Emergence of Antibiotic-Resistant Porphyromonas gingivalis in United States Periodontitis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. P. gingivalis Clinical Isolates
2.3. P. gingivalis In Vitro Antibiotic Resistance Testing
2.4. Data Analysis
3. Results
3.1. Quality Control
3.2. Patients and Subgingival P. gingivalis Recovery
3.3. P. gingivalis In Vitro Antibiotic Resistance Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Interagency Coordination Group on Antimicrobial Resistance. No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Secretary-General of the United Nations, April 2019. Available online: https://www.who.int/docs/default-source/documents/no-time-to-wait-securing-the-future-from-drug-resistant-infections-en.pdf?sfvrsn=5b424d7_6 (accessed on 15 September 2023).
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019; p. 3. [CrossRef]
- Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2019; pp. 1–39. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 15 September 2023).
- Do, P.C.; Assefa, Y.A.; Batikawai, S.M.; Reid, S.A. Strengthening antimicrobial resistance surveillance systems: A scoping review. BMC Infect. Dis. 2023, 23, 593. [Google Scholar] [CrossRef]
- Abe, F.C.; Kodaira, K.; Motta, C.C.B.; Barberato-Filho, S.; Silva, M.T.; Guimarães, C.C.; Martins, C.C.; Lopes, L.C. Antimicrobial resistance of microorganisms present in periodontal diseases: A systematic review and meta-analysis. Front. Microbiol. 2022, 13, 961986. [Google Scholar] [CrossRef]
- Ng, E.; Tay, J.R.H.; Boey, S.K.; Laine, M.L.; Ivanovski, S.; Seneviratne, C.J. Antibiotic resistance in the microbiota of periodontitis patients: An update of current findings. Crit. Rev. Microbiol. 2023. [Google Scholar] [CrossRef]
- Abusleme, L.; Hoare, A.; Hong, B.Y.; Diaz, P.I. Microbial signatures of health, gingivitis, and periodontitis. Periodontology 2000 2021, 86, 57–78. [Google Scholar] [CrossRef]
- Baek, K.; Ji, S.; Choi, Y. Complex intratissue microbiota forms biofilms in periodontal lesions. J. Dent. Res. 2018, 97, 192–200. [Google Scholar] [CrossRef]
- Sochalska, M.; Potempa, J. Manipulation of neutrophils by Porphyromonas gingivalis in the development of periodontitis. Front. Cell. Infect. Microbiol. 2017, 7, 197. [Google Scholar] [CrossRef]
- Zheng, S.; Yu, S.; Fan, X.; Zhang, Y.; Sun, Y.; Lin, L.; Wang, H.; Pan, Y.; Li, C. Porphyromonas gingivalis survival skills: Immune evasion. J. Periodontal Res. 2021, 56, 1007–1018. [Google Scholar] [CrossRef]
- Meghil, M.M.; Ghaly, M.; Cutler, C.W. A tale of two fimbriae: How invasion of dendritic cells by Porphyromonas gingivalis disrupts DC maturation and depolarizes the T-cell-mediated immune response. Pathogens 2022, 11, 328. [Google Scholar] [CrossRef]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and systemic effects of Porphyromonas gingivalis infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Diaz, P.I. Porphyromonas gingivalis: Immune subversion activities and role in periodontal dysbiosis. Curr. Oral Health Rep. 2020, 7, 12–21. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Manoil, D. Microbial community-driven etiopathogenesis of peri-implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Fitzsimonds, Z.R.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontology 2000 2022, 89, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, Y.; Gong, T. The interplay between oral microbiota, gut microbiota and systematic diseases. J. Oral Microbiol. 2023, 15, 2213112. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Slots, J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontology 2000 1999, 20, 122–135. [Google Scholar] [CrossRef]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and its systemic impact: Current status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef]
