Terminalia petiolaris A.Cunn ex Benth. Extracts Have Antibacterial Activity and Potentiate Conventional Antibiotics against β-Lactam-Drug-Resistant Bacteria
Abstract
:1. Introduction
2. Results
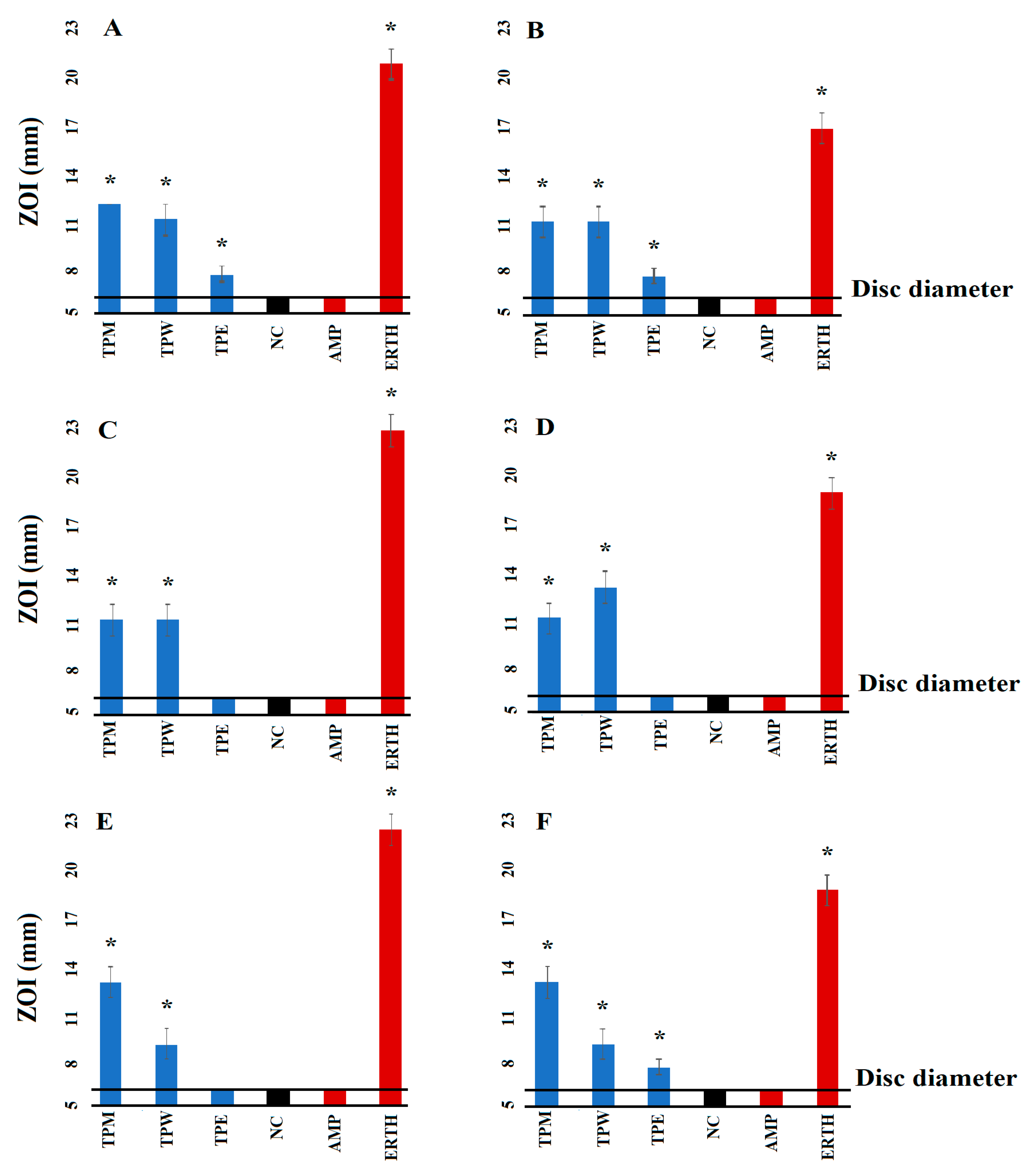
2.1. Antibacterial Susceptibility Studies
2.2. Determination of Fractional Inhibitory Concentration
2.3. Synergistic Interaction of Extract–Antibiotic at Different Ratios
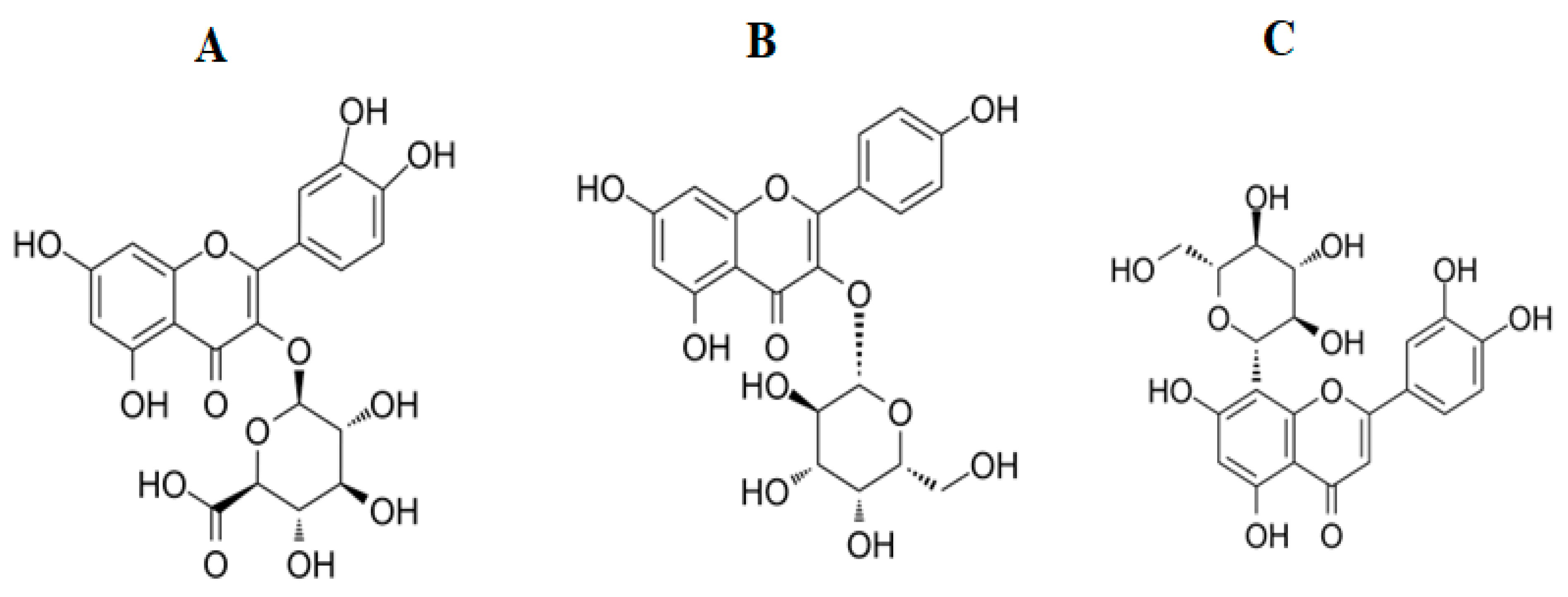
2.4. Identification of Compounds in T. petiolaris Methanol (TPM) and Aqueous (TPW Extracts)
2.5. Toxicity Quantification
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Collection and Extraction
4.3. Antibacterial Studies
4.4. Growth of Bacterial Cultures
4.5. Disc Diffusion Assay Screening
4.6. Liquid Microdilution MIC Assay
4.7. Determination of Combinational Effects
4.8. Determination of Optimal Ratios by Isobologram Analysis
4.9. Non-Targeted Headspace LC-MS Conditions for Quantitative Analysis
4.10. Toxicity Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 22 October 2023).
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2021; WHO: Geneva, Switzerland, 2021.
