Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens
Abstract
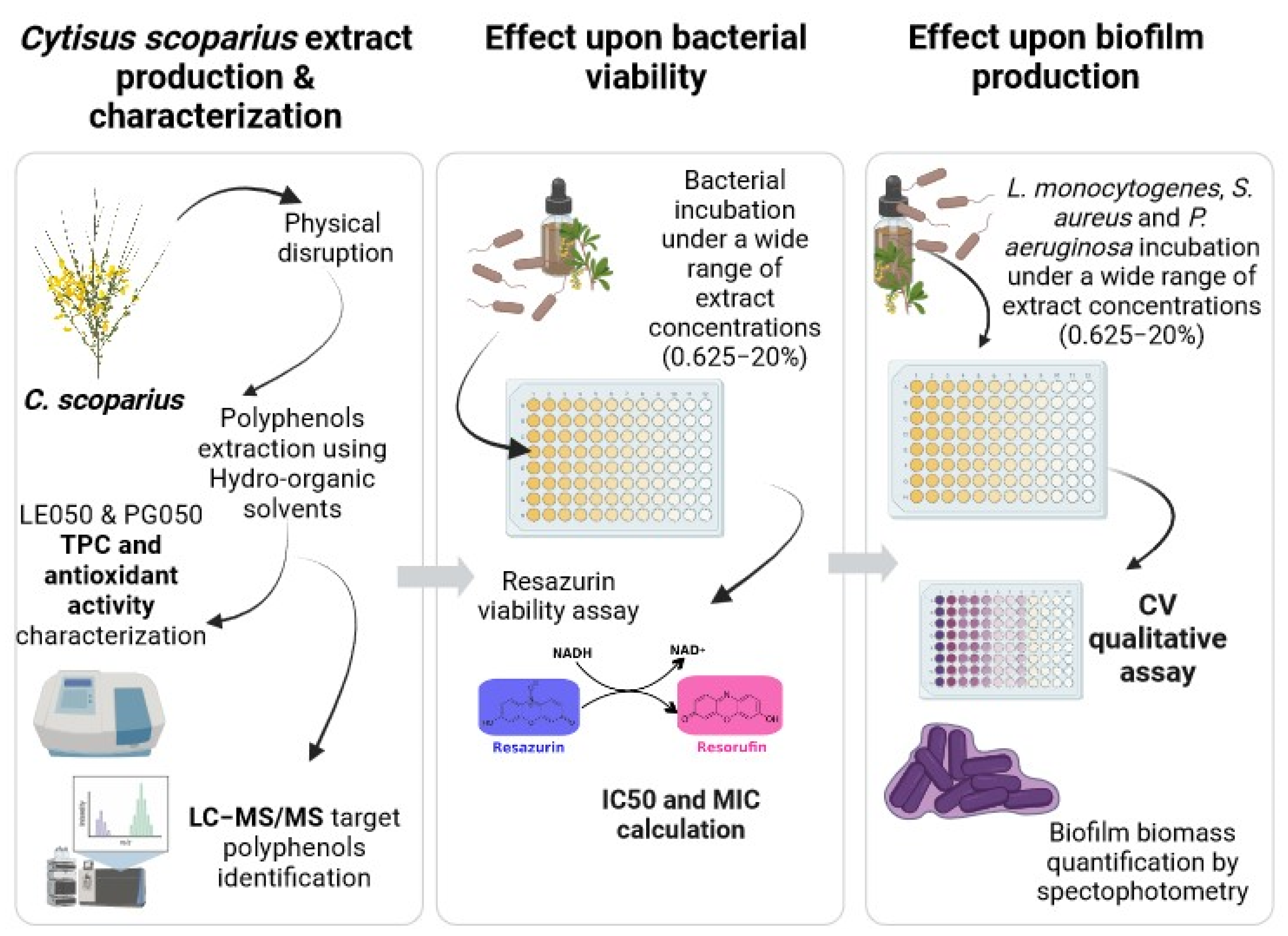
:1. Introduction
2. Results
2.1. Extract Characterization, Total Polyphenol Index (TPC), and Antioxidative Activity Determination
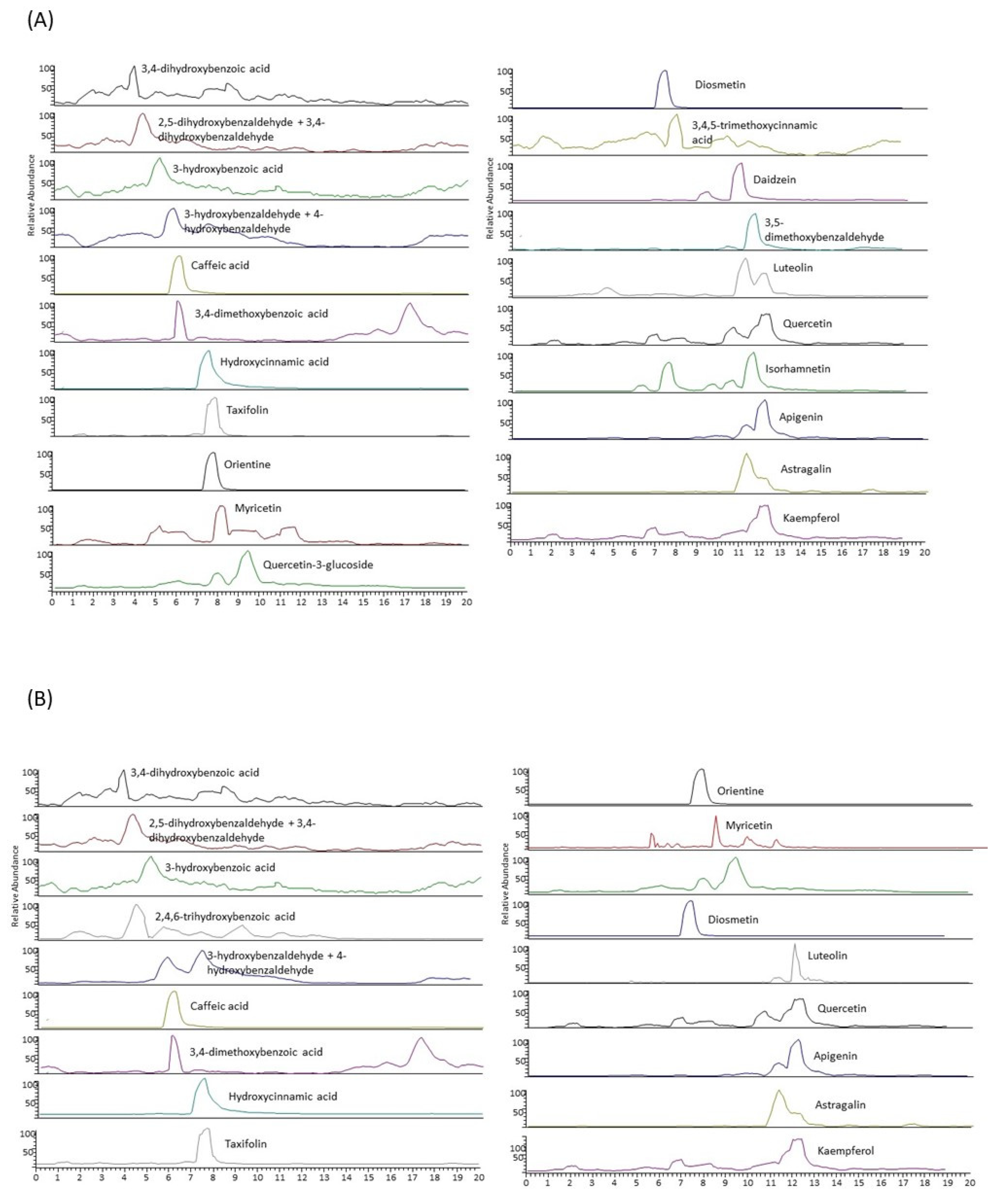
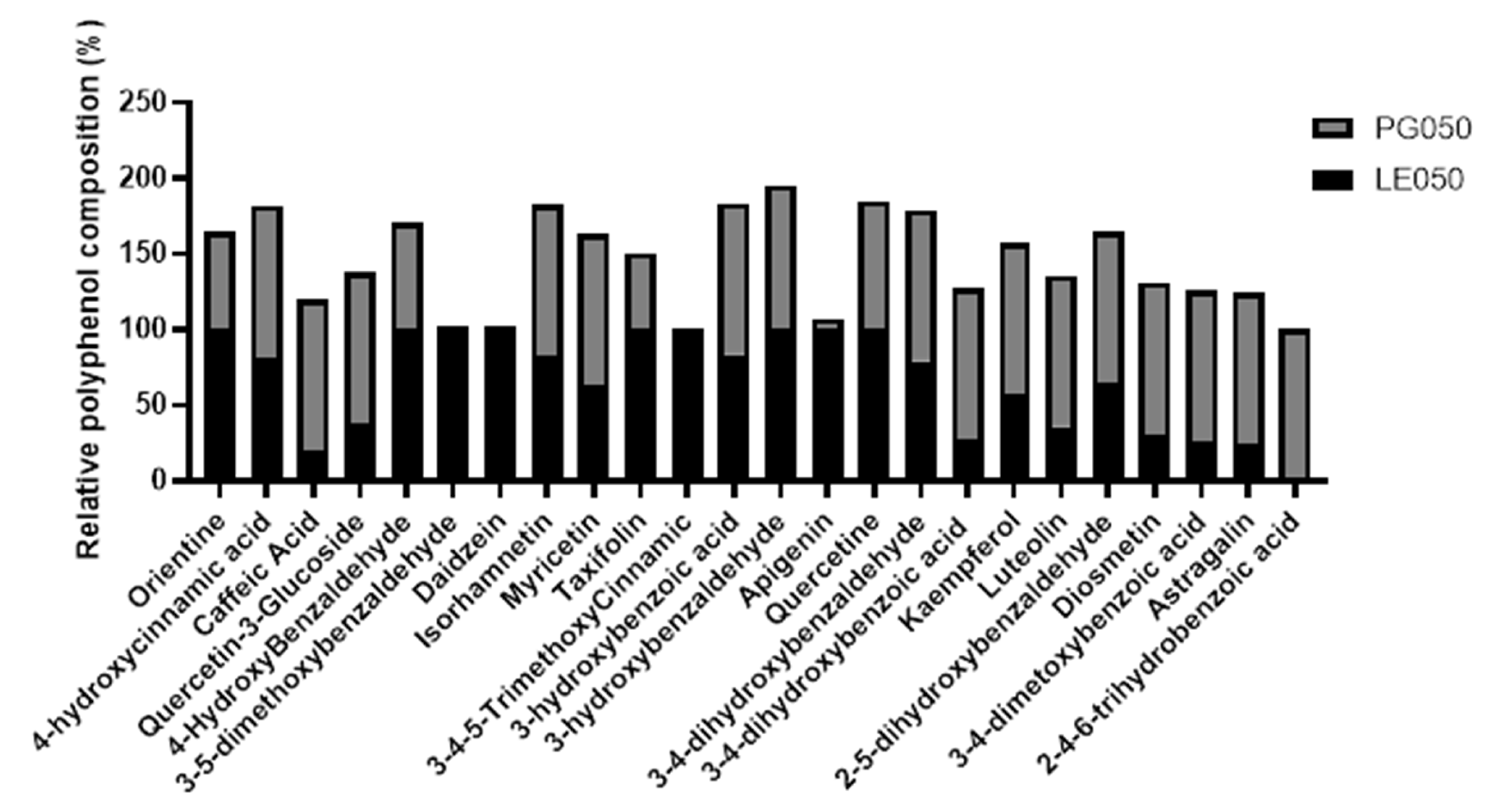
2.2. LC-MS/MS Characterization of the Extracts
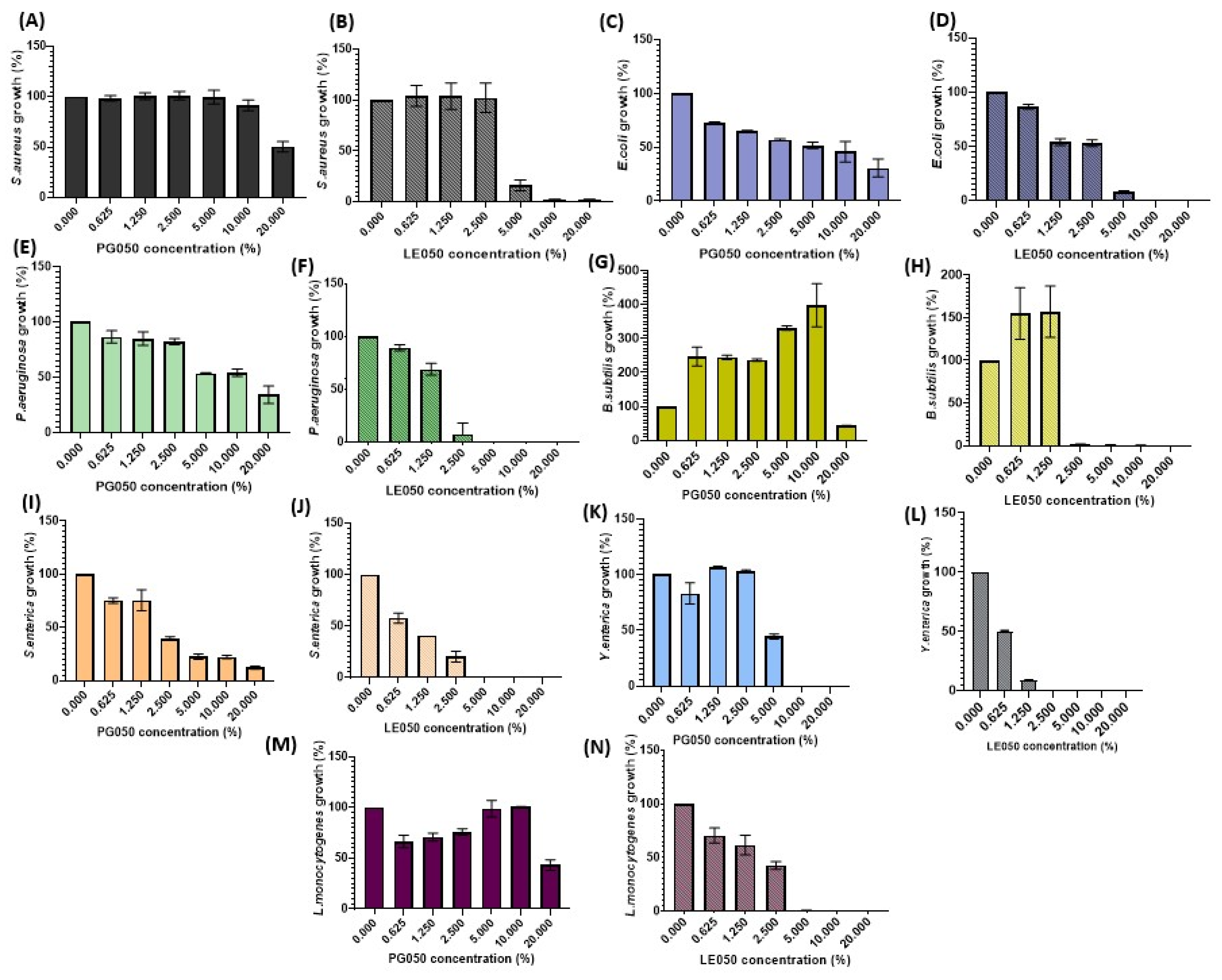
2.3. Antimicrobial Activity
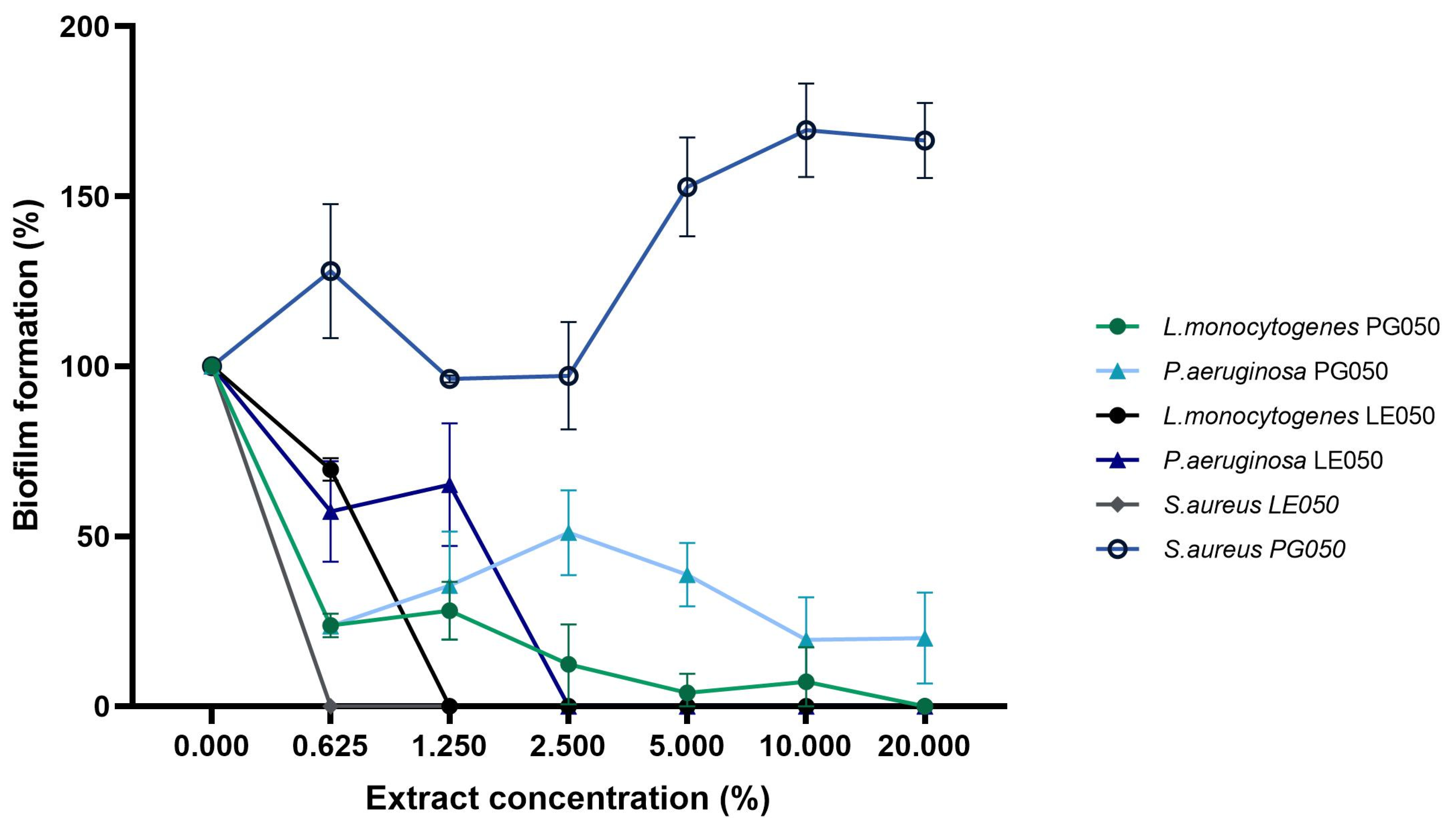
2.4. Inhibition of Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Medium-Scale Ambient Temperature Extract Production
4.3. Extract Characterization
4.4. Total Polyphenolic Index of the Extracts (TPC)
4.5. Antioxidant Activity of the Extracts
4.6. Characterization of Individual Polyphenols by Liquid Chromatography Coupled to a Tandem Mass Spectrometer (LC-MS/MS)
4.7. Bacterial Strains and Culture
4.8. Determination of the Antibacterial Activity by the AlamarBlue Viable Cell Count Method with Fluorometric Reading
4.9. Inhibition of Biofilm Formation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Strain | LE050 | PG050 | ||||
|---|---|---|---|---|---|---|
| IC50 | IC90 | MIC | IC50 | IC90 | MIC | |
| Escherichia coli | 12.63 | 20 | >20 | 20 | >20 | >20 |
| Staphylococcus aureus | 6.89 | 20 | >20 | 9.37 | >20 | >20 |
| Pseudomonas aeruginosa | 5.36 | 13.13 | 20 | >20 | >20 | >20 |
| Bacillus subtilis | 4.77 | 5.86 | 10 | >20 | >20 | >20 |
| Salmonella enterica | 1.58 | 2.75 | 10 | 1.65 | 20 | >20 |
