Antifungal Activities of Compounds Produced by Newly Isolated Acrocarpospora Strains
Abstract
1. Introduction
2. Results
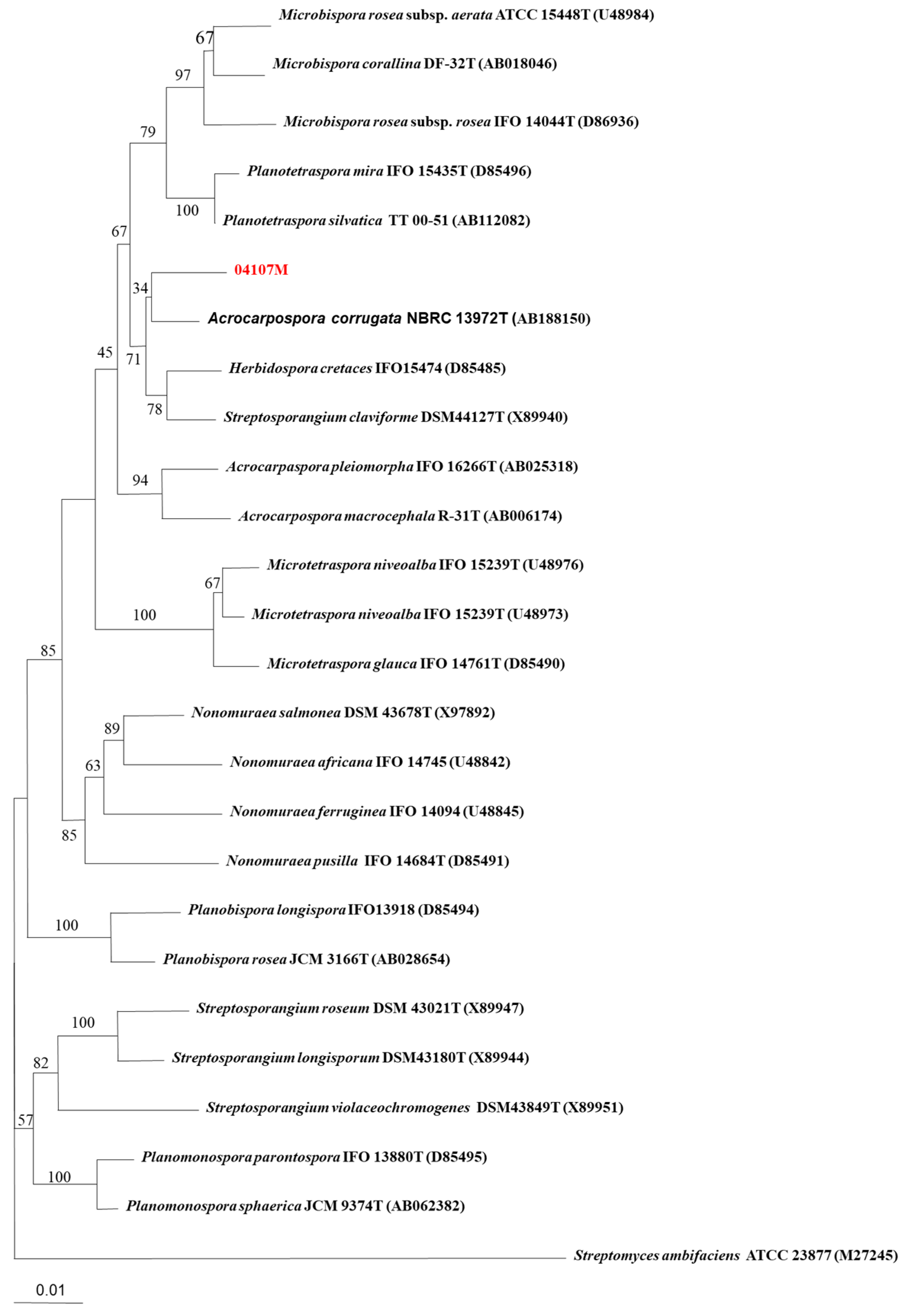
2.1. Taxonomic Identification (Phenotypic and Genotypic Data) of Acrocarpospora punica 04107M
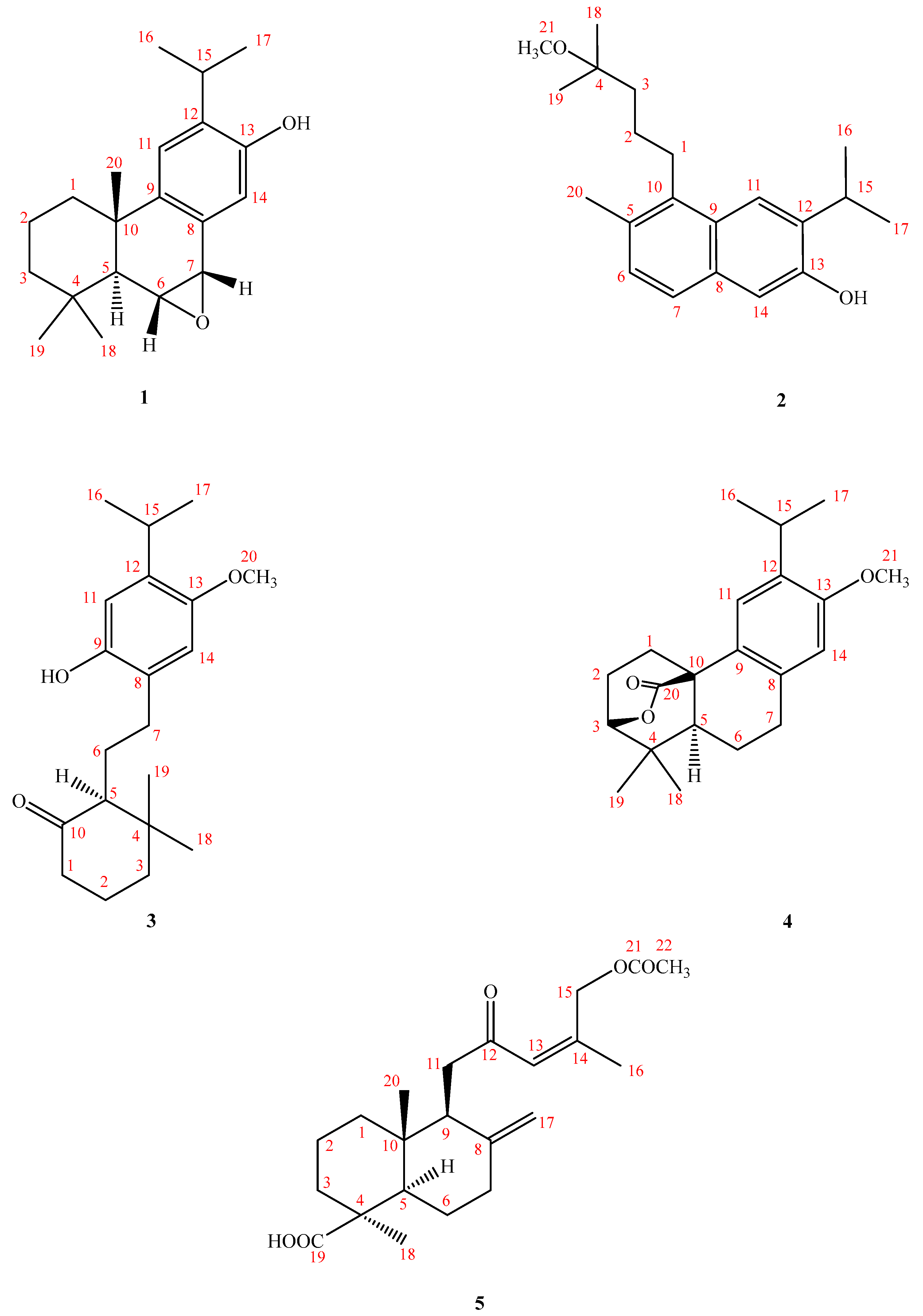
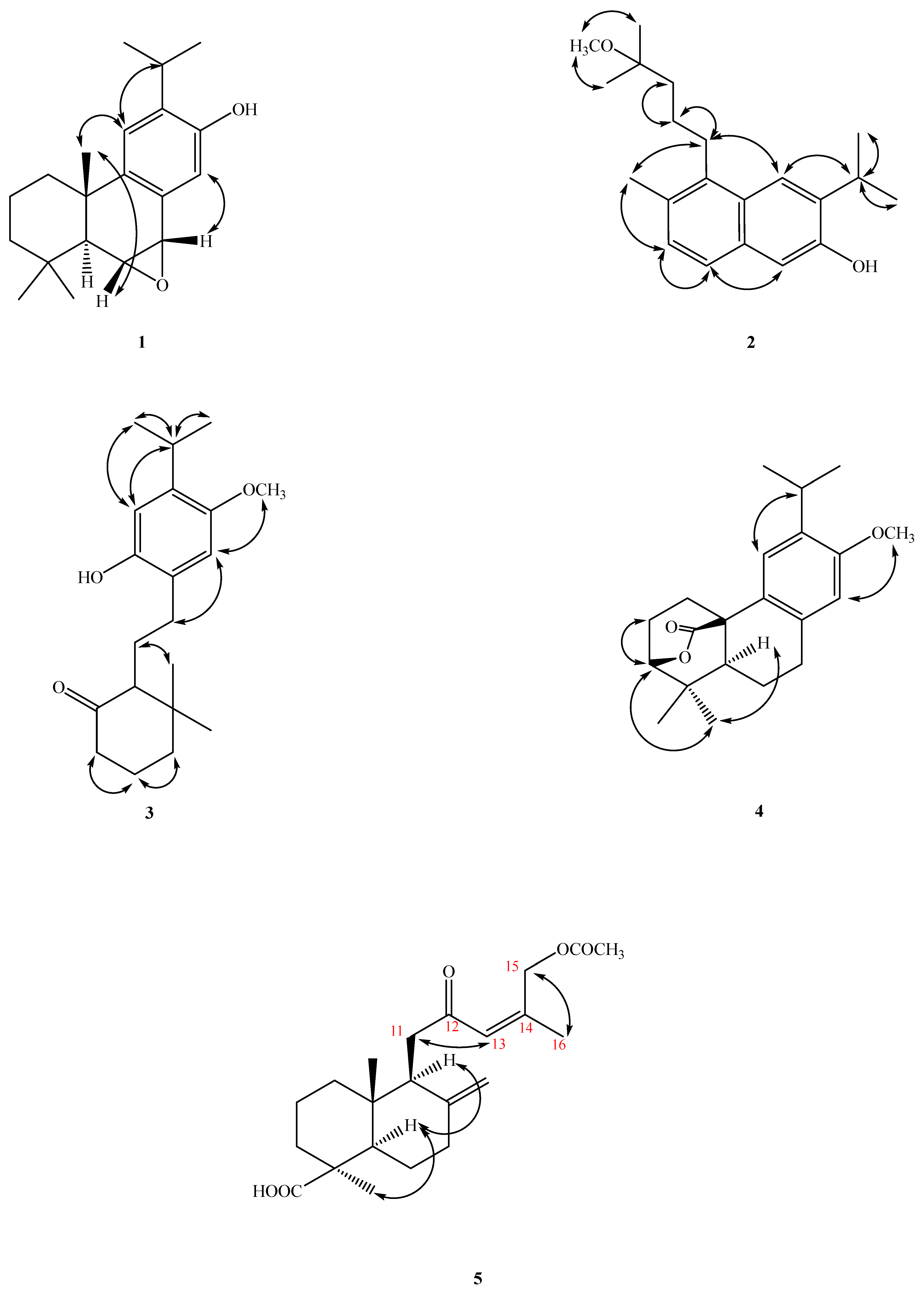
2.2. Structure Elucidation of Compounds
3. Discussion
Biological Studies
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Microorganism, Cultivation, and Preparation of the Actinobacteria Strain
4.3. Isolation and Characterization of Secondary Metabolites
4.4. Antifungal Activity Assays
4.4.1. By Disk Diffusion Assay
4.4.2. By Broth Dilution Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barman, D.; Dkhar, M.S. Seasonal variation influence endophytic Actinobacterial communities of medicinal plants from tropical deciduous forest of Meghalaya and characterization of their plant growth-promoting potentials. Curr. Microbiol. 2020, 77, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dubey, A.K. Diversity and applications of endophytic Actinobacteria of plants in special and other ecological niches. Front. Microbiol. 2018, 9, 1767. [Google Scholar] [CrossRef]
- Chen, C.; Ye, Y.; Wang, R.; Zhang, Y.; Wu, C.; Debnath, S.C.; Ma, Z.; Wang, J.; Wu, M. Streptomyces Nigra Sp. Nov. Is a Novel Actinobacterium Isolated from Mangrove Soil and Exerts a Potent Antitumor Activity in vitro. Front. Microbiol. 2018, 9, 1587. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Pokhrel, A.R.; Nguyen, C.T.; Pham, V.T.T.; Dhakal, D.; Lim, H.N.; Jung, H.J.; Kim, T.S.; Yamaguchi, T.; Sohng, J.K. Streptomyces Sp. VN1, a Producer of Diverse Metabolites Including Non-Natural Furan-Type Anticancer Compound. Sci. Rep. 2020, 10, 1756. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Othman, E.M.; Stopper, H.; Edrada-Ebel, R.A.; Hentschel, U.; Abdelmohsen, U.R. Isolation of Petrocidin a, a New Cytotoxic Cyclic Dipeptide from the Marine Sponge-Derived Bacterium Streptomyces sp. SBT348. Mar. Drugs 2017, 15, 383. [Google Scholar] [CrossRef]
- Jinendiran, S.; Teng, W.; Dahms, H.U.; Liu, W.; Ponnusamy, V.K.; Chiu, C.C.C.; Kumar, B.S.D.; Sivakumar, N. Induction of Mitochondria-Mediated Apoptosis and Suppression of Tumor Growth in Zebrafish Xenograft Model by Cyclic Dipeptides Identified from Exiguobacterium acetylicum. Sci. Rep. 2020, 10, 13721. [Google Scholar] [CrossRef]
- Farnaes, L.; Coufal, N.G.; Kauffman, C.A.; Rheingold, A.L.; Dipasquale, A.G.; Jensen, P.R.; Fenical, W. Napyradiomycin Derivatives, Produced by a Marine-Derived Actinomycete, Illustrate Cytotoxicity by Induction of Apoptosis. J. Nat. Prod. 2014, 77, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Suzuki, S.; Hatano, K. Acrocarpospora gen. nov., a new genus of the order Actinomycetales. Int. J. Syst. Evol. Microbiol. 2000, 50 Pt 3, 1163–1171. [Google Scholar] [CrossRef][Green Version]
- Komaki, H.; Oguchi, A.; Tamura, T.; Hamada, M.; Ichikawa, N. Diversity of nonribosomal peptide synthetase and polyketide synthase gene clusters in the genus Acrocarpospora. J. Gen. Appl. Microbiol. 2021, 66(6), 315–322. [Google Scholar] [CrossRef]
