Physical Approaches to Prevent and Treat Bacterial Biofilm
Abstract
1. Introduction
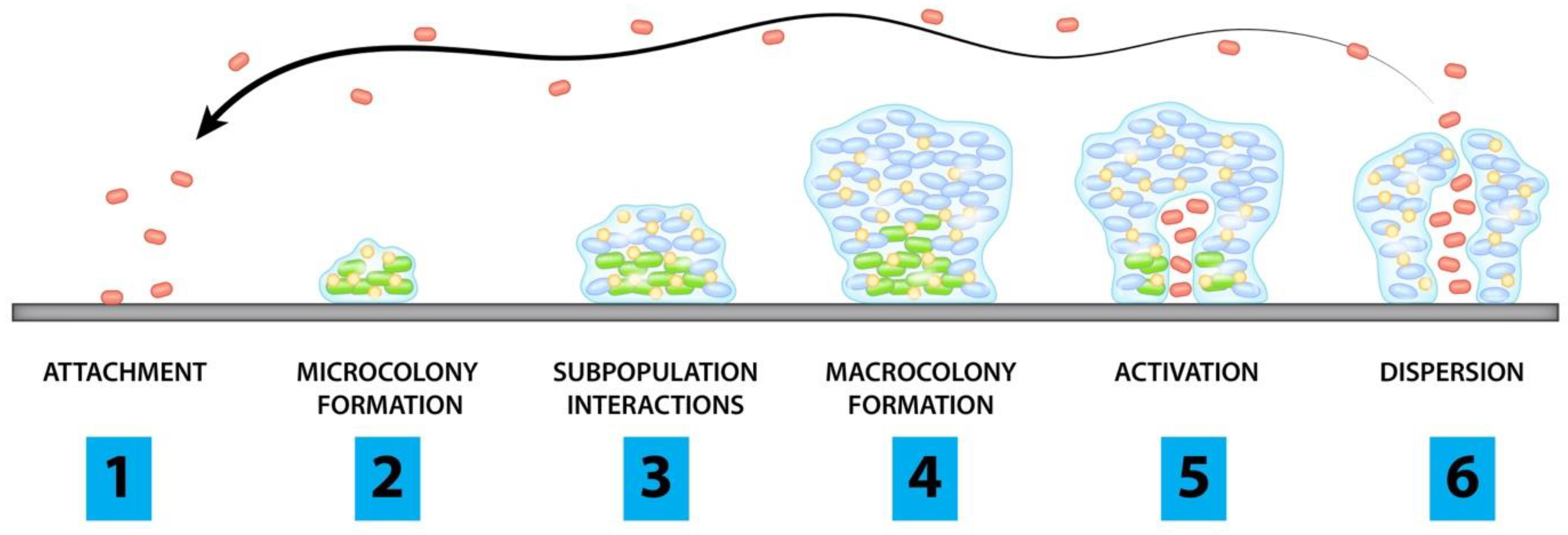
2. Pathogenesis of Biofilms
- Planktonic cells attach to surface of concern
- Cells begin to form microcolonies
- Interactions between subpopulations form microstructures and protective layers
- Biofilm matures and forms microcolonies
- Channels form and allow for accumulation of cells
- Planktonic cells are released from microcolonies
3. Methods of Literature Review
4. Intrinsic Methods
Conclusions for Intrinsic Methods
5. Extrinsic Methods
5.1. Photodynamic Therapy
5.2. Sonication
5.3. Plasma Treatment
5.4. Electric Fields & Currents
5.5. Electromagnetic Fields
5.6. Summary of Extrinsic Methods
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, P.L.; Lowry, N.; Campbell, T.; Reid, T.W.; Webster, D.R.; Tobin, E.; Aslani, A.; Mosley, T.; Dertien, J.; Colmer-Hamood, J.; et al. An Organoselenium Compound Inhibits Staphylococcus aureus Biofilms on Hemodialysis Catheters In Vivo. Antimicrob. Agents Chemother. 2012, 56, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Rohacek, M.; Weisser, M.; Kobza, R.; Schoenenberger, A.W.; Pfyffer, G.E.; Frei, R.; Erne, P.; Trampuz, A. Bacterial colonization and infection of electrophysiological cardiac devices detected with sonication and swab culture. Circulation 2010, 121, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Aboltins, C.; Daffy, J.; Choong, P.; Stanley, P. Current concepts in the management of prosthetic joint infection. Intern. Med. J. 2014, 44, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Nishi, Y.; Seto, K.; Kamashita, Y.; Kaji, A.; Kurono, A.; Nagaoka, E. Survival of microorganisms on complete dentures following ultrasonic cleaning combined with immersion in peroxide-based cleanser solution. Gerodontology 2012, 31, 202–209. [Google Scholar] [CrossRef]
- Lehmann, K.H.; von Segesser, L.; Müller-Glauser, W.; Siebenmann, R.; Schneider, K.; Lüscher, T.F.; Turina, M. Internal-mammary coronary artery grafts: Is their superiority also due to a basically intact endothelium? Thorac. Cardiovasc. Surg. 1989, 37, 187–189. [Google Scholar] [CrossRef]
- Kadry, A.A.; Fouda, S.I.; Shibl, A.M.; Abu El-Asrar, A.A. Impact of slime dispersants and anti-adhesives on in vitro biofilm formation of Staphylococcus epidermidis on intraocular lenses and on antibiotic activities. J. Antimicrob. Chemother. 2009, 63, 480–484. [Google Scholar] [CrossRef]
- Fux, C.A.; Quigley, M.; Worel, A.M.; Post, C.; Zimmerli, S.; Ehrlich, G.; Veeh, R.H. Biofilm-related infections of cerebrospinal fluid shunts. Clin. Microbiol. Infect. 2006, 12, 331–337. [Google Scholar] [CrossRef]
- Auler, M.E.; Morreira, D.; Rodrigues, F.F.O.; Abr Ão, M.S.; Margarido, P.F.R.; Matsumoto, F.E.; Silva, E.G.; Silva, B.C.M.; Schneider, C.R.; Paula, C.R. Biofilm formation on intrauterine devices in patients with recurrent vulvovaginal candidiasis. Med. Mycol. 2010, 48, 211–216. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Tran, N.V.; Petty, P.M.; Johnson, C.H.; Walsh, M.F.; Bite, U.; Clay, R.P.; Mandrekar, J.N.; Piper, K.E.; Steckelberg, J.M.; et al. Pilot study of association of bacteria on breast implants with capsular contracture. J. Clin. Microbiol. 2009, 47, 1333–1337. [Google Scholar] [CrossRef]
