontitis. Sci. Rep. 2014, 4, 6602. [Google Scholar] [CrossRef] [PubMed]
- Tamura, N.; Ochi, M.; Miyakawa, H.; Nakazawa, F. Analysis of Bacterial Flora Associated with Peri-Implantitis Using Obligate Anaerobic Culture Technique and 16S rDNA Gene Sequence. Int. J. Oral Maxillofac. Implant. 2013, 28, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.S.C.; Feres, M.; Figueiredo, L.C.; Shibli, J.A.; Ramiro, F.S.; Faveri, M. Microbiological Diversity of Peri-Implantitis Biofilm by Sanger Sequencing. Clin. Oral Implant. Res. 2014, 25, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T.; Sakamoto, M.; Takeuchi, Y.; Ohkuma, M.; Izumi, Y. Analysis of Microbiota Associated with Peri-Implantitis Using 16S rRNA Gene Clone Library. J. Oral Microbiol. 2010, 2, 5104. [Google Scholar] [CrossRef]
- Faveri, M.; Gonçalves, L.F.H.; Feres, M.; Figueiredo, L.C.; Gouveia, L.A.; Shibli, J.A.; Mayer, M.P.A. Prevalence and Microbiological Diversity of Archaea in Peri-Implantitis Subjects by 16S Ribosomal RNA Clonal Analysis: Archaea in Peri-Implantitis Subjects. J. Periodontal Res. 2011, 46, 338–344. [Google Scholar] [CrossRef]
- Valiente-Mullor, C.; Beamud, B.; Ansari, I.; Francés-Cuesta, C.; García-González, N.; Mejía, L.; Ruiz-Hueso, P.; González-Candelas, F. One Is Not Enough: On the Effects of Reference Genome for the Mapping and Subsequent Analyses of Short-Reads. PLoS Comput. Biol. 2021, 17, e1008678. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, Q.; Zheng, H.; Xu, L.; Wang, X.; Chen, F. Pyrosequencing of the Subgingival Microbiome in Peri-implantitis after Non-surgical Mechanical Debridement Therapy. J. Periodontal Res. 2020, 55, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Di Rienzi, S.C.; Poole, A.C.; Koren, O.; Walters, W.A.; Caporaso, J.G.; Knight, R.; Ley, R.E. Conducting a Microbiome Study. Cell 2014, 158, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Quinque, D.; Horz, H.-P.; Li, M.; Rzhetskaya, M.; Raff, J.A.; Hayes, M.G.; Stoneking, M. Comparative Analysis of the Human Saliva Microbiome from Different Climate Zones: Alaska, Germany, and Africa. BMC Microbiol. 2014, 14, 316. [Google Scholar] [CrossRef]
- Nasidze, I.; Li, J.; Schroeder, R.; Creasey, J.L.; Li, M.; Stoneking, M. High Diversity of the Saliva Microbiome in Batwa Pygmies. PLoS ONE 2011, 6, e23352. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Nagaraja, H.N.; Camerlengo, T.; Joshi, V.; Kumar, P.S. Deep Sequencing Identifies Ethnicity-Specific Bacterial Signatures in the Oral Microbiome. PLoS ONE 2013, 8, e77287. [Google Scholar] [CrossRef]
- Wade, W.G. The Oral Microbiome in Health and Disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Cruz, G.D.; Chen, Y.; Salazar, C.R.; Le Geros, R.Z. The Association of Immigration and Acculturation Attributes with Oral Health among Immigrants in New York City. Am. J. Public Health 2009, 99, S474–S480. [Google Scholar] [CrossRef]
- Menon, R.K.; Gopinath, D. Eliminating Bias and Accelerating the Clinical Translation of Oral Microbiome Research in Oral Oncology. Oral Oncol. 2018, 79, 84–85. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Khammissa, R.A.G.; Feller, L.; Meyerov, R.; Lemmer, J. Peri-Implant Mucositis and Peri-Implantitis: Clinical and Histopathological Characteristics and Treatment. SADJ 2012, 67, 122, 124–126. [Google Scholar] [PubMed]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-Implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Gilli, F.; Wiedemeier, D.B.; Attin, T.; Schmidlin, P.R.; Karygianni, L. The Microbiome of Peri-Implantitis: A Systematic Review and Meta-Analysis. Microorganisms 2020, 8, 661. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, É.B.S.; Romandini, M.; Sadilina, S.; Sant’Ana, A.C.P.; Sanz, M. Microbiota Associated with Peri-Implantitis-A Systematic Review with Meta-Analyses. Clin. Oral Implant. Res. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Lim, Y.S.; Lee, S.; Kim, K.M.; Ryu, C.-M.; Jung, I.Y.; Ahn, M.-Y.; Ann, H.W.; Ahn, J.Y.; Ku, N.S.; et al. A Comparison Between Next-Generation Sequencing and Bacterial Culture for the Detection of Bacteria in Clinical Specimen. Open Forum Infect. Dis. 2015, 2, 1108. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Okada, S.; Yasuda, K.; Kawagoe, M.; Kajiya, M.; Tsuga, K. Microbial Differences between Active and Remission Peri-Implantitis. Sci. Rep. 2022, 12, 5284. [Google Scholar] [CrossRef] [PubMed]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- ROBINS-E Development Group. Risk Of Bias In Non-randomized Studies-of Exposure (ROBINS-E). Launch Version. 20 June 2023. Available online: https://www.riskofbias.info/welcome/robins-e-tool (accessed on 14 August 2023).