| Listeria monocytogenes | 3.05 | 5.12 | 10 | >20 | >20 | >20 |
| Yersinia enterocolitica | 1.37 | 1.84 | 5 | 4.38 | 20 | >20 |
References
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed]
- Brinkac, L.; Voorhies, A.; Gomez, A.; Nelson, K.E. The Threat of Antimicrobial Resistance on the Human Microbiome. Microb. Ecol. 2017, 74, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Sun, Y.; Zeng, Z. Antimicrobial use and antimicrobial resistance in food animals. Environ. Sci. Pollut. Res. Int. 2018, 25, 18377–18384. [Google Scholar] [CrossRef] [PubMed]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef] [PubMed]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Bouarab Chibane, L.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Martinengo, P.; Arunachalam, K.; Shi, C. Polyphenolic antibacterials for food preservation: Review, challenges, and current applications. Foods 2021, 10, 2469. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Lai, P.K.; Roy, J. Antimicrobial and Chemopreventive Properties of Herbs and Spices. Curr. Med. Chem. 2004, 11, 1451–1460. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Interactions of Cyclic Hydrocarbons with Biological Membranes. J. Biol. Chem. 1994, 269, 8022–8028. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta 1993, 1147, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Caturla, N.; Vera-Samper, E.; Villalaín, J.; Mateo, C.; Micol, V. The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes. Free Radic. Biol. Med. 2003, 34, 648–662. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef] [PubMed]