- Peng, X.; Cheng, L.; You, Y.; Tang, C.; Ren, B.; Li, Y.; Xu, X.; Zhou, X. Oral microbiota in human systematic diseases. Int. J. Oral Sci. 2022, 14, 14. [Google Scholar] [CrossRef]
- Bregaint, S.; Boyer, E.; Fong, S.B.; Meuric, V.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Porphyromonas gingivalis outside the oral cavity. Odontology 2022, 110, 1–19. [Google Scholar] [CrossRef]
- Park, S.; Kim, I.; Han, S.J.; Kwon, S.; Min, E.J.; Cho, W.; Koh, H.; Koo, B.N.; Lee, J.S.; Kwon, J.S.; et al. Oral Porphyromonas gingivalis infection affects intestinal microbiota and promotes atherosclerosis. J. Clin. Periodontol. 2023, 50, 1553–1567. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Oduro, P.K.; Sun, T.; Chen, H.; Yi, Y.; Zeng, W.; Wang, Q.; Leng, L.; Yang, L.; et al. The relationship between Porphyromonas gingivalis and rheumatoid arthritis: A meta-analysis. Front. Cell. Infect. Microbiol. 2022, 12, 956417. [Google Scholar] [CrossRef]
- Jia, S.; Li, X.; Du, Q. Host insulin resistance caused by Porphyromonas gingivalis-review of recent progresses. Front. Cell. Infect. Microbiol. 2023, 13, 1209381. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, J.; Dong, J.; Hu, P.; Guo, Q. Periodontopathogens Porphyromonas gingivalis and Fusobacterium nucleatum and their roles in the progression of respiratory diseases. Pathogens 2023, 12, 1110. [Google Scholar] [CrossRef]
- Fu, Y.; Xu, X.; Zhang, Y.; Yue, P.; Fan, Y.; Liu, M.; Chen, J.; Liu, A.; Zhang, X.; Bao, F. Oral Porphyromonas gingivalis infections increase the risk of Alzheimer’s disease: A review. Oral Health Prev. Dent. 2023, 21, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Emrich, L.J.; Genco, R.J.; Rosling, B.G. Relationship between some subgingival bacteria and periodontal pocket depth and gain or loss of periodontal attachment after treatment of adult periodontitis. J. Clin. Periodontol. 1985, 12, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Schmid, B.; Rutar, A.; Lang, N.P. Persistence patterns of Porphyromonas gingivalis, Prevotella intermedia/nigrescens, and Actinobacillus actinomycetemcomitans after mechanical therapy of periodontal disease. J. Periodontol. 2000, 71, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Rhemrev, G.E.; Timmerman, M.F.; Veldkamp, I.; van Winkelhoff, A.J.; Van der Velden, U. Immediate effect of instrumentation on the subgingival microflora in deep inflamed pockets under strict plaque control. J. Clin. Periodontol. 2006, 33, 42–48. [Google Scholar] [CrossRef]
- Chaves, E.S.; Jeffcoat, M.K.; Ryerson, C.C.; Snyder, B. Persistent bacterial colonization of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in periodontitis and its association with alveolar bone loss after 6 months of therapy. J. Clin. Periodontol. 2000, 27, 897–903. [Google Scholar] [CrossRef]
- Rams, T.E.; Slots, J. Antimicrobial chemotherapy for recalcitrant severe human periodontitis. Antibiotics 2023, 12, 265. [Google Scholar] [CrossRef]