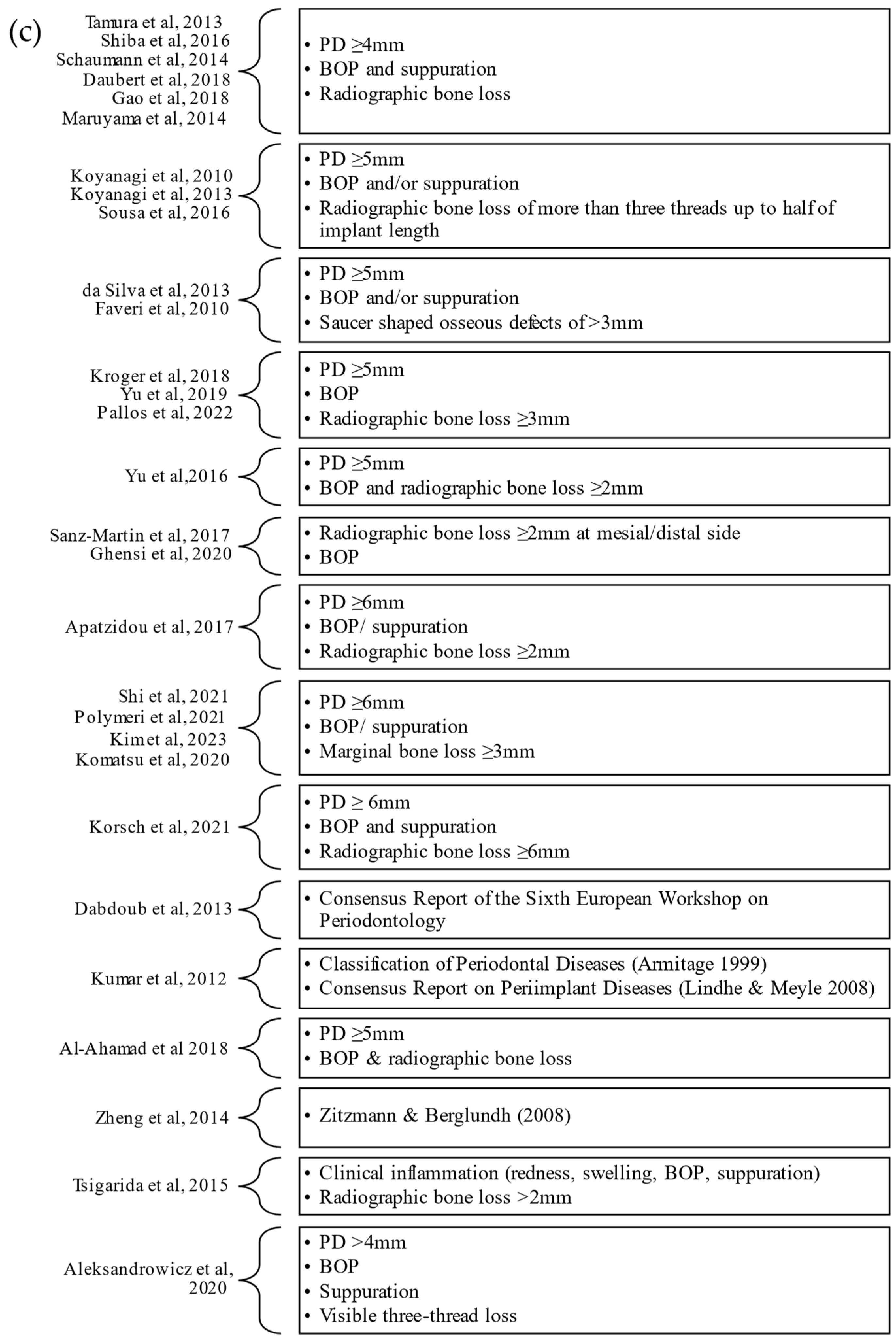
| Author, Year | Confounding Variables | Measurement of the Exposure | Selection of Participants | Post-Exposure Interventions | Missing Data | Measurement of Outcome | Selection of Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Kim et al., 2023 [32] | S | L | S | S | L | L | L | S |
| Song et al., 2022 [33] | L | L | S | L | L | L | L | S |
| Pallos et al., 2022 [34] | H | L | L | L | L | L | L | H |
| Barbagallo et al., 2022 [35] | H | S | S | L | L | L | L | H |
| Shi et al., 2021 [36] | S | L | L | L | L | L | L | L |
| Polymeri et al., 2021 [37] | L | L | L | L | L | L | L | L |
| Korsch et al., 2021 [38] | L | L | L | L | L | L | L | L |
| Komatsu et al., 2020 [39] | S | L | L | L | L | L | L | L |
| Ghensi et al., 2020 [40] | S | S | S | L | L | L | L | S |
| Aleksandrowicz et al., 2020 [41] | S | L | L | L | L | L | L | L |
| Yu et al., 2019 [42] | S | L | L | L | L | L | L | L |
| Kröger et al., 2018 [43] | L | L | H | L | L | L | S | H |
| Gao et al., 2018 [44] | L | L | L | L | L | L | L | L |
| Daubert et al., 2018 [45] | L | S | L | L | L | L | L | S |
| Al-Ahmad et al., 2018 [46] | L | L | L | L | L | L | L | L |
| Sousa et al., 2016 [47] | L | L | L | L | L | L | L | L |
| Sanz-Martin et al., 2017 [20] | L | L | L | L | L | L | L | L |
| Apatzidou et al., 2017 [23] | S | S | L | L | L | L | L | S |
| Yu et al., 2016 [48] | S | L | L | L | L | L | L | L |
| Shiba et al., 2016 [49] | S | L | S | L | L | L | L | S |
| Tsigarida et al., 2015 [50] | L | L | L | L | L | L | L | L |
| Jakobi et al., 2015 [51] | S | L | S | L | L | L | L | S |
| Zheng et al., 2014 [52] | L | L | L | L | L | L | L | L |
| Schaumann et al., 2014 [53] | S | L | S | L | L | L | L | S |
| Maruyama et al., 2014 [54] | S | S | S | L | L | L | L | S |
| Tamura et al., 2013 [55] | L | L | L | L | L | L | L | L |
| Koyanagi et al., 2013 [21] | S | L | L | L | L | L | L | L |
| Dabdoub et al., 2013 [25] | L | L | L | L | L | L | L | L |
| da Silva et al., 2013 [56] | L | L | L | L | L | L | L | L |
| Kumar et al., 2012 [22] | H | S | S | L | L | L | L | H |
| Koyanagi et al., 2010 [57] | S | L | L | L | L | L | L | L |
| Faveri et al., 2010 [58] | L | L | L | L | L | L | L | L |
| Certainty Assessment | Summary of Findings | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Participants (Studies) Follow-Up | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Overall Certainty of Evidence | Study Event Rates (%) | Impact | |
| With Conventional Methods | With Next-Generation Sequencing | ||||||||
| Outcome: Diversity and Richness | |||||||||
| 1069 (32 observational studies) | serious a | serious b | serious c | not serious | All plausible residual confounding would reduce the demonstrated effect | Low | The diversity and richness of the microbiome is heterogeneous and inconsistent across all 32 studies. | ||
| Outcome: Abundance of Taxa | |||||||||
| 1069 (32 observational studies) | serious a | serious b | serious c | not serious | All plausible residual confounding would suggest a spurious effect, while no effect was observed | Low | A heterogeneous pattern of taxa can be seen across all 32 studies reviewed.. The evidence suggests that next-generation sequencing has detected previously uncultured bacteria in diseased sites. | ||
| Author, Year | Number of Subjects | Number of Implants | Study Setting | Duration of Implant | Case Definition for Peri-Implantitis/Peri-Implant Mucositis | Samples Collected | Collection Method |
|---|---|---|---|---|---|---|---|
| Kim et al., 2023 [32] | 109 | 30 H, 30 PI | Korea | Not stated | PD ≥ 6 mm BOP Radiographic bone loss ≥3 mm | Supra- and subgingival plaque | Sterile Gracey curette |
| Song et al., 2022 [33] | 14 | 14 H, 14 PI | China | Not stated | PD ≥ 6 mm Radiographic bone loss ≥3 mm | Subgingival plaque | Sterile paper point |
| Pallos et al., 2022 [34] | 42 | 21 H, 21 PI | Brazil | ≥2 years | PD ≥ 5 mm BOP ± suppuration Radiographic bone loss ≥3 mm | Unstimulated saliva | Sterile plastic tube |
| Barbagallo et al., 2022 [35] | 24 | 10 H, 24 PI | Italy | ≥1 year | Increasing PD since loading Evidence of radiographic bone loss BOP | Subgingival plaque | Sterile paper point |
