A Clinical Case of Nosocomial Pneumonia as a Complication of COVID-19: How to Balance Benefits and Risks of Immunosuppressive Therapy?
Abstract
1. Introduction
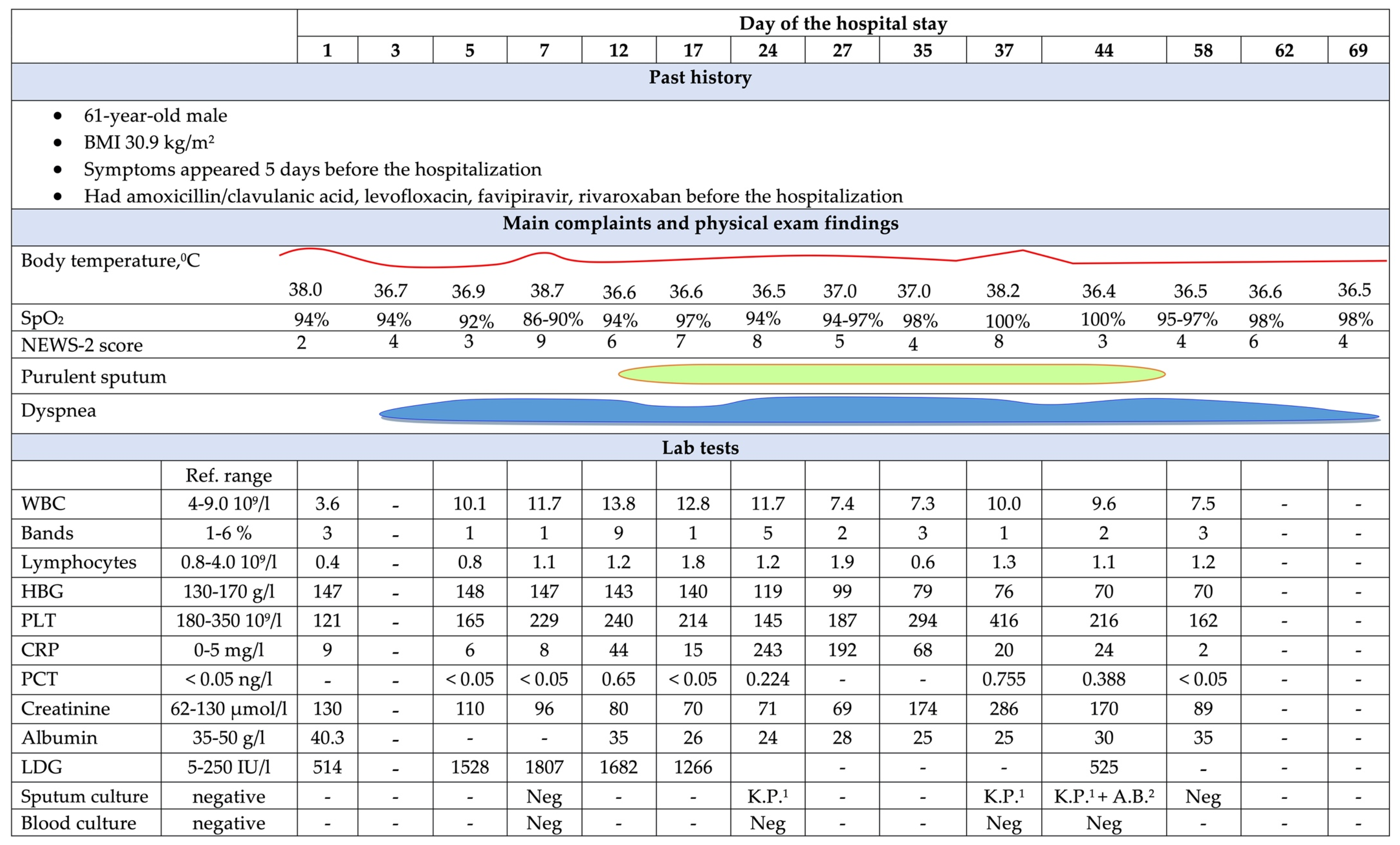
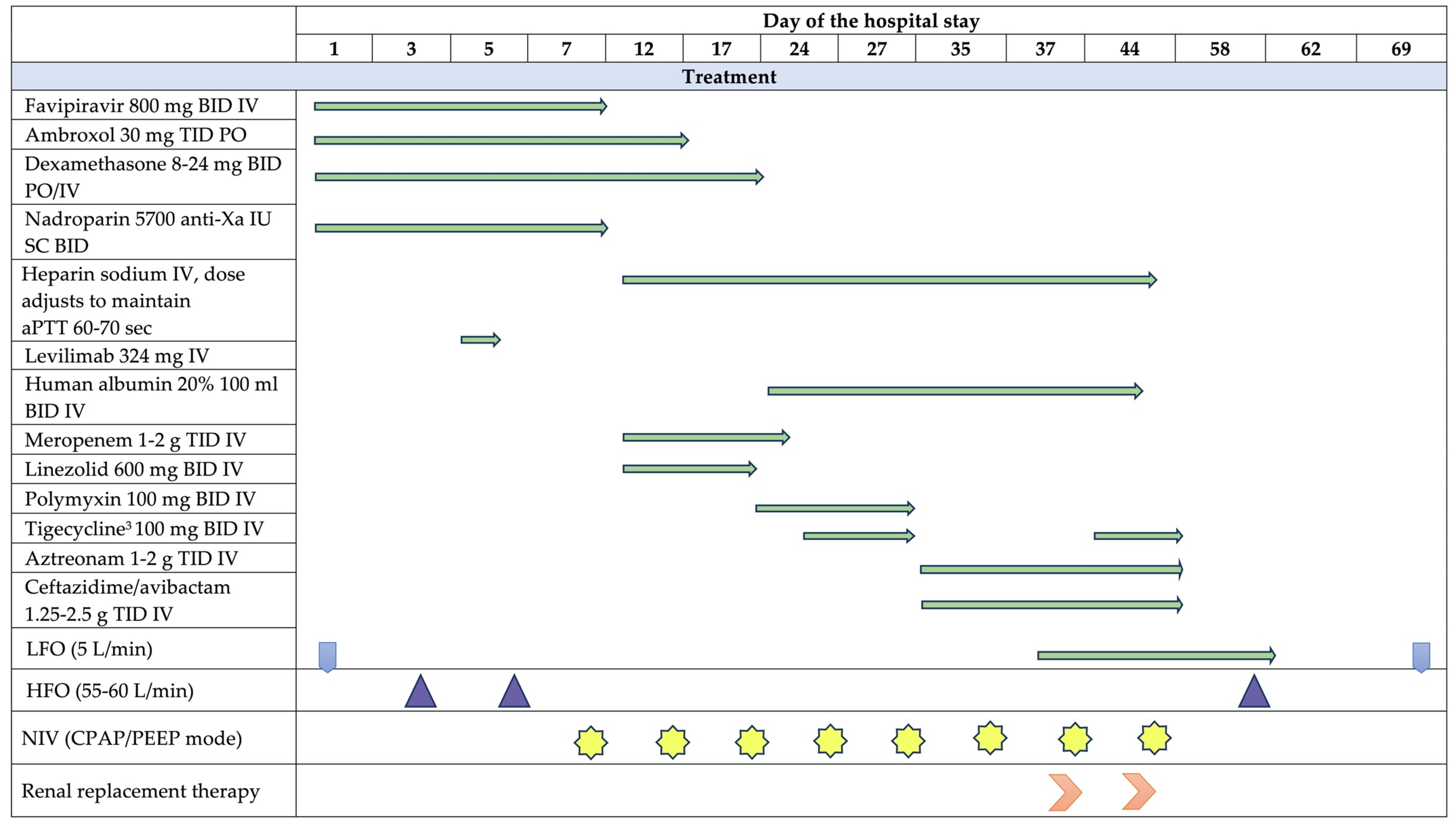
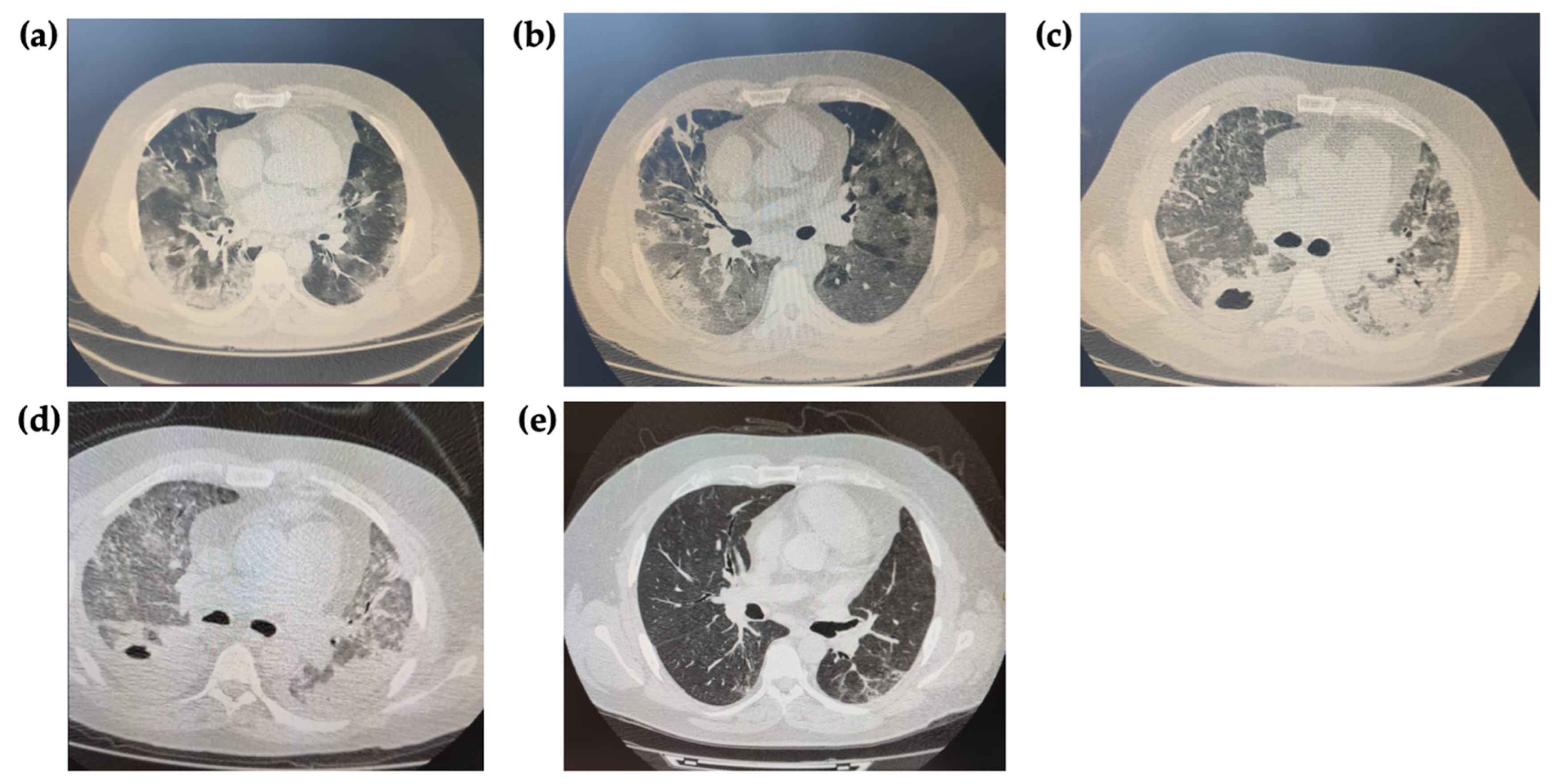
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukova, M.; Edelstein, M.; Ivanchik, N.; Skleenova, E.; Shajdullina, E.; Azyzov, I.; Shek, E.; Kuzmenkov, A.; Dekhnich, A.; Kozlov, R.; et al. Antimicrobial resistance of nosocomial Enterobacterales isolates in Russia: Results of multicenter epidemiological study “MARATHON 2015–2016”. Clin. Microbiol. Antimicrob. Chemother. 2019, 21, 147–159. [Google Scholar] [CrossRef]
- Sukhorukova, M.V.; Edelstein, M.V.; Skleenova, E.Y.; Ivanchik, N.V.; Shek, E.A.; Dekhnich, A.V.; Kozlov, R.S. Antimicrobial resistance of nosocomial Acinetobacter spp. isolates in Russia: Results of multicenter epidemiological study “MARATHON 2015–2016”. Clin. Microbiol. Antimicrob. Chemother. 2019, 21, 171–180. [Google Scholar] [CrossRef]
- Romashov, O.M.; Ni, O.G.; Bykov, A.O.; Kruglov, A.N.; Protsenko, D.N.; Tyurin, I.N. Antimicrobial resistance and antimicrobial therapy modification during COVID-19 pandemic in large tertiary hospital. Clin. Microbiol. Antimicrob. Chemother. 2021, 23, 293–303. [Google Scholar] [CrossRef]
- Porretta, A.D.; Baggiani, A.; Arzilli, G.; Casigliani, V.; Mariotti, T.; Mariottini, F.; Scardina, G.; Sironi, D.; Totaro, M.; Barnini, S.; et al. Increased risk of acquisition of New Delhi metallo-beta-lactamase–producing carbapenem-resistant Enterobacterales (NDM-CRE) among a cohort of COVID-19 patients in a teaching hospital in Tuscany, Italy. Pathogens 2020, 9, 635. [Google Scholar] [PubMed]