- Rams, T.E.; Listgarten, M.A.; Slots, J. Utility of 5 major putative periodontal pathogens and selected clinical parameters to predict periodontal breakdown in patients on maintenance care. J. Clin. Periodontol. 1996, 23, 346–354. [Google Scholar] [CrossRef]
- Rams, T.E.; Listgarten, M.A.; Slots, J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis subgingival presence, species-specific serum immunoglobulin G antibody levels, and periodontitis disease recurrence. J. Periodontal Res. 2006, 41, 228–234. [Google Scholar] [CrossRef]
- Byrne, S.J.; Dashper, S.G.; Darby, I.B.; Adams, G.G.; Hoffmann, B.; Reynolds, E.C. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol. Immunol. 2009, 24, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Charalampakis, G.; Dahlén, G.; Carlén, A.; Leonhardt, A. Bacterial markers vs. clinical markers to predict progression of chronic periodontitis: A 2-yr prospective observational study. Eur. J. Oral Sci. 2013, 121, 394–402. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Rams, T.E.; Slots, J. Systemic antibiotic therapy in periodontics. Periodontology 2000 1996, 10, 45–78. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; American Academy of Periodontology Research, Science and Therapy Committee. Systemic antibiotics in periodontics. J. Periodontol. 2004, 75, 1553–1565. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J. Systemic antibiotics in periodontal therapy. Aust. Dent. J. 2009, 54, S96–S101. [Google Scholar] [CrossRef]
- Sheridan, R.A.; Wang, H.L.; Eber, R.; Oh, T.J. Systemic chemotherapeutic agents as adjunctive periodontal therapy: A narrative review and suggested clinical recommendations. J. Int. Acad. Periodontol. 2015, 17, 123–134. [Google Scholar]
- Walters, J.; Lai, P.C. Should antibiotics be prescribed to treat chronic periodontitis? Dent. Clin. N. Am. 2015, 59, 919–933. [Google Scholar] [CrossRef]
- Smiley, C.J.; Tracy, S.L.; Abt, E.; Michalowicz, B.S.; John, M.T.; Gunsolley, J.; Cobb, C.M.; Rossmann, J.; Harrel, S.K.; Forrest, J.L.; et al. Evidence-based clinical practice guideline on the nonsurgical treatment of chronic periodontitis by means of scaling and root planing with or without adjuncts. J. Am. Dent. Assoc. 2015, 146, 525–535. [Google Scholar] [CrossRef]
- Sigusch, B.; Beier, M.; Klinger, G.; Pfister, W.; Glockmann, E. A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J. Periodontol. 2001, 72, 275–283. [Google Scholar] [CrossRef]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontology 2000 2015, 67, 131–186. [Google Scholar] [CrossRef]
- Pretzl, B.; Sälzer, S.; Ehmke, B.; Schlagenhauf, U.; Dannewitz, B.; Dommisch, H.; Eickholz, P.; Jockel-Schneider, Y. Administration of systemic antibiotics during non-surgical periodontal therapy-a consensus report. Clin. Oral Investig. 2019, 23, 3073–3085. [Google Scholar] [CrossRef] [PubMed]
- Luchian, I.; Goriuc, A.; Martu, M.A.; Covasa, M. Clindamycin as an alternative option in optimizing periodontal therapy. Antibiotics 2021, 10, 814. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human chronic periodontitis microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Comparative in vitro resistance of human periodontal bacterial pathogens to tinidazole and four other antibiotics. Antibiotics 2020, 9, 68. [Google Scholar] [CrossRef]
- Walker, C.B. The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000 1996, 10, 79–88. [Google Scholar] [CrossRef]