| Shi et al., 2021 [36] | 64 | 27 PM, 37 PI | China | ≥1 year | PD ≥ 6 mm BOP/suppuration Marginal bone loss ≥3 mm | Subgingival plaque | Sterile paper point |
| Polymeri et al., 2021 [37] | 41 | 41 PI | The Netherlands | ≥1 year | PD ≥ 6 mm Clinical inflammation Radiographic bone loss ≥3 mm | Subgingival plaque | Sterile paper point |
| Korsch et al., 2021 [38] | 48 | 31 PI, 22 H | Germany | ≤3 months or ≥3 years | PD ≥ 6 mm BOP and suppuration Radiographic bone loss ≥6 mm | Subgingival plaque | Sterile paper point |
| Komatsu et al., 2020 [39] | 21 | 21 PI | Japan | ≥1 year | PD ≥ 6 mm BOP ± suppuration Radiographic bone loss ≥3 mm | Subgingival plaque | Sterile paper point |
| Ghensi et al., 2020 [40] | 72 | 35 H, 37 PM, 41 PI | Italy | ≥1 year | BOP Radiographic bone loss > 2 mm | Subgingival plaque | Sterile Gracey curette |
| Aleksandrowicz et al., 2020 [41] | 139 | 37 H, 41 PI | Poland | Not stated | PD > 4 mm BOP Suppuration Visible three-thread loss | Subgingival plaque | Sterile Gracey curette |
| Yu et al., 2019 [42] | 18 | 18 PI, 18 H | China | Not stated | PD ≥ 5 mm BOP and radiographic bone loss | Subgingival/submucosal plaque | Sterile paper point |
| Kröger et al., 2018 [43] | 30 | 45 PI | Germany | Not stated | PD ≥ 5 mm BOP Radiographic bone loss ≥3 mm | Subgingival plaque | Sterile paper point |
| Gao et al., 2018 [44] | 40 | 20 H, 20 PI | China | ≥6 months | PD ≥ 4 mm BOP Radiographic bone loss ≥2 mm | Subgingival plaque | Sterile paper point |
| Daubert et al., 2018 [45] | 9 | 5 H, 6 PI | USA | Not stated | PD ≥ 4 mm BOP ± suppuration Radiographic bone loss > 2 mm | Subgingival plaque | Sterile ½ mini Gracey curette |
| Al-Ahmad et al., 2018 [46] | 10 | 10 H, 10 PI | Germany | Not stated | PD ≥ 5 mm BOP and radiographic bone loss | Subgingival plaque | Sterile paper point |
| Sousa et al., 2016 [47] | 18 | 2 H, 2 PM, 2 PI | UK | Not stated | PD ≥ 5 mm Radiographic bone loss of more than three threads up to half of the implant length or ≥2.5 mm BOP | Subgingival plaque | Sterile Gracey curette |
| Sanz-Martin et al., 2017 [20] | 67 | 35 PI, 32 H | Switzerland | ≥1 year | Radiographic bone loss ≥2 mm at the mesial/distal side BOP | Subgingival plaque | Sterile Gracey curette |
| Apatzidou et al., 2017 [23] | 10 | 4 H, 10 PI | Greece | ≥1 year | PD ≥ 6 mm BOP/suppuration Radiographic bone loss ≥2 mm | Subgingival plaque | Sterile paper point |
| Yu et al., 2016 [48] | 18 | 18 PI, 18 H | China | Not stated | PD ≥ 5 mm BOP and radiographic bone loss ≥2 mm | Subgingival plaque | Sterile paper point |
| Shiba et al., 2016 [49] | 12 | 12 PI, 12 P | Japan | 8.6 ± 7.2 | PD ≥ 4 mm BOP and/or suppuration Radiographic bone loss | Subgingival plaque | Sterile paper point |
| Tsigarida et al., 2015 [50] | 80 | 40 H, 20 PM, 20 PI | USA | ≥4 years | Clinical inflammation (redness, swelling, BOP, suppuration) Radiographic bone loss > 2 mm | Subgingival plaque | Sterile paper point |
| Jakobi et al., 2015 [51] | 18 | 9 H, 9 PI, 9 P | Germany | >6 months | Presence of mobility BOP ± suppuration | Subgingival plaque | Sterile paper point |
| Zheng et al., 2014 [52] | 24 | 10 H, 8 PM, 6 PI | China | Not stated | Zitzmann & Berglundh (2008) | Subgingival plaque | Periodontal probe |
| Schaumann et al., 2014 [53] | 7 | 4.7 ± 3.6 PI | Germany | ≥1 year | PD ≥ 4 mm BOP Radiographic bone loss | Supra- and subgingival plaque | Sterile paper point |
| Maruyama et al., 2014 [54] | 20 | 20 PI, 20 P | Japan | ≥1 year | PD ≥ 4 mm BOP ± suppuration Presence of radiographic bone loss | Subgingival plaque | Sterile paper point |
| Tamura et al., 2013 [55] | 30 | 15 H, 15 PI | Japan | >6 months | PD ≥ 4 mm BOP and suppuration Radiographic bone loss | Subgingival plaque | Sterile paper point |
| Koyanagi et al., 2013 [21] | 6 | 6 PI | Japan | Not stated | PD ≥ 5 mm BOP and/or suppuration Radiographic bone loss of more than three threads up to half of the implant length | Subgingival plaque | Sterile paper point |
| Dabdoub et al., 2013 [25] | 81 | 33 H, 20 PM, 20 PI | USA | ≥1 year | Consensus Report of the Sixth European Workshop on Periodontology | Subgingival plaque | Sterile paper point |
| da Silva et al., 2013 [56] | 20 | 10 PI, 20 H | Brazil | Not stated | PD ≥ 5 mm BOP and/or suppuration Saucer-shaped osseous defects of >3 mm | Subgingival plaque | Sterile Gracey curette |