- Tiri, B.; Sensi, E.; Marsiliani, V.; Cantarini, M.; Priante, G.; Vernelli, C.; Martella, L.A.; Costantini, M.; Mariottini, A.; Andreani, P.; et al. Antimicrobial stewardship program, COVID-19, and infection control: Spread of carbapenem-resistant Klebsiella pneumoniae colonization in ICU COVID-19 patients. What did not work? J. Clin. Med. 2020, 9, 2744. [Google Scholar] [CrossRef]
- Segala, F.V.; Bavaro, D.F.; Di Gennaro, F.; Salvati, F.; Marotta, C.; Saracino, A.; Murri, R.; Fantoni, M. Impact of SARS-CoV-2 Epidemic on Antimicrobial Resistance: A Literature Review. Viruses 2021, 13, 2110. [Google Scholar] [CrossRef]
- Zavala-Flores, E.; Salcedo-Matienzo, J. Pre-hospitalary medication in COVID-19 patients from a public hospital in Lima-Peru. Acta Med. Peru 2020, 37, 393–395. [Google Scholar]
- Avdeev, S.; Rachina, S.; Belkova, Y.; Kozlov, R.; Versporten, A.; Pauwels, I.; Goossens, H.; Bochanova, E.; Elokhina, E.; Portnjagina, U.; et al. Antimicrobial Prescribing Patterns in Patients with COVID-19 in Russian MultiField Hospitals in 2021: Results of the Global-PPS Project. Trop. Med. Infect. Dis. 2022, 7, 75. [Google Scholar] [CrossRef]
- Patel, A.; Emerick, M.; Cabunoc, M.K.; Williams, M.H.; Preas, M.A.; Schrank, G.; Rabinowitz, R.; Luethy, P.; Johnson, J.K.; Leekha, S. Rapid Spread and Control of Multidrug-Resistant Gram-Negative Bacteria in COVID-19 Patient Care Units. Emerg. Infect. Dis. 2021, 27, 1234–1237. [Google Scholar] [CrossRef]