- Hastey, C.J.; Boyd, H.; Schuetz, A.N.; Anderson, K.; Citron, D.M.; Dzink-Fox, J.; Hackel, M.; Hecht, D.W.; Jacobus, N.V.; Jenkins, S.G.; et al. Changes in the antibiotic susceptibility of anaerobic bacteria from 2007–2009 to 2010–2012 based on the CLSI methodology. Anaerobe 2016, 42, 27–30. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Machtei, E.E.; Christersson, L.A.; Zambon, J.J.; Hausmann, E.; Grossi, S.G.; Dunford, R.; Genco, R.J. Alternative methods for screening periodontal disease in adults. J. Clin. Periodontol. 1993, 20, 81–87. [Google Scholar] [CrossRef]
- Rauch, C.A.; Nichols, J.H. Laboratory accreditation and inspection. Clin. Lab. Med. 2007, 27, 845–858. [Google Scholar] [CrossRef]
- Dahlén, G.; Pipattanagovit, P.; Rosling, B.; Möller, A.J. A comparison of two transport media for saliva and subgingival samples. Oral Microbiol. Immunol. 1993, 8, 375–382. [Google Scholar] [CrossRef]
- Möller, Å.J.R. Microbiological examination of root canals and periapical tissues of human teeth. Methodological studies. Odontol. Tidskr. 1966, 74, 1–380. [Google Scholar]
- Slots, J.; Reynolds, H.S. Long-wave UV light fluorescence for identification of black-pigmented Bacteroides spp. J. Clin. Microbiol. 1982, 16, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Detection of colonies of Bacteroides gingivalis by a rapid fluorescence assay for trypsin-like activity. Oral Microbiol. Immunol. 1987, 2, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Rams, T.E.; Sautter, J.D.; Getreu, A.; van Winkelhoff, A.J. Phenotypic identification of Porphyromonas gingivalis validated with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Microb. Pathog. 2016, 94, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Slots, J. Microbiological tests for Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontology 2000 1999, 20, 53–64. [Google Scholar] [CrossRef]
- Feres, M.; Haffajee, A.D.; Goncalves, C.; Allard, K.A.; Som, S.; Smith, C.; Goodson, J.M.; Socransky, S.S. Systemic doxycycline administration in the treatment of periodontal infections (II). Effect on antibiotic resistance of subgingival species. J. Clin. Periodontol. 1999, 26, 784–792. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; pp. 95–96. [Google Scholar]
- French Society for Microbiology Antibiogram Committee. Comité de l′Antibiogramme de la Société Française de Microbiologie report 2003. Int. J. Antimicrob. Agents 2003, 21, 364–391. [Google Scholar] [CrossRef]
- Veloo, A.C.; Seme, K.; Raangs, E.; Rurenga, P.; Singadji, Z.; Wekema-Mulder, G.; van Winkelhoff, A.J. Antibiotic susceptibility profiles of oral pathogens. Int. J. Antimicrob. Agents 2012, 40, 450–454. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic resistance in human peri-implantitis microbiota. Clin. Oral Implant. Res. 2014, 25, 82–90. [Google Scholar] [CrossRef]
- Morehead, M.S.; Scarbrough, C. Emergence of global antibiotic resistance. Prim. Care 2018, 45, 467–484. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J.; Herrera Gonzales, D.; Winkel, E.G.; Dellemijn-Kippuw, N.; Vandenbroucke-Grauls, C.M.; Sanz, M. Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J. Clin. Periodontol. 2000, 27, 79–86. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Herrera, D.; Oteo, A.; Sanz, M. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in The Netherlands and Spain. J. Clin. Periodontol. 2005, 32, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Z.; Ding, P.; Lu, J.; Mao, L.; Ngiam, L.; Yuan, Z.; Engelstädter, J.; Schembri, M.A.; Guo, J. Antidepressants can induce mutation and enhance persistence toward multiple antibiotics. Proc. Natl. Acad. Sci. USA 2023, 120, e2208344120. [Google Scholar] [CrossRef] [PubMed]