- Vaara, M. Agents That Increase the Permeability of the Outer Membrane. Microbiol. Rev. 1992, 56, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.; E Richmond, G.; Piddock, L.J. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol. 2014, 9, 1165–1177. [Google Scholar] [CrossRef]
- Slobodníková, L.; Fialová, S.; Rendeková, K.; Kováč, J.; Mučaji, P. Antibiofilm activity of plant polyphenols. Molecules 2016, 21, 1717. [Google Scholar] [CrossRef]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef]
- Bucur, F.I.; Grigore-Gurgu, L.; Crauwels, P.; Riedel, C.U.; Nicolau, A.I. Resistance of Listeria monocytogenes to Stress Conditions Encountered in Food and food processing environments. Front. Microbiol. 2018, 9, 2700. [Google Scholar] [CrossRef] [PubMed]
- Macé, C.; Seyer, D.; Chemani, C.; Cosette, P.; Di-Martino, P.; Guery, B.; Filloux, A.; Fontaine, M.; Molle, V.; Junter, G.-A.; et al. Identification of biofilm-associated cluster (bac) in Pseudomonas aeruginosa involved in biofilm formation and virulence. PLoS ONE 2008, 3, e3897. [Google Scholar] [CrossRef]
- Lores, M.; Pájaro, M.; Álvarez-Casas, M.; Domínguez, J.; García-Jares, C. Use of ethyl lactate to extract bioactive compounds from Cytisus scoparius: Comparison of pressurized liquid extraction and medium scale ambient temperature systems. Talanta 2015, 140, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Caramelo, D.; Barroca, C.; Guiné, R.; Gallardo, E.; Anjos, O.; Gominho, J. Potential Applications of the Cytisus Shrub Species: Cytisus multiflorus, Cytisus scoparius, and Cytisus striatus. Processes 2022, 10, 1287. [Google Scholar] [CrossRef]
- Sundararajan, R.; Haja, N.A.; Venkatesan, K.; Mukherjee, K.; Saha, B.P.; Bandyopadhyay, A.; Mukherjee, P.K. Cytisus scoparius link—A natural antioxidant. BMC Complement. Altern. Med. 2006, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Benabderrahmane, W.; Amrani, A.; Benaissa, O.; Lores, M.; Lamas, J.P.; de Miguel, T.; Benayache, F.; Benayache, S. Chemical constituents, in vitro antioxidant and antimicrobial properties of ethyl acetate extract obtained from Cytisus triflorus l’Her. Nat. Prod. Res. 2020, 34, 1586–1590. [Google Scholar] [CrossRef] [PubMed]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyzowska, A. Plant extracts rich in polyphenols: Antibacterial agents and natural preservatives for meat and meat products. Crit. Rev. Food Sci. Nutr. 2021, 61, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Thakali, A.; MacRae, J.D. A review of chemical and microbial contamination in food: What are the threats to a circular food system? Environ. Res. 2021, 194, 110635. [Google Scholar] [CrossRef]