- Meyers, P.R. Analysis of recombinase A (recA/RecA) in the actinobacterial family Streptosporangiaceae and identification of molecular signatures. Syst. Appl. Microbiol. 2015, 38(8), 567–577. [Google Scholar] [CrossRef]
- Niemhom, N.; Suriyachadkun, C.; Tamura, T.; Thawai, C. Acrocarpospora phusangensis sp. nov., isolated from a temperate peat swamp forest soil. Int. J. Syst. Evol. Microbiol. 2013, 63, 2174–2179. [Google Scholar] [CrossRef] [PubMed]
- Akita, H.; Oishi, T. Aromatic substitution in dehydroabietane derivatives. Chem. Pharm. Bull. 1981, 29, 1567–1579. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanaka, Y.; Terao, H.; Takeda, Y.; Wada, M. The synthesis of salvinolone, saprorthoquinone, and 4-hydroxysapri-paraquinone from (+)-dehydroabietic acid. Bull. Chem. Soc. Jpn. 1993, 66, 3053–3057. [Google Scholar] [CrossRef]
- Matsumoto, T.; Usui, S.; Kawashima, H.; Mitsuki, M. Synthesis of (+)-hinokiol, (+)-hinokione, (+)-salviol, and (+)-2-oxoferruginol. Bull. Chem. Soc. Jpn. 1981, 54, 581–584. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nishino, C. Biological activities of pisiferic acid and O-methylpisiferic acid. Agric. Biol. Chem. 1986, 50, 2405–2407. [Google Scholar] [CrossRef]
- Cheng, M.J.; Cheng, Y.C.; Hsieh, M.T.; Chen, I.S.; Tseng, M.; Yuan, G.F.; Chang, H.S. Chemical Constituents of Metabolites Produced by the Actinomycete Acrocarpospora punica. Chem. Nat. Compd. 2014, 50, 606–610. [Google Scholar] [CrossRef]
- Gonza’lez, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Hanson, J.R. Diterpenoids of terrestrial origin. Nat. Prod. Rep. 2017, 34, 1233–1243. [Google Scholar] [CrossRef]