- Almeida, V.S.M.; Azevedo, J.; Leal, H.F.; Queiroz, A.T.L.; da Silva Filho, H.P.; Reis, J.N. Bacterial diversity and prevalence of antibiotic resistance genes in the oral microbiome. PLoS ONE 2020, 15, e0239664. [Google Scholar] [CrossRef]
- Brooks, L.; Narvekar, U.; McDonald, A.; Mullany, P. Prevalence of antibiotic resistance genes in the oral cavity and mobile genetic elements that disseminate antimicrobial resistance: A systematic review. Mol. Oral Microbiol. 2022, 37, 133–153. [Google Scholar] [CrossRef]
- Soares, G.M.; Figueiredo, L.C.; Faveri, M.; Cortelli, S.C.; Duarte, P.M.; Feres, M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J. Appl. Oral Sci. 2012, 20, 295–309. [Google Scholar] [CrossRef]
- Ardila, C.M.; Granada, M.I.; Guzmán, I.C. Antibiotic resistance of subgingival species in chronic periodontitis patients. J. Periodontal Res. 2010, 45, 557–563. [Google Scholar] [CrossRef]
- Serrano, C.; Torres, N.; Valdivieso, C.; Castaño, C.; Barrera, M.; Cabrales, A. Antibiotic resistance of periodontal pathogens obtained from frequent antibiotic users. Acta Odontol. Latinoam. 2009, 22, 99–104. [Google Scholar]
- Conrads, G.; Klomp, T.; Deng, D.; Wenzler, J.S.; Braun, A.; Abdelbary, M.M.H. The antimicrobial susceptibility of Porphyromonas gingivalis: Genetic repertoire, global phenotype, and review of the literature. Antibiotics 2021, 10, 1438. [Google Scholar] [CrossRef]
- Reissier, S.; Penven, M.; Guérin, F.; Cattoir, V. Recent trends in antimicrobial resistance among anaerobic clinical isolates. Microorganisms 2023, 11, 1474. [Google Scholar] [CrossRef]
- Fine, D.H. Microbial identification and antibiotic sensitivity testing, an aid for patients refractory to periodontal therapy. A report of 3 cases. J. Clin. Periodontol. 1994, 21, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.R. Antimicrobial susceptibility tests: Testing methods and interpretive problems. In Antimicrobial Susceptibility Testing; Poupard, J.A., Walsh, L.R., Kleger, B., Eds.; Plenum Press: New York, NY, USA, 1994; pp. 15–25. [Google Scholar]
- Dubreuil, L.; Veloo, A.C.; Sóki, J.; ESCMID Study Group for Anaerobic Infections (ESGAI). Correlation between antibiotic resistance and clinical outcome of anaerobic infections; mini-review. Anaerobe 2021, 72, 102463. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.; Walker, C.; Hovliaras, C.; Socransky, S. Efficacy of clindamycin hydrochloride in refractory periodontitis: 24-month results. J. Periodontol. 1990, 61, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Gordon, J. The effect of clindamycin on the microbiota associated with refractory periodontitis. J. Periodontol. 1990, 61, 692–698. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Zhu, D.; Xu, M.; Yu, Y.; Yu, L.; Zhang, W. Microbiota in human periodontal abscess revealed by 16S rDNA sequencing. Front. Microbiol. 2019, 10, 1723. [Google Scholar] [CrossRef]
- Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Antibiotic resistance of human periodontal pathogen Parvimonas micra over 10 years. Antibiotics 2020, 9, 709. [Google Scholar] [CrossRef]