- Guaglianone, E.; Cardines, R.; Vuotto, C.; Di Rosa, R.; Babini, V.; Mastrantonio, P.; Donelli, G. Microbial biofilms associated with biliary stent clogging. FEMS Immunol. Med. Microbiol. 2010, 59, 410–420. [Google Scholar] [CrossRef]
- Tollefson, D.F.; Bandyk, D.F.; Kaebnick, H.W.; Seabrook, G.R.; Towne, J.B. Surface Biofilm Disruption: Enhanced Recovery of Microorganisms from Vascular Prostheses. Arch. Surg. 1987, 122, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Bianco, M.; Ruiz-Castañé, E.; Iafrate, M. Treatment of Penile Prosthesis Implant’s Infection. Urol. Int. 2020, 104, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Ruch, Y.; Meyer, N.; Perrier, S.; Minh, T.H.; Schneider, C.; Lavigne, T.; Marguerite, S.; Ajob, G.; Cristinar, M.; et al. Left ventricular assist device-associated infections: Incidence and risk factors. J. Thorac. Dis. 2020, 12, 2654–2662. [Google Scholar] [CrossRef] [PubMed]
- Obremskey, W.T.; Metsemakers, W.J.; Schlatterer, D.R.; Tetsworth, K.; Egol, K.; Kates, S.; McNally, M. Musculoskeletal Infection in Orthopaedic Trauma: Assessment of the 2018 International Consensus Meeting on Musculoskeletal Infection. J. Bone Jt. Surg. Am. 2020, 102, e44. [Google Scholar] [CrossRef] [PubMed]
- Wannemuehler, T.J.; Lobo, B.C.; Johnson, J.D.; Deig, C.R.; Ting, J.Y.; Gregory, R.L. Vibratory stimulus reduces in vitro biofilm formation on tracheoesophageal voice prostheses. Laryngoscope 2016, 126, 2752–2757. [Google Scholar] [CrossRef]
- Paredes, J.; Alonso-Arce, M.; Schmidt, C.; Valderas, D.; Sedano, B.; Legarda, J.; Arizti, F.; Gómez, E.; Aguinaga, A.; Del Pozo, J.L.; et al. Smart central venous port for early detection of bacterial biofilm related infections. Biomed Microdevices 2014, 16, 365–374. [Google Scholar] [CrossRef]
- Høiby, N.; Ciofu, O.; Bjarnsholt, T. Pseudomonas aeruginosa biofilms in cystic fibrosis. Future Microbiol. 2010, 5, 1663–1674. [Google Scholar] [CrossRef]
- Martínez-Solano, L.; Macia, M.D.; Fajardo, A.; Oliver, A.; Martinez, J.L. Chronic Pseudomonas aeruginosa Infection in Chronic Obstructive Pulmonary Disease. Clin. Infect. Dis. 2008, 47, 1526–1533. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.Z.; Hoiby, N.; Song, Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2014, 7, 1–7. [Google Scholar] [CrossRef]
- Jain, R.; Douglas, R. When and how should we treat biofilms in chronic sinusitis? Curr. Opin. Otolaryngol. Head Neck Surg. 2014, 22, 16–21. [Google Scholar] [CrossRef]
- Hunt, A.M.A.; Gibson, J.A.; Larrivee, C.L.; O’Reilly, S.; Navitskaya, S.; Busik, J.V.; Waters, C.M. Come to the Light Side: In Vivo Monitoring of Pseudomonas aeruginosa Biofilm Infections in Chronic Wounds in a Diabetic Hairless Murine Model. J. Vis. Exp. 2017, 10, e55991. [Google Scholar] [CrossRef]
- Darouiche, R.O. Treatment of Infections Associated with Surgical Implants. N. Engl. J. Med. 2004, 350, 1422–1429. [Google Scholar] [CrossRef]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. Pharmacokinetics/Pharmacodynamics of Colistin and Imipenem on Mucoid and Nonmucoid Pseudomonas aeruginosa Biofilms. Antimicrob. Agents Chemother. 2011, 55, 4469–4474. [Google Scholar] [CrossRef]