- Scaravilli, V.; Guzzardella, A.; Madotto, F.; Beltrama, V.; Muscatello, A.; Bellani, G.; Monti, G.; Greco, M.; Pesenti, A.; Bandera, A.; et al. Impact of dexamethasone on the incidence of ventilator-associated pneumonia in mechanically ventilated COVID-19 patients: A propensity-matched cohort study. Critical Care 2022, 26, 176. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vidal, C.; Sanjuan, G.; Moreno-García, E.; Puerta-Alcalde, P.; Garcia-Pouton, N.; Chumbita, M.; Fernandez-Pittol, M.; Pitart, C.; Inciarte, A.; Bodro, M.; et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. Am. J. Respir. Crit Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef]
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Bardi, T.; Pintado, V.; Gomez-Rojo, M.; Escudero-Sanchez, R.; Azzam Lopez, A.; Diez-Remesal, Y.; Martinez Castro, N.; Ruiz-Garbajosa, P.; Pestaña, D. Nosocomial infections associated to COVID-19 in the intensive care unit: Clinical characteristics and outcome. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 495–502. [Google Scholar] [CrossRef]
- Llitjos, J.F.; Bredin, S.; Lascarrou, J.B.; Soumagne, T.; Cojocaru, M.; Leclerc, M.; Lepetit, A.; Gouhier, A.; Charpentier, J.; Piton, G.; et al. Increased susceptibility to intensive care unit-acquired pneumonia in severe COVID-19 patients: A multicentre retrospective cohort study. Ann. Intensive Care 2021, 11, 20. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit Care 2021, 25, 25. [Google Scholar] [CrossRef]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- Bhatt, P.J.; Shiau, S.; Brunetti, L.; Xie, Y.; Solanki, K.; Khalid, S.; Mohayya, S.; Au, P.H.; Pham, C.; Uprety, P.; et al. Risk Factors and Outcomes of Hospitalized Patients With Severe Coronavirus Disease 2019 (COVID-19) and Secondary Bloodstream Infections: A Multicenter Case-Control Study. Clin. Infect. Dis. 2021, 72, e995–e1003. [Google Scholar] [CrossRef]
- Naranje, P.; Bhalla, A.S.; Jana, M.; Garg, M.; Nair, A.D.; Singh, S.K.; Banday, I. Imaging of Pulmonary Superinfections and Co-Infections in COVID-19. Curr. Probl. Diagn. Radiol. 2022, 51, 768–778. [Google Scholar] [CrossRef]
- Kooistra, E.J.; van Berkel, M.; van Kempen, N.F.; van Latum, C.R.; Bruse, N.; Frenzel, T.; van den Berg, M.J.; Schouten, J.A.; Kox, M.; Pickkers, P. Dexamethasone and tocilizumab treatment considerably reduces the value of C-reactive protein and procalcitonin to detect secondary bacterial infections in COVID-19 patients. Crit. Care 2021, 25, 281. [Google Scholar] [CrossRef] [PubMed]
- van de Veerdonk, F.L.; Giamarellos-Bourboulis, E.; Pickkers, P.; Derde, L.; Leavis, H.; van Crevel, R.; Engel, J.J.; Wiersinga, W.J.; Vlaar, A.P.; Shankar-Hari, M.; et al. A guide to immunotherapy for COVID-19. Nat. Med. 2022, 28, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Anka, A.U.; Tahir, M.I.; Abubakar, S.D.; Alsabbagh, M.; Zian, Z.; Hamedifar, H.; Sabzevari, A.; Azizi, G. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 2021, 93, e12998. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [PubMed]