- D’Andrea, M.M.; Fraziano, M.; Thaller, M.C.; Rossolini, G.M. The urgent need for novel antimicrobial agents and strategies to fight antibiotic resistance. Antibiotics 2019, 8, 254. [Google Scholar] [CrossRef]
- Bassetti, S.; Tschudin-Sutter, S.; Egli, A.; Osthoff, M. Optimizing antibiotic therapies to reduce the risk of bacterial resistance. Eur. J. Intern. Med. 2022, 99, 7–12. [Google Scholar] [CrossRef]
- UNICEF. Levels and Trends in Child Mortality: Report 2019; World Bank Group: Washington, DC, USA, 2019; p. 52. Available online: https://www.unicef.org/reports/levels-and-trends-child-mortality-report-2019 (accessed on 24 January 2023).
- Sankar, M.J.; Chaurasia, S.; Sivanandan, S.; Agarwal, R.; Ellis, S.; Sharland, M. Neonatal sepsis in South Asia: Huge burden and spiralling antimicrobial resistance. Bmj-Brit. Med. J. 2019, 364, k5314. [Google Scholar] [CrossRef]
- Alvarez, A.; Fernández, L.; Gutiérrez, D.; Iglesias, B.; Rodríguez, A.; García, P. Methicillin-resistant Staphylococcus aureus in hospitals: Latest trends and treatments based on bacteriophages. J. Clin. Microbiol. 2019, 57, e01006-19. [Google Scholar] [CrossRef]
- Ensinck, G.; Lazarte, G.; Ernst, A.; Romagnoli, A.; Papucci, S.L.; Aletti, A.; Chiossone, A.; Pigozzi, F.; Sguassero, Y. Community-acquired methicillin-resistant pneumonia in a children’s hospital. Our ten-year experience. Arch. Argent. Pediatr. 2021, 119, 11–17. [Google Scholar] [CrossRef]
- Ali MF, B.; Marzouq, M.A.; Hussein, S.A.; Salman, B.I. A bio-analytically validated HPLC-UV method for simultaneous determination of doripenem and ertapenem in pharmaceutical dosage forms and human plasma: A dual carbapenem regimen for treatment of drug-resistant strain of Klebsiella pneumoniae. RSC Adv. 2021, 11, 3125–3133. [Google Scholar] [CrossRef]
- Levine, M.M.; Nasrin, D.; Acácio, S.; Bassat, Q.; Powell, H.; Tennant, S.M.; Sow, S.O.; Sur, D.; Zaidi, A.K.M.; Faruque, A.S.G.; et al. Diarrhoeal disease and subsequent risk of death in infants and children residing in low-income and middle-income countries: Analysis of the GEMS case-control study and 12-month GEMS-1A follow-on study. Lancet Glob. Health 2020, 8, E204–E214. [Google Scholar] [CrossRef] [PubMed]
- Yigit, H.; Queenan, A.M.; Anderson, G.J.; Domenech-Sanchez, A.; Biddle, J.W.; Steward, C.D.; Alberti, S.; Bush, K.; Tenover, F.C. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2001, 45, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- McDanel, J.; Schweizer, M.; Crabb, V.; Nelson, R.; Samore, M.; Khader, K.; Blevins, A.E.; Diekema, D.; Chiang, H.Y.; Nair, R.; et al. Incidence of Extended-Spectrum β-Lactamase (ESBL)-producing Escherichia coli and Klebsiella infections in the United States: A systematic literature review. Infect. Control. Hosp. Epidemiol. 2017, 38, 1209–1215. [Google Scholar] [CrossRef]
- Kawamura, K.; Nagano, N.; Suzuki, M.; Wachino, J.-I.; Kimura, K.; Arakawa, Y. ESBL-producing Escherichia coli and its rapid rise among healthy people. Food Saf. 2017, 5, 122–150. [Google Scholar]