- Levack, A.E.; Cyphert, E.L.; Bostrom, M.P.; Hernandez, C.J.; von Recum, H.A.; Carli, A.V. Current Options and Emerging Biomaterials for Periprosthetic Joint Infection. Curr. Rheumatol. Rep. 2018, 20, 33. [Google Scholar] [CrossRef] [PubMed]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Infectious Diseases Society of America. Executive summary: Diagnosis and management of prosthetic joint infection: Clinical practice guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kandel, C.E.; Jenkinson, R.; Daneman, N.; Backstein, D.; Hansen, B.E.; Muller, M.P.; Katz, K.C.; Widdifield, J.; Bogoch, E.; Ward, S.; et al. Predictors of Treatment Failure for Hip and Knee Prosthetic Joint Infections in the Setting of 1- and 2-Stage Exchange Arthroplasty: A Multicenter Retrospective Cohort. Open Forum Infect. Dis. 2019, 6, ofz452. [Google Scholar] [CrossRef] [PubMed]
- McConoughey, S.J.; Howlin, R.; Granger, J.F.; Manring, M.M.; Calhoun, J.H.; Shirtliff, M.; Kathju, S.; Stoodley, P. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014, 9, 987–1007. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, A.; Kolin, D.A.; Farley, K.X.; Wilson, J.M.; McLawhorn, A.S.; Cross, M.B.; Sculco, P.K. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J. Arthroplast. 2021, 36, 1484–1489.e3. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Hao, Y.; Liu, Y.; Dong, Z.; Li, K. Biological and Physiochemical Methods of Biofilm Adhesion Resistance Control of Medical-Context Surface. Int. J. Biol. Sci. 2021, 17, 1769–1781. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Wu, H.; Song, Z.; Høiby, N.; Molin, S.; Givskov, M. Combating biofilms. FEMS Immunol. Med. Microbiol. 2012, 65, 146–157. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microb. Biofilms 2015, 223–247. [Google Scholar] [CrossRef]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial biofilm: Structure, function, and antimicrobial resistance. Endod. Top. 2010, 22, 2–16. [Google Scholar] [CrossRef]
- Arciola, C.R.; Campoccia, D.; Ravaioli, S.; Montanaro, L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, C.T.; Miller, L.C.; Siryaporn, A.; Drescher, K.; Semmelhack, M.F.; Bassler, B.L. A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. USA 2013, 110, 17981–17986. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Cirioni, O.; Giacometti, A.; Ghiselli, R.; Braunstein, J.B.; Silvestri, C.; Mocchegiani, F.; Saba, V.; Scalise, G. Treatment of Staphylococcus aureus Biofilm Infection by the Quorum-Sensing Inhibitor RIP. Antimicrob. Agents Chemother. 2007, 51, 2226–2229. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Anguita-Alonso, P.; Hanssen, A.D.; Osmon, D.R.; Trampuz, A.; Steckelberg, J.M.; Patel, R. High rate of aminoglycoside resistance among staphylococci causing prosthetic joint infection. Clin. Orthop. Relat. Res. 2005, 439, 43–47. [Google Scholar] [CrossRef]
- Dunne, N.; Hill, J.; McAfee, P.; Todd, K.; Kirkpatrick, R.; Tunney, M.; Patrick, S. In vitro study of the efficacy of acrylic bone cement loaded with supplementary amounts of gentamicin: Effect on mechanical properties, antibiotic release, and biofilm formation. Acta Orthop. 2007, 78, 774–785. [Google Scholar] [CrossRef]
- McConoughey, S.J.; Howlin, R.P.; Wiseman, J.; Stoodley, P.; Calhoun, J.H. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 870–877. [Google Scholar] [CrossRef]