- Helfenstein, A.; Vahermo, M.; Nawrot, D.A.; Demirci, F.; İşcan, G.; Krogerus, S.; Yli-Kauhaluoma, J.; Moreira, V.M.; Tammela, P. Antibacterial profiling of abietane-type diterpenoids. Bioorg. Med. Chem. 2017, 25, 132–137. [Google Scholar] [CrossRef]
- Gonza’lez, M.A. Aromatic abietane diterpenoids: Total syntheses and synthetic studies. Tetrahedron 2015, 71, 1883–1908. [Google Scholar] [CrossRef]
- Shin, B.; Kim, B.Y.; Cho, E.; Oh, K.B.; Shin, J.; Goodfellow, M.; Oh, D.C. Actinomadurol, an Antibacterial Norditerpenoid from a Rare Actinomycete, Actinomadura sp. KC 191. J. Nat. Prod. 2016, 79, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline; CLSI document M44-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2004. [Google Scholar]
- Clinical and Laboratory Standards Institute. Zone Diameter Interpretive Standards, Corresponding Minimal Inhibitory Concentration (MIC) Interpretive Breakpoints, and Quality Control Limits for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Informational Supplement, 2nd ed.; CLSI document M44-S2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Filamentous Fungi; Proposed Guideline; CLSI document M51-P; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard, 3rd ed.; CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Informational Supplement, 3rd ed.; CLSI document M27-S3; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard, 2nd ed.; CLSI document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Johansson, M.; Kopcke, B.; Anke, H.; Sterner, O. Biologically active secondary metabolites from the ascomycete A111-95. 2. Structure elucidation. J. Antibiot. 2002, 55, 104–106. [Google Scholar] [CrossRef] [PubMed]
- Berdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.K. Pharmaceutically active secondary metabolites of marine Actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef] [PubMed]

| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 39.6 | 29.3 | 41.8 | 32.6 | 39.2 |
| 2 | 18.8 | 24.2 | 23.0 | 21.7 | 19.8 |
| 3 | 45.5 | 40.6 | 40.2 | 84.2 | 37.9 |
| 4 | 34.8 | 74.8 | 40.0 | 38.0 | 44.1 |
| 5 | 47.9 | 133.7 | 60.0 | 50.4 | 55.9 |
| 6 | 43.9 | 126.8 | 24.8 | 22.9 | 25.6 |
| 7 | 76.9 | 125.5 | 30.2 | 30.0 | 37.9 |
| 8 | 126.9 | 128.3 | 118.3 | 128.5 | 148.9 |
| 9 | 149.1 | 131.7 | 154.1 | 132.5 | 50.8 |
| 10 | 38.8 | 132.3 | 216.9 | 46.1 | 39.7 |
| 11 | 109.1 | 106.0 | 99.7 | 110.8 | 33.1 |
| 12 | 153.5 | 152.3 | 156.4 | 155.1 | 200.9 |
| 13 | 132.8 | 135.2 | 127.9 | 136.2 | 134.6 |
| 14 | 128.1 | 125.8 | 126.6 | 126.4 | 140.1 |
| 15 | 26.7 | 27.4 | 26.2 | 26.6 | 61.4 |
| 16 | 22.9 | 22.6 | 22.9 | 22.5 | 12.1 |
| 17 | 22.3 | 22.6 | 22.9 | 22.7 | 106.3 |
| 18 | 36.5 | 24.9 | 29.9 | 27.6 | 28.9 |
| 19 | 23.2 | 24.9 | 21.0 | 21.7 | 181.4 |
| 20 | 26.2 | 20.1 | 55.5 | 177.1 | 13.4 |
| 21 | 49.1 | 55.8 | 171.0 | ||
| 22 | 29.0 |
| No. | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 1.50 (m, H-α) 2.28 (br d, J = 13.2 Hz, H-β) | 1.23 (m, H-α) 2.89 (m, H-β) | 2.46(br d, J = 15.0 Hz, H-β) 2.35 (m, H-α) | 1.84 (1H, m, H-α) 2.32 (1H, td, J = 13.0, 5.0 Hz, H1-β) | 1.18/1.61 (each 1H, m) |
| 2 | 1.71 (1H, m) 1.59 (1H, m) | 1.62 (2H, m) | 1.82 (1H, m) 1.96 (1H, m) | 2.15 (2H, m) | 1.50/1.85 (each 1H, m) |
| 3 | 1.39 (2H, m) | 1.69 (2H, m) | 1.59 (1H, m) 1.74 (1H, m) | 4.20 (1H, d, J = 4.0 Hz, H-β) | 1.05/2.20 (each 1H, m) |