| Kumar et al., 2012 [22] | 40 | 10 H, 10 PI | USA | ≥1 year | Classification of Periodontal Diseases (Armitage 1999) Consensus Report on Peri-Implant Diseases (Lindhe & Meyle 2008) | Subgingival plaque | Sterile paper point |
| Koyanagi et al., 2010 [57] | 3 | 3 H, 3 PI | Japan | 3–10 | PD ≥ 5 mm BOP and/or suppuration Radiographic bone loss of more than three threads up to half of the implant length | Subgingival plaque | Sterile paper point |
| Faveri et al., 2010 [58] | 50 | 25 H, 25 PI | Brazil | Not stated | PD ≥ 5 mm Saucer-shaped osseous defects of >3 mm BOP and/or suppuration | Subgingival plaque | Sterile Gracey curette |
| Author, Year | Method of DNA Extraction | DNA Amplification and Targeted Region | Sequencing Technique | Reference Database |
|---|---|---|---|---|
| Kim et al., 2023 [32] | Lucigen DNA kit, LGC Biosearch Technologies, Middleton, USA | PCR amplification of the 16s rRNA gene at the V3–V4 region | Illumina MiSeq | Human Oral Microbiome Database |
| Song et al., 2022 [33] | TIANamp Micro DNA Isolation Kit, TIANGEN BIOTECH, Beijing, China | PCR amplification at the V3–V4 hypervariable region of 16S rRNA with the primers 338F and 806R | Illumina MiSeq | Human Oral Microbiome database |
| Pallos et al., 2022 [34] | NucliSENS easyMAG, bioMérieux, Missouri, USA | V4 hypervariable region of the 16S rRNA gene was amplified using F515 and R80 | Ion 318™ Chip kit v2 400-base chemistry | HOMD and Greengene amd NCBI 16s rRNA reference sequence |
| Barbagallo et al., 2022 [35] | PureLink Genomic DNA kit, Thermo Fisher Scientific, USA | PCR amplification of the 16s rRNA gene at V3–V4 region | Illumina Miseq | Human Oral Microbiome database |
| Shi et al., 2021 [36] | DNeasy PowerSoil kit, QIAGEN, Venlo, The Netherlands | PCR amplification of the 16S rRNA genes at V3–V4 region | Illumina MiSeq | Silva database |
| Polymeri et al., 2021 [37] | AGOWA mag Mini DNA Isolation Kit, LGC Genomics, Teddington, United Kingdom | PCR amplification of the 16S rRNA gene hypervariable region V5–V7. | 454 GS-FLX + Titanium system was used for pyrosequencing | Ribosomal Database Project & Human Oral Microbiome Database |
| Korsch et al., 2021 [38] | Qiagen DNA MiniAmp Kit, QIAGEN, Venlo, The Netherlands | PCR amplification of the 16s rRNA gene at V1–V2 region | Illumina MiSeq | Silva database |
| Komatsu et al., 2020 [39] | Mora-extract, AMR Inc., Tokyo, Japan | Not stated | Illumina Miseq | Human Oral Microbiome database |
| Ghensi et al., 2020 [40] | Qiagen DNA MiniAmp kit, QIAGEN, Venlo, The Netherlands | Not stated | Illumina Hiseq | MetaPhlAn 2 and HUMAnN2 |
| Aleksandrowicz et al., 2020 [41] | Genomic Mini kit, A&A Biotechnology, Gdańsk, Poland | The 2720 Thermal Cycler was used for the amplification of archaeal and bacterial DNA. Oligonucleotide-specific primers were used to target the specific 16s rRNA gene | 3130xl Genetic Analyzer | GenBank |
| Yu et al., 2019 [42] | Qiagen DNA MiniAmp kit, QIAGEN, Venlo, The Netherlands | PCR amplification at the hypervariable region V3–V4 of 16s rRNA | Paired-end MiSeq sequencing | Human Oral Microbiome Database |
| Kröger et al., 2018 [43] | Sigma-Aldrich GenElute Bacterial Genomic DNA Kit, Sigma-Aldrich, Munich, Germany | PCR amplification of the 16s rRNA gene at V3–V4 regions | Illumina MiSeq | Human Oral Microbiome Database |
| Gao et al., 2018 [44] | Not stated | PCR amplification of the 16S V3–V4 regions with primers 343F and 798R | Illumina Miseq | Human Oral Microbiome database |
| Daubert et al., 2018 [45] | Chelex-100, Bio-Rad, Hercules, USA | PCR amplification was used to amplify prokaryotic 16S rRNA genes using universal primers (27F and 1392R). Region of amplification not stated | Roche 454 | Human Oral Microbiome database |
| Al-Ahmad et al., 2018 [46] | DNeasy Blood and Tissue kit, QIAGEN, Venlo, The Netherlands | PCR amplification of 16s rRNA using the universal primers 27F-YM and 1492R, region not stated | Ridom TraceEdit software, version 1.1.0 | GenBank |
| Sousa et al., 2016 [47] | Not stated | Amplification with PCR using the 16S rRNA gene with V5–V7 primers | Illumina MiSeq | Greengenes |