- Lam, M.M.C.; Wyres, K.L.; Wick, R.R.; Judd, L.M.; Fostervold, A.; Holt, K.E.; Löhr, I.H. Convergence of virulence and MDR in a single plasmid vector in MDR ST15. J. Antimicrob. Chemother. 2019, 74, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.J.; Wang, Y.J.; Hsia, Y.F.; Sharland, M.; Heath, P.T. Systematic review of carbapenem-resistant causing neonatal sepsis in China. Ann. Clin. Microb. Antimicrob. 2019, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- McGaw, L.J.; Rabe, T.; Sparg, S.G.; Jäger, A.K.; Eloff, J.N.; van Staden, J. An investigation on the biological activity of species. J. Ethnopharmacol. 2001, 75, 45–50. [Google Scholar] [CrossRef]
- Cock, I.E. The medicinal properties and phytochemistry of plants of the genus Terminalia (Combretaceae). Inflammopharmacology 2015, 23, 203–229. [Google Scholar] [CrossRef]
- Konczak, I.; Zabaras, D.; Dunstan, M.; Aguas, P. Antioxidant capacity and hydrophilic phytochemicals in commercially grown native Australian fruits. Food Chem. 2010, 123, 1048–1054. [Google Scholar] [CrossRef]
- Courtney, R.; Cock, I.E. Comparison of the antibacterial activity of Australian Terminalia spp. extracts against Klebsiella pneumoniae: A potential treatment for ankylosing spondylitis. Inflammopharmacology 2022, 30, 207–223. [Google Scholar] [CrossRef]
- Eloff, J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J. Ethnopharmacol. 1998, 60, 1–8. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Alcorn, S.; Verma, V.; Cock, I.E. An assessment of the growth inhibition profiles of Hamamelis virginiana L. extracts against Streptococcus and Staphylococcus spp. J. Tradit. Complement. Med. 2021, 11, 457–465. [Google Scholar] [CrossRef]
- van Vuuren, S.; Viljoen, A. Plant-based antimicrobial studies-methods and approaches to study the interaction between natural products. Planta Med. 2011, 77, 1168–1182. [Google Scholar] [CrossRef]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef]
- Rayan, P.; Matthews, B.; McDonnell, P.A.; Cock, I.E. Terminalia ferdinandiana extracts as inhibitors of proliferation: A new treatment for giardiasis. Parasitol. Res. 2015, 114, 2611–2620. [Google Scholar] [CrossRef]
- Xie, Y.X.; Yang, W.J.; Tang, F.; Chen, X.Q.; Ren, L.C. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2015, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure-activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Simo, I.K.; Ngameni, B.; Bigoga, J.D.; Watchueng, J.; Kapguep, R.N.; Etoa, F.X.; Tchaleu, B.N.; Beng, V.P. Antimicrobial activity of the methanolic extract, fractions and four flavonoids from the twigs of Dorstenia angusticornis Engl. (Moraceae). J. Ethnopharmacol. 2007, 112, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Ngameni, B.; Kuete, V.; Simo, I.K.; Ambassa, P.; Roy, R.; Bezabih, M.; Etoa, F.X.; Ngadjui, B.T.; Abegaz, B.M.; et al. Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 2008, 116, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.H.; Fu, Y.M.; Zhang, W.L.; Chen, X.B.; Zhao, J.Z.; Song, W.Q.; Li, Y.J.; Huang, Y.; Wu, Z.; Sun, R.; et al. Synergistic effects of baicalein with cefotaxime against through inhibiting CTX-M-1 gene expression. BMC Microbiol. 2016, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Kong, J.L.; Dong, B.Y.; Huang, H.; Wang, K.; Wu, L.H.; Hou, C.C.; Liang, Y.; Li, B.; Chen, Y.Q. Baicalein attenuates the quorum sensing-controlled virulence factors of and relieves the inflammatory response in infected macrophages by downregulating the MAPK and NFκB signal-transduction pathways. Drug Des. Dev. Ther. 2016, 10, 183–203. [Google Scholar] [CrossRef]
- Geoghegan, F.; Wong, R.W.K.; Rabie, A.B.M. Inhibitory effect of quercetin on periodontal pathogens. Phytother. Res. 2010, 24, 817–820. [Google Scholar] [CrossRef]
- Siriwong, S.; Teethaisong, Y.; Thumanu, K.; Dunkhunthod, B.; Eumkeb, G. The synergy and mode of action of quercetin plus amoxicillin against amoxicillin-resistant Staphylococcus epidermidis. BMC Pharmacol. Toxicol. 2016, 17, 39. [Google Scholar] [CrossRef]
- Wu, T.; He, M.Y.; Zang, X.X.; Zhou, Y.; Qiu, T.F.; Pan, S.Y.; Xu, X.Y. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Et Biophys. Acta Biomembr. 2013, 1828, 2751–2756. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]


| Extract and Antibiotic | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| E. coli | ESBL E. coli | S. aureus | MRSA | K. pneumoniae | ESBL K. pneumoniae | |
| TPM | 109.37 | 437.5 | 437.5 | 437.5 | 437.5 | 437.5 |
| TPW | 218.75 | 875 | 875 | 875 | 218.75 | 212.5 |
| TPE | 250 | 250 | 250 | 250 | 250 | 250 |
| Tetracycline | - | - | 1.25 | - | - | - |
| Chloramphenicol | - | - | 0.31 | - | 1.25 | 1.25 |
| Ciprofloxacin | 2.5 | - | 0.62 | 2.5 | 2.5 | 1.25 |
| Gentamicin | 0.039 | 0.039 | 0.03 | 0.03 | 0.03 | 0.03 |
| Erythromycin | - | - | 1.25 | - | 2.5 | - |
| Negative control | - | - | - | - | - | - |
| Bacteria | Extract | Tetracycline | Chloramphenicol | Ciprofloxacin | Gentamicin | Erythromycin |
|---|---|---|---|---|---|---|
| E. coli | TPM | - | - | 0.52 | 3.60 | - |
| TPW | - | - | 2.25 | 0.71 | ||
| TPE | - | - | 0.75 | 1.36 | - | |
| ESBL E. coli | TPM | - | - | - | 2.85 | - |
| TPW | - | - | - | 5.33 | - | |
| TPE | - | - | - | 2.66 | - | |
| S. aureus | TPM | 0.62 | 1 | 1.50 | 0.71 | 0.62 |
| TPW | 0.50 | 1.25 | 1.04 | 0.66 | 1 | |
| TPE | 1 | 1.25 | 1.5 | 0.66 | 1 | |
| MRSA | TPM | - | - | 2.25 | 2.85 | - |
| TPW | - | - | 0.75 | 0.66 | - | |
| TPE | - | - | 0.75 | 2.60 | - | |
| K. pneumoniae | TPM | - | 0.62 | 1.12 | 2.85 | 0.56 |
| TPW | - | 0.62 | 2.25 | 0.71 | 1.12 | |
| TPE | - | 2 | 0.75 | 0.66 | 0.40 | |
| ESBL K. pneumoniae | TPM | - | 0.62 | 1.25 | 2.85 | - |
| TPW | - | 0.63 | 2.55 | 1.42 | - | |
| TPE | - | 2 | 1 | 0.66 | - |
| Retention Time (Min) | Empirical Formula | Molecular Mass | Putative Identification | Relative Abundance (% Total Area) | |