| 4 | |||||
| 5 | 1.97 (1H, d, J = 10.8 Hz) | 2.21 (1H, m) | 1.78 (d, J = 13.0) | 1.47 (1H, dd, J = 12.8, 2.6 Hz) | |
| 6 | 3.40 (1H, dd, J = 10.8, 4.2 Hz) | 7.09 (d, J = 8.4) | 1.62 (2H, m) | 1.38 (1H, m) 1.86 (1H, m) | 1.85–1.98 (m) |
| 7 | 5.18 (1H, br d, J = 4.2 Hz) | 7.48 (d, J = 8.4) | 2.38 (1H, m) 2.29 (1H, m) | 2.76 (1H, m) 2.67 (1H, m) | 2.15–2.40 (m) |
| 8 | |||||
| 9 | 2.60 (m) | ||||
| 10 | |||||
| 11 | 6.68 (s) | 7.22 (s) | 6.82 (s) | 6.89 (1H, s) | 2.59–2.63 (m, H-11β) 2.96 (dd, J = 16.5, 10.5 Hz, H-11α) |
| 12 | |||||
| 13 | 6.60 (br t, J = 1.2 Hz)) | ||||
| 14 | 7.25 (s) | 7.52 (s) | 6.51 (s) | 6.65 (1H, s) | |
| 15 | 3.06 (1H, sep, J = 7.4 Hz) | 3.31 (1H, sep, J = 6.8 Hz) | 3.17 (1H, sep, J = 7.0 Hz) | 3.19 (1H, sep, J = 7.2 Hz) | 4.80 (br d, J = 1.2 Hz) |
| 16 | 1.23 (3H, d, J = 7.4 Hz) | 1.33 (3H, d, J = 6.8 Hz) | 1.15 (3H, d, J = 7.0 Hz) | 1.21 (3H, d, J = 7.2 Hz) | 1.80 (br s) |
| 17 | 1.23 (3H, d, J = 7.4 Hz) | 1.33 (3H, d, J = 6.8 Hz) | 1.17 (3H, d, J = 7.0 Hz) | 1.21 (3H, d, J = 7.0 Hz) | 4.70/4.22 (each 1H, br s) |
| 18 | 1.26 (3H, s) | 1.15 (3H, s) | 1.08 (3H, s) | 1.15 (3H, s) | 1.24 (s) |
| 19 | 1.29 (3H, s) | 1.15 (3H, s) | 0.71 (3H, s) | 1.06 (3H, s) | |
| 20 | 1.22 (3H, s) | 2.41 (3H, s) | 3.79 (3H, s) | 0.67 (3H, s) | |
| 21 | 3.20 (3H, s) | 3.79 (3H, s) | |||
| 22 | 2.09 (3H, s) |
| Test Microorganism | Isolated Compounds | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Ketoconazole | |
| A. niger | 27.4 ±1.7 | 22.7 ± 1.2 | 22.3 ± 1.9 | 22.0 ± 2.8 | 27.5 ± 2.8 | 36 ± 1.7 |
| P. italicum | 18.7 ± 0.3 | 18.2 ± 2.6 | 19.4 ± 1.2 | 26.3 ± 1.8 | 19.3 ± 0.3 | 33 ± 1.8 |