- Piscoya, A.; del Riego, A.P.; Cerna-Viacava, R.; Rocco, J.; Roman, Y.M.; Escobedo, A.A.; Pasupuleti, V.; White, C.M.; Hernandez, A.V. Efficacy and harms of tocilizumab for the treatment of COVID-19 patients: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0269368. [Google Scholar] [CrossRef]
- Mattos-Silva, P.; Felix, N.S.; Silva, P.L.; Robba, C.; Battaglini, D.; Pelosi, P.; Rocco, P.R.; Cruz, F.F. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir. Physiol. Neurobiol. 2020, 280, 103492. [Google Scholar] [CrossRef]
- Malgie, J.; Schoones, J.W.; Pijls, B.G. Decreased Mortality in Coronavirus Disease 2019 Patients Treated With Tocilizumab: A Rapid Systematic Review and Meta-analysis of Observational Studies. Clin. Infect. Dis. 2021, 72, e742–e749. [Google Scholar] [CrossRef]
- Malgie, J.; Schoones, J.W.; Zeegers, M.P.; Pijls, B.G. Decreased mortality and increased side effects in COVID-19 patients treated with IL-6 receptor antagonists: Systematic review and meta-analysis. Sci. Rep. 2021, 11, 21522. [Google Scholar] [CrossRef]
- Søvik, S.; Barratt-Due, A.; Kåsine, T.; Olasveengen, T.; Strand, M.W.; Tveita, A.A.; Berdal, J.E.; Lehre, M.A.; Lorentsen, T.; Heggelund, L.; et al. Corticosteroids and superinfections in COVID-19 patients on invasive mechanical ventilation. J. Infect. 2022, 85, 57–63. [Google Scholar] [CrossRef]
- Beloborodov, V.B.; Goloschapov, O.V.; Gusarov, V.G.; Dekhnich, A.V.; Zamyatin, M.N.; Zubareva, N.A.; Zyryanov, S.K.; Kamyshova, D.A.; Klimko, N.N.; Kozlov, R.S.; et al. Guidelines "Diagnosis and antimicrobial therapy of infections caused by multiresistant microorganisms". Messenger Anesthesiol. Resusc. 2020, 17, 52–83. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachina, S.; Kiyakbaev, G.; Antonova, E.; Mescheryakov, A.; Kupryushina, O.; Hewathanthirige, G.; Palagin, I.; Kozhevnikova, E.; Sukhorukova, M.; Strelkova, D. A Clinical Case of Nosocomial Pneumonia as a Complication of COVID-19: How to Balance Benefits and Risks of Immunosuppressive Therapy? Antibiotics 2023, 12, 53. https://doi.org/10.3390/antibiotics12010053
Rachina S, Kiyakbaev G, Antonova E, Mescheryakov A, Kupryushina O, Hewathanthirige G, Palagin I, Kozhevnikova E, Sukhorukova M, Strelkova D. A Clinical Case of Nosocomial Pneumonia as a Complication of COVID-19: How to Balance Benefits and Risks of Immunosuppressive Therapy? Antibiotics. 2023; 12(1):53. https://doi.org/10.3390/antibiotics12010053
Chicago/Turabian StyleRachina, Svetlana, Gairat Kiyakbaev, Elena Antonova, Alexey Mescheryakov, Olga Kupryushina, Girindu Hewathanthirige, Ivan Palagin, Elena Kozhevnikova, Marina Sukhorukova, and Daria Strelkova. 2023. "A Clinical Case of Nosocomial Pneumonia as a Complication of COVID-19: How to Balance Benefits and Risks of Immunosuppressive Therapy?" Antibiotics 12, no. 1: 53. https://doi.org/10.3390/antibiotics12010053
APA StyleRachina, S., Kiyakbaev, G., Antonova, E., Mescheryakov, A., Kupryushina, O., Hewathanthirige, G., Palagin, I., Kozhevnikova, E., Sukhorukova, M., & Strelkova, D. (2023). A Clinical Case of Nosocomial Pneumonia as a Complication of COVID-19: How to Balance Benefits and Risks of Immunosuppressive Therapy? Antibiotics, 12(1), 53. https://doi.org/10.3390/antibiotics12010053