- Sakellari, D.; Goodson, J.M.; Kolokotronis, A.; Konstantinidis, A. Concentration of 3 tetracyclines in plasma, gingival crevice fluid and saliva. J. Clin. Periodontol. 2000, 27, 53–60. [Google Scholar] [CrossRef]
- Zamora-Cintas, M.; Marín, M.; Quiroga, L.; Martínez, A.; Fernández-Chico, M.A.; Bouza, E.; Rodríguez-Sánchez, B.; Alcalá, L. Identification of Porphyromonas isolates from clinical origin using MALDI-TOF mass spectrometry. Anaerobe 2018, 54, 197–200. [Google Scholar] [CrossRef]
- Sparbrod, M.; Gager, Y.; Koehler, A.K.; Jentsch, H.; Stingu, C.S. Relationship between phenotypic and genotypic resistance of subgingival biofilm samples in patients with periodontitis. Antibiotics 2022, 12, 68. [Google Scholar] [CrossRef]
- Thompson, W.; Teoh, L.; Pulcini, C.; Sanderson, S.; Williams, D.; Carter, V.; Pitkeathley, C.; Walsh, T. International consensus on a dental antibiotic stewardship core outcome set. Int. Dent. J. 2023, 73, 456–462. [Google Scholar] [CrossRef]
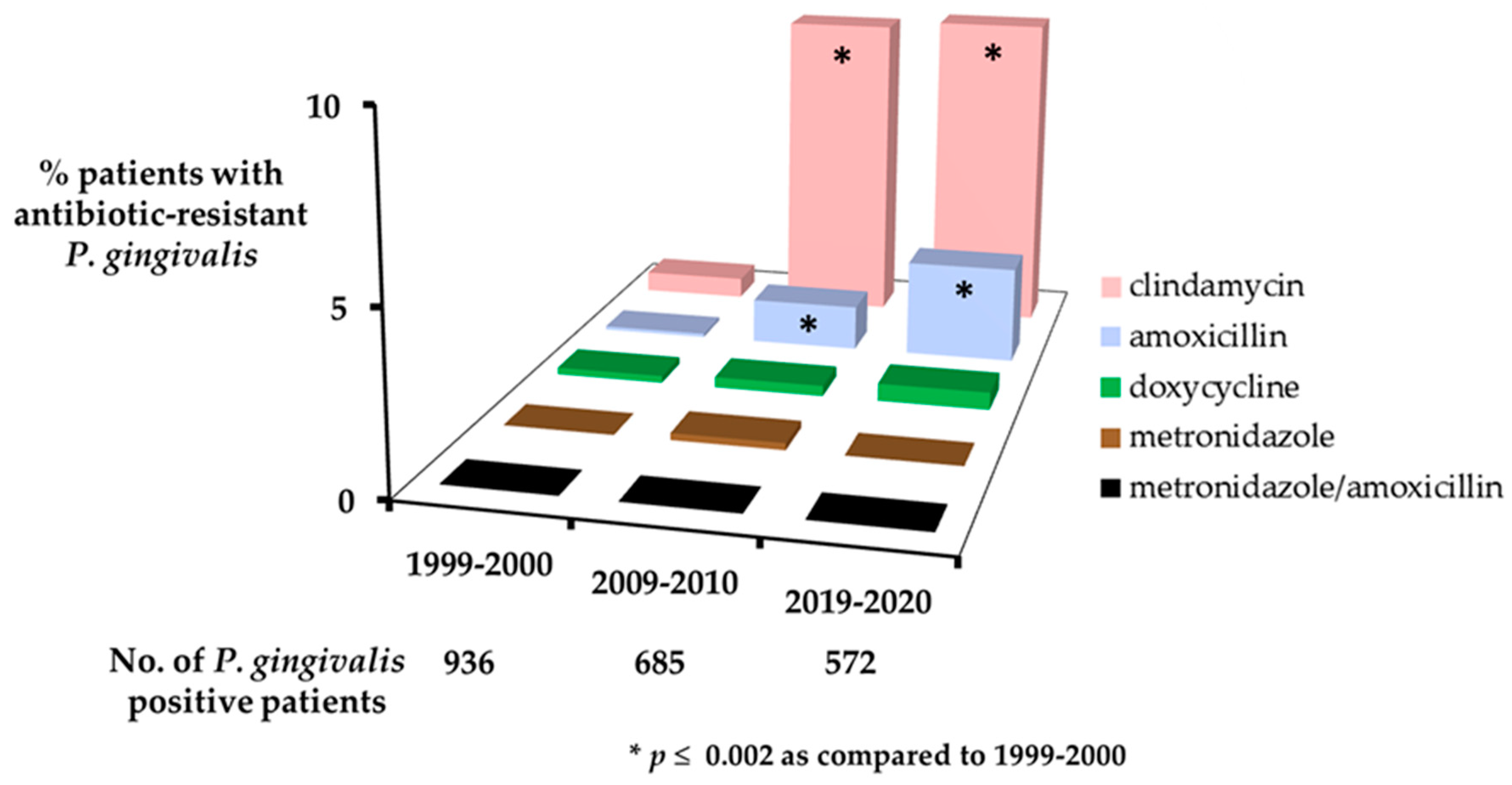
| Feature | Patient Group | ||
|---|---|---|---|
| Time period | 1999–2000 | 2009–2010 | 2019–2020 |
| No. of patients | 936 | 685 | 572 |
| % male | 49.7 | 48.6 | 47.6 |
| Mean age, years ± SD | 53.1 ± 10.6 * | 55.9 ± 11.2 | 55.7 ± 12.7 |
| Age range, years | 35–81 | 35–86 | 35–87 |
| Mean% P. gingivalis ± SD | 13.3 ± 15.3 | 12.9 ± 12.6 | 10.6 ± 12.1 * |
| Range% P. gingivalis | 0.1–78.9 | 0.1–68.9 | 0.1–69.3 |
| Patient Group | |||
|---|---|---|---|
| Antibiotic | 1999–2000 (N = 936) | 2009–2010 (N = 685) | 2019–2020 (N = 572) |
| No. (%) patients with clindamycin-resistant P. gingivalis | 6 (0.6) | 62 (9.1) * | 53 (9.3) * |
| Mean % drug-resistant P. gingivalis ± SD | 19.2 ± 16.8 ‡ | 7.2 ± 9.2 | 11.3 ± 15.4 |
| No. (%) patients with amoxicillin-resistant P. gingivalis | 1 (0.1) | 9 (1.3) * | 16 (2.8) * |
| Mean % drug-resistant P. gingivalis ± SD | 1.7 | 9.3 ± 11.5 | 10.0 ± 17.2 |
| No. (%) patients with doxycycline-resistant P. gingivalis | 2 (0.2) | 2 (0.3) | 3 (0.5) |
| Mean % drug-resistant P. gingivalis ± SD | 14.5 ± 6.4 | 7.7 ± 3.4 | 9.7 ± 6.4 |
| No. (%) patients with metronidazole-resistant P. gingivalis | 0 | 1 (0.2) | 0 |
| Mean % drug-resistant P. gingivalis ± SD | 0 | 1.6 | 0 |
| No. (%) patients with metronidazole/amoxicillin-resistant P. gingivalis | 0 | 0 | 0 |
| Mean drug-resistant % P. gingivalis ± SD | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rams, T.E.; Sautter, J.D.; van Winkelhoff, A.J. Emergence of Antibiotic-Resistant Porphyromonas gingivalis in United States Periodontitis Patients. Antibiotics 2023, 12, 1584. https://doi.org/10.3390/antibiotics12111584
Rams TE, Sautter JD, van Winkelhoff AJ. Emergence of Antibiotic-Resistant Porphyromonas gingivalis in United States Periodontitis Patients. Antibiotics. 2023; 12(11):1584. https://doi.org/10.3390/antibiotics12111584
Chicago/Turabian StyleRams, Thomas E., Jacqueline D. Sautter, and Arie J. van Winkelhoff. 2023. "Emergence of Antibiotic-Resistant Porphyromonas gingivalis in United States Periodontitis Patients" Antibiotics 12, no. 11: 1584. https://doi.org/10.3390/antibiotics12111584
APA StyleRams, T. E., Sautter, J. D., & van Winkelhoff, A. J. (2023). Emergence of Antibiotic-Resistant Porphyromonas gingivalis in United States Periodontitis Patients. Antibiotics, 12(11), 1584. https://doi.org/10.3390/antibiotics12111584