| C. albicans | 27.2 ± 1.4 | 17.0 ± 1.2 | 16.2 ± 2.6 | 29 ± 2.3 | 28.0 ± 1.3 | 39 ± 4.4 |
| S. cerevisiae | 29.2 ± 2.4 | 28.1 ± 1.5 | 11.2 ± 2.7 | 28 ± 2.3 | 27.3 ± 1.4 | 34 ± 1.9 |
| Compounds | A. niger | P. italicum | C. albicans | S. cerevisiae |
|---|---|---|---|---|
| 1 | 54.87 ± 6.13 a | 53.98 ± 2.34 a | 49.56 ± 6.49 a | >100 |
| 2 | >100 | >100 | >100 | 57.38 ± 7.28 a |
| 3 | >100 | >100 | >100 | >100 |
| 4 | >100 | 42.78 ± 5.23 a | 38.89 ± 3.31 a | 40.58 ± 0.23 a |
| 5 | 59.78 ± 7.04 a | >100 | 51.32 ± 10.60 a | 56.92 ± 13.19 a |
| Ketoconazole | 3.25 ± 1.48 a | 6.72 ± 2.23 a | 11.79 ± 4.81 a | 3.16 ± 1.51 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-J.; Chen, J.-J.; Wu, M.-D.; Leu, J.-Y.; Tseng, M. Antifungal Activities of Compounds Produced by Newly Isolated Acrocarpospora Strains. Antibiotics 2023, 12, 95. https://doi.org/10.3390/antibiotics12010095
Cheng M-J, Chen J-J, Wu M-D, Leu J-Y, Tseng M. Antifungal Activities of Compounds Produced by Newly Isolated Acrocarpospora Strains. Antibiotics. 2023; 12(1):95. https://doi.org/10.3390/antibiotics12010095
Chicago/Turabian StyleCheng, Ming-Jen, Jih-Jung Chen, Ming-Der Wu, Jyh-Yih Leu, and Min Tseng. 2023. "Antifungal Activities of Compounds Produced by Newly Isolated Acrocarpospora Strains" Antibiotics 12, no. 1: 95. https://doi.org/10.3390/antibiotics12010095
APA StyleCheng, M.-J., Chen, J.-J., Wu, M.-D., Leu, J.-Y., & Tseng, M. (2023). Antifungal Activities of Compounds Produced by Newly Isolated Acrocarpospora Strains. Antibiotics, 12(1), 95. https://doi.org/10.3390/antibiotics12010095