| Sanz-Martin et al., 2017 [20] | Masterpure purification kit, Epicentre, Wisconsin, USA | PCR amplification of the 16s rRNA gene at V3–V4 region | Illumina MiSeq | Ribosomal Database Project (RDP) |
| Apatzidou et al., 2017 [23] | Proteinase K (100 mcg/mL) at 60 °C for 60 min, later boiled for 10 min Concentration measured with the Nanodrop NP-1000 spectrophotometer (Thermo Fisher Scientific, Renfrew, UK) Final concentration adjusted to 5 ng/mcL | PCR amplification of the V3–V4 region of the 16s rRNA gene | Illumina MiSeq | Greengenes database |
| Yu et al., 2016 [48] | Qiagen DNA MiniAmp kit, QIAGEN, Venlo, The Netherlands | PCR amplification of 16s rRNA at ca. 650 bp regions corresponding to the V2–V5 region | M13 forward primer | Human Oral Microbiome Database |
| Shiba et al., 2016 [49] | Not stated | PCR amplification of 16s rRNA, region not stated | Illumina MiSeq | Human Oral Microbiome Database |
| Tsigarida et al., 2015 [50] | Qiagen DNA MiniAmp kit, QIAGEN, Venlo, The Netherlands | PCR amplification of the V1 to V3 and V7 to V9 regions | The TTitanium platform was used to perform multiplexed bacterial-tag-encoded FLX amplicon pyrosequencing. | Human Oral Microbiome Database |
| Jakobi et al., 2015 [51] | Qiagen DNA MiniAmp kit, QIAGEN, Venlo, The Netherlands | PCR amplification of 16s rDNA | Not stated | Ribosomal Database Project |
| Zheng et al., 2014 [52] | Not stated | PCR was used to amplify the V1–V3 regions of the 16s rRNA gene | The 454-GS-FLX sequencing platform was used for pyrosequencing | Ribosomal Database Project |
| Schaumann et al., 2014 [53] | QIAamp DNA MiniAmp Kit, QIAGEN, Venlo, The Netherlands | PCR amplification of 16s rRNA at the V1–V3 regions | Pyrosequencing was performed via the GS FLX sequencer | Greengenes |
| Maruyama et al., 2014 [54] | Mora-extract, AMR Inc. Tokyo, Japan | PCR amplification of the 16S V3–V4 regions with primers 806R and 515F | Roche 454 | Ribosomal Database Project, Human Oral Microbiome Database, and NCBI |
| Tamura et al., 2013 [55] | Not stated | PCR amplification of the 16s rRNA gene with the forward primers 16S27F and 16S341F and the reverse primers 16S1492R and 16S907R | Takara Bio | GenBank database |
| Koyanagi et al., 2013 [21] | Mora-extract, AMR Inc. Tokyo, Japan | PCR amplification of the 16s rRNA gene with the primers 27F and 1492R | The 27F and 520R primers (BigDye Terminator Cycle Sequencing kit) were used, and 3130xl Genetic Analyzer | Ribosomal Database Project-II (RDP-II) |
| Dabdoub et al., 2013 [25] | Qiagen DNA MiniAmp kit, QIAGEN, Venlo, The Netherlands | PCR amplification of the 16s rRNA gene at two regions: V1–V3 and V7–V9 | Pyrotag sequencing was performed | Greengenes |
| da Silva et al., 2013 [56] | Masterpure DNA purification kit, Epicentre, Wisconsin, USA | Two step PCR was performed. The first step involved two sets of forward primers in a 1:1 ratio and the reverse primer 1541R. The second step involved the same two sets of forward primers and the reverse primer 1492R. | ABI Prism fluorescent bases | Ribosomal Data Project (RDP) & GenBank |
| Kumar et al., 2012 [22] | Qiagen DNA MiniAmp kit, QIAGEN, Venlo, The Netherlands | PCR amplification of 16s rRNA at the V1–V3 and V7–V9 regions | The Titanium platform was used to perform multiplexed bacterial-tag-encoded FLX amplicon pyrosequencing. | Greengenes |
| Koyanagi et al., 2010 [57] | Mora-extract, AMR Inc. Tokyo, Japan | PCR amplification of plasmid DNA | 27F and 520R primers (BigDye Terminator Cycle Sequencing kit) were used and the 3130xl Genetic Analyzer | Ribosomal Database Project-II (RDP-II) |
| Faveri et al., 2010 [58] | Proteinase K (200 mg/mL) was added to the buffer and then inactivated at 95 °C | PCR amplification with the universal primer pair for Euryarchaea and the reverse primer 954rEyAr | ABI Prism fluorescent bases | Ribosomal Data Project (RDP) & GenBank |
| Author, Year | Groups | Results | |
|---|---|---|---|
| Diversity and Richness | Abundance of Taxa | ||
| Kim et al., 2023 [32] | Peri-implantitis Periodontitis | PI = P a PI > P b | PI&P: P. gingivalis, Prevotella spp., Treponema spp., F. alocis, and F. fastidiosum PI > P: Anaerotignum lactatifermentans, Bacteroides vulgatus, Faecalibacterium prausnitzii, Olsenella uli, Parasutterella excrementihominis, Prevotella buccae, P. alactolyticus, and Slackia exigua |