- Checkouri, E.; Reigner, F.; Silva, C.R.-D.; Meilhac, O. Evaluation of Polyphenol Content and Antioxidant Capacity of Aqueous Extracts from Eight Medicinal Plants from Reunion Island: Protection against Oxidative Stress in Red Blood Cells and Preadipocytes. Antioxidants 2020, 9, 959. [Google Scholar] [CrossRef]
- Nešović, M.; Gašić, U.; Tosti, T.; Horvacki, N.; Nedić, N.; Sredojević, M.; Blagojević, S.; Ignjatović, L.; Tešić, Ž. Distribution of polyphenolic and sugar compounds in different buckwheat plant parts. RSC Adv. 2021, 11, 25816–25829. [Google Scholar] [CrossRef]
- Castillo, A.; Celeiro, M.; Rubio, L.; Bañobre, A.; Otero-Otero, M.; Garcia-Jares, C.; Lores, M. Optimization of bioactives extraction from grape marc via a medium scale ambient temperature system and stability study. Front. Nutr. 2022, 9, 1008457. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, J.; Gómez-Brandón, M.; Martínez-Cordeiro, H.; Lores, M. Bioconversion of Scotch broom into a high-quality organic fertiliser: Vermicomposting as a sustainable option. Waste Manag. Res. 2018, 36, 1092–1099. [Google Scholar] [CrossRef] [PubMed]
- Elbehiry, A.; Abalkhail, A.; Marzouk, E.; Elmanssury, A.E.; Almuzaini, A.M.; Alfheeaid, H.; Alshahrani, M.T.; Huraysh, N.; Ibrahem, M.; Alzaben, F.; et al. An Overview of the Public Health Challenges in Diagnosing and Controlling Human Foodborne Pathogens. Vaccines 2023, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Rama, J.-L.R.; Mallo, N.; Biddau, M.; Fernandes, F.; de Miguel, T.; Sheiner, L.; Choupina, A.; Lores, M. Exploring the powerful phytoarsenal of white grape marc against bacteria and parasites causing significant diseases. Environ. Sci. Pollut. Res. 2020, 28, 24270–24278. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Sun, Q.; Gong, S.; Bi, X.; Jiang, W.; Xue, W.; Fei, P. Antimicrobial Activity and Action Approach of the Olive Oil Polyphenol Extract Against Listeria monocytogenes. Front. Microbiol. 2019, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Song, H.; Xie, J.; Liu, T.; Zhao, X.; Chen, X.; He, X.; Wu, S.; Zhang, Y.; Zheng, X. Research progress in the biological activities of 3,4,5-trimethoxycinnamic acid (TMCA) derivatives. Eur. J. Med. Chem. 2019, 173, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Woo, E.; Lee, D.G. Apigenin promotes antibacterial activity via regulation of nitric oxide and superoxide anion production. J. Basic Microbiol. 2020, 60, 862–872. [Google Scholar] [CrossRef]
- Arunachalam, K.; Ravi, J.; Tian, X.; Shunmugiah, K.P.; Shanmugaraj, G.; Shi, C. Antibacterial activity of 2-hydroxy-4-methoxybenzaldehyde and its possible mechanism against Staphylococcus aureus. J. Appl. Microbiol. 2023, 134, lxad144. [Google Scholar] [CrossRef]
- Charlebois, A.; Jacques, M.; Archambault, M. Biofilm formation of Clostridium perfringens and its exposure to low-dose antimicrobials. Front. Microbiol. 2014, 5, 183. [Google Scholar] [CrossRef]
- Gato, E.; Rosalowska, A.; Martínez-Guitián, M.; Lores, M.; Bou, G.; Pérez, A. Anti-adhesive activity of a Vaccinium corymbosum polyphenolic extract targeting intestinal colonization by Klebsiella pneumoniae. Biomed. Pharmacother. 2020, 132, 110885. [Google Scholar] [CrossRef]