- Howlin, R.P.; Brayford, M.J.; Webb, J.S.; Cooper, J.J.; Aiken, S.S.; Stoodley, P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob. Agents Chemother. 2015, 59, 111–120. [Google Scholar] [CrossRef]
- Veiranto, M.; Suokas, E.; Ashammakhi, N.; Törmälä, P. Novel bioabsorbable antibiotic releasing bone fracture fixation implants. Adv. Exp. Med. Biol. 2004, 553, 197–208. [Google Scholar] [PubMed]
- Seo, Y.; Hwang, J.; Lee, E.; Kim, Y.J.; Lee, K.; Park, C.; Choi, Y.; Jeon, H.; Choi, J. Engineering copper nanoparticles synthesized on the surface of carbon nanotubes for anti-microbial and anti-biofilm applications. Nanoscale 2018, 10, 15529–15544. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Aw, M.S.; Losic, D. Drug-eluting Ti wires with titania nanotube arrays for bone fixation and reduced bone infection. Nanoscale Res. Lett. 2011, 6, 571. [Google Scholar] [CrossRef] [PubMed]
- Brennan, S.A.; Ní Fhoghlú, C.; Devitt, B.M.; O’Mahony, F.J.; Brabazon, D.; Walsh, A. Silver nanoparticles and their orthopaedic applications. Bone Jt. J. 2015, 97, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.P.; McHale, K.J.; Parvizi, J.; Mehta, S. Nanotechnology: Current concepts in orthopaedic surgery and future directions. Bone Jt. J. 2014, 96, 569–573. [Google Scholar] [CrossRef]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; Chiesa, R.; Arciola, C.R.; Rimondini, L. Biofilm formation on titanium implants counteracted by grafting gallium and silver ions. J. Biomed. Mater. Res. A 2015, 103, 1176–1187. [Google Scholar] [CrossRef]
- Sussman, E.M.; Casey, B.J.; Dutta, D.; Dair, B.J. Different cytotoxicity responses to antimicrobial nanosilver coatings when comparing extract-based and direct-contact assays. J. Appl. Toxicol. 2015, 35, 631–639. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Pan, R.; Han, D.; Chen, T.; Geng, Z.; Xiong, Y.; Chen, Y. Electrodeposition of chitosan/gelatin/nanosilver: A new method for constructing biopolymer/nanoparticle composite films with conductivity and antibacterial activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 222–228. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Wang, X.; Shao, M.; Xu, J.; Wang, J.; Li, L.; Zhang, R.; Guo, X. Stable and efficient loading of silver nanoparticles in spherical polyelectrolyte brushes and the antibacterial effects. Colloids Surf. B Biointerfaces 2015, 127, 148–154. [Google Scholar] [CrossRef]
- Pishbin, F.; Mouriño, V.; Gilchrist, J.B.; McComb, D.W.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Single-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nano-silver composite system. Acta Biomater. 2013, 9, 7469–7479. [Google Scholar] [CrossRef]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; Von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Zaatreh, S.; Haffner, D.; Strauss, M.; Dauben, T.; Zamponi, C.; Mittelmeier, W.; Quandt, E.; Kreikemeyer, B.; Bader, R. Thin magnesium layer confirmed as an antibacterial and biocompatible implant coating in a co-culture model. Mol. Med. Rep. 2017, 15, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Hoang Thi, T.H.; Chai, F.; Leprêtre, S.; Blanchemain, N.; Martel, B.; Siepmann, F.; Hildebrand, H.F.; Siepmann, J.; Flament, M.P. Bone implants modified with cyclodextrin: Study of drug release in bulk fluid and into agarose gel. Int. J. Pharm. 2010, 400, 74–85. [Google Scholar] [CrossRef]