| Song et al., 2022 [33] | Peri-implantitis | PI = HI b PI > HI e HI ≠ PI c (Significant difference between groups) | PI: Bacteroidetes, Spirochaetes, and Synergistetes, as well as the genera of Porphyromonas, Treponema, Filifactor, Fretibacterium, Lachnospiraceae G-8, and Peptostreptococcaceae XIG-1 HI: Proteobacteria, Neisseria, Streptococcus, Haemophilus, and Rothia |
| Pallos et al., 2022 [34] | Peri-implantitis | HI > PI a,e HI = PI c | PI > HI: Stenotrophomonas, Enterococcus, Leuconostoc genus, Faecalibacterium prausnitzii, Haemophilus parainfluenzae, Prevotella copri, Bacteroides vulgatus, and Bacteroides stercoris |
| Barbagallo et al., 2022 [35] | Peri-implantitis Periodontitis | PI > P a PI = P b | PI: Peptostreptococcaceae, Dialister, Mongibacterium, Atopobium, and Filifactor P: Bacteroidales |
| Shi et al., 2021 [36] | Peri-implantitis Peri-implant mucositis | PI = PM (No significant difference between groups) a,b,c | PI = PM: No significant difference, Bacteroidetes (45.08% in PM, 42.89% in PI), Firmicutes (21.03% in PM, 19.44% in PI), Proteobacteria (11.16% in PM, 10.41% in PI) Fusobacteria (11.12% in PM, 14.7% in PI), Spirochetes (8.38% in PM, 9.68% in PI), Porphyromonas (17.04% in PM, 16.54% in PI), Fusobacterium (9.78% in PM, 12.39% in PI), Treponema (8.37% in PM, 9.59% in PI) and Prevotella (7.43% in PM, 7.04% in PI). PI > PM: Holdemanella and Cardiobacterium PM > PI: Oribacterium, Staphylococcus, and Ramlibacter |
| Polymeri et al., 2021 [37] | Peri-implantitis Peri-implant mucositis | HI = PM = PI (No significant differences between groups) a,b,g | PI: Fusobacterium nucleatum and Treponema denticola PM: Rothia mucilaginosa and Streptococcus salivarius |
| Korsch et al., 2021 [38] | Peri-implantitis | PI > HI d | PI: Fusobacterium nucleatum and Porphyromonas gingivalis HI: Streptococcus, Neisseria, Rothia and Veillonella |
| Komatsu et al., 2020 [39] | Peri-implantitis Periodontitis | PI > P a PI = P c,g | PI: Solobacterium moorei and Prevotella denticola P: F. nucleatum, P. stomatis and Leptotrichia sp. |
| Ghensi et al., 2020 [40] | Peri-implantitis Peri-implant mucositis | PI < HI a,b | PI: Treponema maltophilum, Fretibacterium fastidiosum, Pseudoramibacter alactolyticus, T. lecithinolyticum, P. gingivalis, T. forsythia, Treponema denticola, P. endodontalis, Filifactor alocis, and Desulfobulbus spp. HI: C. gingivalis, C. granulosa, C. ochracea, S. noxia, S. artemidis, Actinomyces, Capnocytophaga, Neisseria, Rothia, and Streptococcus |
| Aleksandrowicz et al., 2020 [41] | Peri-implantitis Periodontitis | Nil | PI: F nucleatum and T denticola |
| Yu et al., 2019 [42] | Peri-implantitis Periodontitis | PI = HI (No significant difference between groups) d,f | PI=HI: Streptococcus infantis/mitis/oralis (HMT-070/HMT-071/HMT-638/HMT-677) and Fusobacterium sp. HMT-203/HMT-698 PI (Low abundance): Aquificae, Chlamydiae, Gemmatimonadetes, Nitrospirae, TM6, Verrucomicrobia, and WPS2 phyla |
| Kröger et al., 2018 [43] | Peri-implantitis | PI > HI g | PI: Eubacteriaceae [XV], Fretibacterium sp. HMT 362, Fretibacterium fastidiosum, Peptostreptococcaceae [XI][G-6], Alloprevotella sp. HMT 473, Fastidiosipila sanguinis, Filifactor alocis, Peptostreptococcaceae [XI][G-4], Bacteriodetes [G-3] bacterium HMT 365, Treponema parvum, Clostridiales [F-1][G-1] bacterium HMT 093, and Orobacterium |
| Gao et al., 2018 [44] | Peri-implantitis | PI > HI b HI ≠ PI (Significant difference between groups) c | PI: Moraxella, Micrococcus, and Acinetobacter HI: Neisseria, Haemophilus, Prevotella, Streptococcus, Porphyromonas, Clostridium, Capnocytophaga, Leptothrix, Actinomycetes, and Actinomyces |
| Daubert et al., 2018 [45] | Peri-implantitis | HI > PI a,b,c | PI: Veillonella and Neisseria. |
| Al-Ahmad et al., 2018 [46] | Peri-implantitis | Not reported | PI: Bacteroidetes (phylum), Fusobacterium nucleatum |
| Sousa et al., 2016 [47] | Peri-implantitis Aggressive periodontitis Peri-implant mucositis | P > PI a,b,f | PI: Propionibacterium, Paludibacterium, Staphylococcus, Filifactor, Mogibacterium, Bradyrhizobium, and Acinetobacter |