- Huber, B.; Eberl, L.; Feucht, W.; Polster, J. Influence of Polyphenols on Bacterial Biofilm Formation and Quorum-sensing. Z. Naturforsch. 2023, 58, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Plyuta, V.; Zaitseva, J.; Lobakova, E.; Zagoskina, N.; Kuznetsov, A.; Khmel, I. Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. APMIS 2013, 121, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Lores, M.; García-Jares, C.; Álvarez-Casas, M.; Llompart, M. Polyphenol Extract from White-Grape Residue. European Patent EP2875822A1, 27 May 2015. [Google Scholar]
- Rubio, L.; Valiño, M.d.C.; Expósito, M.J.; Lores, M.; Garcia-Jares, C. Sourcing New Ingredients for Organic Cosmetics: Phytochemicals of Filipendula vulgaris Flower Extracts. Cosmetics 2022, 9, 132. [Google Scholar] [CrossRef]
- Celeiro, M.; Lamas, J.P.; Arcas, R.; Lores, M. Antioxidants profiling of by-products from eucalyptus greenboards manufacture. Antioxidants 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review HHS Public Access. Res. Rev. J. Eng. Technol. 2017, 6, PMC6133255. [Google Scholar]

| Strain | LE050 | PG050 | ||||
|---|---|---|---|---|---|---|
| IC50 | IC90 | MIC | IC50 | IC90 | MIC | |
| Escherichia coli | 2.09 | 2.79 | 10 | 18.12 | 20 | ≥20 |
| Staphylococcus aureus | 3.27 | 5.19 | 10 | 19.5 | 20 | ≥20 |
| Pseudomonas aeruginosa | 1.41 | 2.43 | 5 | 12.03 | 20 | ≥20 |