|---|---|---|---|---|---|
| TPM | TPW | ||||
| 7.06 | C21H20O11 | 448 | Trifolin | 1.36 | |
| 6.35 | C27H30O16 | 610 | Quercetin | 0.18 | |
| 5.54 | C21H20O11 | 448 | Orientin | 5.17 | |
| 5.94 | C21H18O13 | 478 | Miquelianin | 0.07 | |
| 6.87 | C28H24O15 | 600 | Isoorientin 2″-O-gallate | 0.04 | |
| 6.37 | C24H26O9 | 458 | 7-Hydroxy-5,4′-dimethoxy-8-methylisoflavone 7-O-rhamnoside | 0.05 | |
| 7.37 | C23H22O13 | 506 | 6-Methoxyluteolin 7-glucuronide methyl ester | 2.73 | |
| 7.16 | C22H20O13 | 492 | 6-Methoxyluteolin 7-glucuronide | 0.12 | |
| 0.32 | C20H18O13 | 466 | 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-8-{[(2R,3R,4S,5S,6R)-3,4,5,6-tetrahydroxytetrahydro-2H-pyran-2-yl]oxy}-4H-chromen-4-one | 0.42 | |
| 7.97 | C15H10O7 | 302 | 2-(2,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one | 0.05 | |
| 6.73 | C28H24O14 | 584 | 2″-O-Galloylisovitexin | 0.10 | |
| 6.18 | C21H20O10 | 432 | 1,5-Anhydro-1-[5,7-dihydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]hexitol | 1.30 | |
| 6.21 | C28H24O15 | 600 | (2S,3R,4R,5S,6S)-2-{[2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl]oxy}-3,5-dihydroxy-6-methyloxan-4-yl 3,4,5-trihydroxybenzoate | 0.98 | |
| 6.17 | C20H22O10 | 422 | (2R,3S)-7-{[(2S,3R,4R,5S)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5-diol | 2.55 | |
| 0.52 | C15H12O7 | 322 | (2R,3R)-2-(2,6-Dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one | 0.24 | |
| 6.29 | C21H20O11 | 448 | Orientin | 1.55 | |
| 6.95 | C28H24O15 | 600 | (2S,3R,4R,5S,6S)-2-{[2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chromen-3-yl]oxy}-3,5-dihydroxy-6-methyloxan-4-yl 3,4,5-trihydroxybenzoate | 0.37 | |
| 7.36 | C21H20O10 | 432 | 1,5-Anhydro-1-[5,7-dihydroxy-3-(4-hydroxyphenyl)-4-oxo-4H-chromen-8-yl]hexitol | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zai, M.J.; Cheesman, M.J.; Cock, I.E. Terminalia petiolaris A.Cunn ex Benth. Extracts Have Antibacterial Activity and Potentiate Conventional Antibiotics against β-Lactam-Drug-Resistant Bacteria. Antibiotics 2023, 12, 1643. https://doi.org/10.3390/antibiotics12111643
Zai MJ, Cheesman MJ, Cock IE. Terminalia petiolaris A.Cunn ex Benth. Extracts Have Antibacterial Activity and Potentiate Conventional Antibiotics against β-Lactam-Drug-Resistant Bacteria. Antibiotics. 2023; 12(11):1643. https://doi.org/10.3390/antibiotics12111643
Chicago/Turabian StyleZai, Muhammad Jawad, Matthew James Cheesman, and Ian Edwin Cock. 2023. "Terminalia petiolaris A.Cunn ex Benth. Extracts Have Antibacterial Activity and Potentiate Conventional Antibiotics against β-Lactam-Drug-Resistant Bacteria" Antibiotics 12, no. 11: 1643. https://doi.org/10.3390/antibiotics12111643
APA StyleZai, M. J., Cheesman, M. J., & Cock, I. E. (2023). Terminalia petiolaris A.Cunn ex Benth. Extracts Have Antibacterial Activity and Potentiate Conventional Antibiotics against β-Lactam-Drug-Resistant Bacteria. Antibiotics, 12(11), 1643. https://doi.org/10.3390/antibiotics12111643