| Sanz-Martin et al., 2017 [20] | Peri-implantitis | PI > HI c | PI: Bacteroides, Spirochetes, and Synergistetes, Tannerella forsythia, Treponema denticola, and Porphyromonas gingivalis, Filifactor alocis, Fretibacterium fastidiosum, and Treponema maltophilum HI: Proteobacteria and Actinobacteria PI > HI: Porphyromonas (phylum Bacteroidetes), Treponema (phylum Spirochetes), Filifactor (phylum Firmicutes), Fretibacterium (phylum Synergistetes), Tannerella (phylum Bacteroidetes), T. forsythia, P. gingivalis, and T. denticola). HI > PI: Streptococcus (phylum Firmicutes), Veillonella (phylum Firmicutes), Rothia (phylum Actinobacteria), Haemophilus (phylum Proteobacteria) and Neisseria spp. |
| Apatzidou et al., 2017 [23] | Peri-implantitis | PI > HI a HI = PI (No significant difference between groups) b | HI: Actinobacillus and Streptococcus PI: Prevotella, Porphyromonas, Synergistetes |
| Yu et al., 2016 [48] | Peri-implantitis Periodontitis | PI ≠ HI (Significant difference between groups) f | PI: High abundance of F. fastidiosum and Fretibacterium |
| Shiba et al., 2016 [49] | Peri-implantitis Smoking Periodontitis | PI = P (No significant difference between groups) a,g PI ≠ P (Significant difference between groups) c | PI = P: High rc-rRNA abundances Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia |
| Tsigarida et al., 2015 [50] | Peri-implantitis Smoking Peri-implant mucositis | HI = PI b HI ≠ PI (Significant difference between groups) c | PI: Aggregatibacter, Capnocytophaga, Corynebacterium mucifaciens, Fretibacterium, Lachnoanaerobaculum, Lactobacillus panis, Neisseria, Prevotella HI: Actinomyces, Alloprevotella, Capnocytophaga, Enterobacter cancerogenus, Fusobacterium gonidiaformans, Fusobacterium, Lactobacillus johnsonii, Neisseria lactamica, Porphyromonas asaccharolytica, Prevotella enoeca, Prevotella, Pseudomonas, Pseudomonas pseudoalcaligenes, SR1 [G-1], Streptococcus, Tannerella |
| Jakobi et al., 2015 [51] | Peri-implantitis Periodontitis | Not reported | PI and P: Enterococcus, Streptococcus, Porphyromonas, Fusobacterium, Prevotella, Bacillus, and Fretibacterium Exclusive to PI: Neisseria and Kingella Exclusive to P: Tannerella, Rothia, Parabacteroides, Parvimonas, and Filifactor HI: Enterococcus, Bacillus, Streptococcus, Fusobacterium, Prevotella, Porphyromonas, Rothia and Proteus |
| Zheng et al., 2014 [52] | Peri-implantitis Peri-implant mucositis | PM = PI (No significant differences among groups) f HI > PM f HI > PI f PI > HI a,b,g | PI: Leptotrichia hofstadii, Eubacterium infirmum, Kingella denitrificans, Actinomyces cardiffensis, Eubacterium minutum, Treponema lecithinolyticum, and Gemella sanguinis, Gemella sanguinis, Eubacterium minutum, and Actinomyces cardiffensis |
| Schaumann et al., 2014 [53] | Peri-implantitis Periodontitis | PI = P (No significant difference between groups) a | PI: Porphyromonadaceae, Lachnospiraceae, and Streptococcaceae; Genera Rothia, Actinomyces, Paenibacillus, Microbacterium, Pseudoramibacter, Leptotrichia, Parascardovia, Tannerella, Granulicatella, Tessaracoccus, Clostridium, Aeromonadales, Veillonella, Capnocytophaga, Prevotella, TG5, Fusobacterium, Exiguobacterium, Enterococcus, Porphyromonas and Streptococcus. |
| Maruyama et al., 2014 [54] | Peri-implantitis Periodontitis | PI = P a,b,c,g (no significant difference) | PI: Prevotella nigrescens, Olsenella, Sphingomonas, Peptostreptococcus, and Neisseriaceae P: Peptostreptococcaceae sp. and Desulfomicrobium orale |
| Tamura et al., 2013 [55] | Peri-implantitis | Not reported | PI: E nodatum, P intermedia, F nucleatum, Filifactor alocis, E brachy, Parascardovia denticolens, Parvimonas micra HI: Veillonella sp., Propionibacterium acnes, Pseudoramibacter alactolyticus, Parvimonas micra |
| Koyanagi et al., 2013 [21] | Peri-implantitis Periodontitis | PI > P a,b | PI and P: Firmicutes and Bacteroidetes, Fusobacterium spp. and Streptococcus spp., Exclusive to PI: Parvimonas micra, Peptostreptococcus stomatis, Pseudoramibacter alactolyticus, and Solobacterium moorei PI > P sites: Dialister spp., Eubacterium spp., Porphyromonas spp., P. gingivalis. PI = P sites: T. forsythia, T. denticola |
| Dabdoub et al., 2013 [25] | Peri-implantitis Periodontitis | P > PI a | PI = P: No significant difference in the number of shared species |