| Bacillus subtilis | 1.93 | 2.53 | 2.5 | 19.44 | 20 | ≥20 |
| Salmonella enterica | 1.2 | 3.55 | 5 | 1.48 | 4.15 | ≥20 |
| Listeria monocytogenes | 3 | 4.37 | 5 | 20 | 20 | ≥20 |
| Yersinia enterocolitica | 0.63 | 1.21 | 2.5 | 4.87 | 5.57 | 10 |
| Strain | LE050 (%v/v) | PG050 (%v/v) |
|---|---|---|
| Staphylococcus aureus | 0.625 | nd |
| Pseudomonas aeruginosa | 2.5 | nd |
| Listeria monocytogenes | 1.25 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo, L.G.; Castillo, A.; Villarino, R.-A.; Rama, J.L.R.; Abril, A.G.; de Miguel, T. Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens. Antibiotics 2023, 12, 1645. https://doi.org/10.3390/antibiotics12111645
Calvo LG, Castillo A, Villarino R-A, Rama JLR, Abril AG, de Miguel T. Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens. Antibiotics. 2023; 12(11):1645. https://doi.org/10.3390/antibiotics12111645
Chicago/Turabian StyleCalvo, Lorena G., Aly Castillo, Rosa-Antía Villarino, José Luis R. Rama, Ana G. Abril, and Trinidad de Miguel. 2023. "Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens" Antibiotics 12, no. 11: 1645. https://doi.org/10.3390/antibiotics12111645
APA StyleCalvo, L. G., Castillo, A., Villarino, R.-A., Rama, J. L. R., Abril, A. G., & de Miguel, T. (2023). Study of the Antibacterial Activity of Rich Polyphenolic Extracts Obtained from Cytisus scoparius against Foodborne Pathogens. Antibiotics, 12(11), 1645. https://doi.org/10.3390/antibiotics12111645