| da Silva et al., 2013 [56] | Peri-implantitis | Not reported | HI: Actinomyces, Atopobium, Gemella, Kingella and Rothia, Campylobacter, Desulfobulbus, Dialister, Eubacterium, Filifactor, Mitsukella, Porphyromonas and Pseudoramibacter. PI > HI: Fusobacterium nucleatum, Dialister invisus, Streptococcus sp. human oral taxon (HOT) 064, Filifactor alocis, and Mitsuokella sp. HOT 131 HI > PI: Veillonella dispar, Actinomyces meyeri, and Granulicatella adiacens |
| Kumar et al., 2012 [22] | Peri-implantitis Periodontitis | HI > PI c P > PI a | PI: Actinomyces, Peptococcus, Campylobacter, nonmutans Streptococcus, Butyrivibrio, and Streptococcus mutans, B. fibrisolvens |
| Koyanagi et al., 2010 [57] | Peri-implantitis Periodontitis | PI > P a,b | PI: Chloroflexi, Tenericutes, and Synergistetes phyla Exclusive to PI: Parvimonas micra, Peptostreptococcus stomatis, Pseudoramibacter alactolyticus, Fusobacterium nucleatum, and Solobacterium moorei Detected in P: Fusobacterium nucleatum, Granulicatella adiacens |
| Faveri et al., 2010 [58] | Peri-implantitis | Not reported | PI: Archaea detected at significantly higher abundance |
| Database | Search Terms |
|---|---|
| Medline | (Peri-implantiti$ OR Peri adj2 Implantiti$ OR Peri-implant$ adj2 inflam$ OR Peri-implant$ adj2 infect$ OR Peri-implant$ adj2 disease$ OR exp Peri-Implantitis/or exp Dental Implants/or exp Dental Implantation, Endosseous/OR peri-implant adj2 mucositi$ OR peri adj2 implant adj2 mucositi$ OR periimplant adj2 mucositi$ OR periimplant$ adj2 mucos$) AND (exp sequence analysis/or exp sequence analysis, dna/or exp sequence analysis, rna/or exp rna-seq/OR exp RNA, Ribosomal, 16S/OR exp Microbiota/OR exp Bacteria/) |
| Cochrane | (peri-implantiti* OR periimplantiti* OR (Peri-Implantitis):ti,ab,kw OR Peri-implant* NEAR/2 inflam* OR Peri-implant* NEAR/2 infect* OR peri-implant muco*sitis OR peri-implant NEAR/2 disease* OR peri-implant infect* OR MeSH descriptor: [Peri-Implantitis] explode all trees OR periimplant* NEAR/2 mucos*) AND (dental implant* OR dental implant, endosseous OR endosseous dental implant*) AND (MeSH descriptor: [Sequence Analysis, DNA] explode all trees OR MeSH descriptor: [Sequence Analysis] explode all trees OR MeSH descriptor: [Sequence Analysis, RNA] explode all trees OR MeSH descriptor: [RNA-Seq] explode all trees OR MeSH descriptor: [RNA, Ribosomal, 16S] explode all trees OR MeSH descriptor: [Microbiota] explode all trees OR MeSH descriptor: [Bacteria] explode all trees) |
| Scopus | (peri-implant* OR peri W/2 implant* OR peri-implant* W/2 inflam* OR peri-implant* W/2 infect* OR peri-implant* W/2 disease* OR peri-implant W/2 mucositi* OR peri W/2 implant W/2 mucositi* OR periimplant W/2 mucositi* OR periimplant* W/2 mucos*) AND (dental AND implants OR dental AND implantation AND endosseous) AND ((sequence AND analysis) OR (sequence AND analysis AND dna) OR (sequence AND analysis AND rna) OR rna-seq OR (rna AND ribosomal AND 16s)) AND (microbiota OR bacteria) |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Observational and case-control studies investigating the microbiome of peri-implant tissues through next-generation DNA sequencing methods. Human studies in English | Culture-based studies, conference papers, review articles, studies regarding peri-implantitis associated with other systematic factors (diabetes mellitus, immune disorders, etc.) Articles that examined only specific microorganisms. Non-English language articles and research conducted on non-human specimens. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chun Giok, K.; Menon, R.K. The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies. Antibiotics 2023, 12, 1610. https://doi.org/10.3390/antibiotics12111610
Chun Giok K, Menon RK. The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies. Antibiotics. 2023; 12(11):1610. https://doi.org/10.3390/antibiotics12111610
Chicago/Turabian StyleChun Giok, Koay, and Rohit Kunnath Menon. 2023. "The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies" Antibiotics 12, no. 11: 1610. https://doi.org/10.3390/antibiotics12111610
APA StyleChun Giok, K., & Menon, R. K. (2023). The Microbiome of Peri-Implantitis: A Systematic Review of Next-Generation Sequencing Studies. Antibiotics, 12(11), 1610. https://doi.org/10.3390/antibiotics12111610