- Taha, M.; Chai, F.; Blanchemain, N.; Neut, C.; Goube, M.; Maton, M.; Matel, B.; Hildebrand, H.F. Evaluation of sorption capacity of antibiotics and antibacterial properties of a cyclodextrin-polymer functionalized hydroxyapatite-coated titanium hip prosthesis. Int. J. Pharm. 2014, 477, 380–389. [Google Scholar] [CrossRef]
- Leprêtre, S.; Chai, F.; Hornez, J.C.; Vermet, G.; Neut, C.; Descamps, M.; Hildebrand, H.F.; Martel, B. Prolonged local antibiotics delivery from hydroxyapatite functionalisedfunctionalised with cyclodextrin polymers. Biomaterials 2009, 30, 6086–6093. [Google Scholar] [CrossRef] [PubMed]
- Cyphert, E.L.; Zuckerman, S.T.; Korley, J.N.; von Recum, H.A. Affinity interactions drive post-implantation drug filling, even in the presence of bacterial biofilm. Acta Biomater. 2017, 57, 95–102. [Google Scholar] [CrossRef]
- Halpern, J.M.; Gormley, C.A.; Keech, M.; von Recum, H.A. Thermomechanical Properties, Antibiotic Release, and Bioactivity of a Sterilized Cyclodextrin Drug Delivery System. J. Mater. Chem. B 2014, 2, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Harth, K.C.; Rosen, M.J.; Thatiparti, T.R.; Jacobs, M.R.; Halaweish, I.; Bajaksouzian, S.; Furlan, J.; von Recum, H.A. Antibiotic-releasing mesh coating to reduce prosthetic sepsis: An in vivo study. J. Surg. Res. 2010, 163, 337–343. [Google Scholar] [CrossRef]
- Grafmiller, K.T.; Zuckerman, S.T.; Petro, C.; Liu, L.; von Recum, H.A.; Rosen, M.J.; Korley, J.N. Antibiotic-releasing microspheres prevent mesh infection in vivo. J. Surg. Res. 2016, 206, 41–47. [Google Scholar] [CrossRef]
- Min, J.; Choi, K.Y.; Dreaden, E.C.; Padera, R.F.; Braatz, R.D.; Spector, M.; Hammond, P.T. Designer Dual Therapy Nanolayered Implant Coatings Eradicate Biofilms and Accelerate Bone Tissue Repair. ACS Nano 2016, 10, 4441–4450. [Google Scholar] [CrossRef]
- Williams, D.L.; Haymond, B.S.; Beck, J.P.; Savage, P.B.; Chaudhary, V.; Epperson, R.T.; Kawaguchi, B.; Bloebaum, R.D. In vivo efficacy of a silicone—cationic steroid antimicrobial coating to prevent implant-related infection. Biomaterials 2012, 33, 8641–8656. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Sinclair, K.D.; Jeyapalina, S.; Bloebaum, R.D. Characterization of a novel active release coating to prevent biofilm implant-related infections. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Haymond, B.S.; Woodbury, K.L.; Beck, J.P.; Moore, D.E.; Epperson, R.T.; Bloebaum, R.D. Experimental model of biofilm implant-related osteomyelitis to test combination biomaterials using biofilms as initial inocula. J. Biomed. Mater. Res. Part A 2012, 100, 1888–1900. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Pham, T.X.; Williams, D.L.; Farnsworth, R.W.; Loc-Carrillo, C.M.; Bloebaum, R.D. Model development for determining the efficacy of a combination coating for the prevention of perioperative device related infections: A pilot study. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Lerdahl, J.M.; Haymond, B.S.; Bloebaum, R.D. In vitro efficacy of a novel active-release antimicrobial coating to eradicate biofilms of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 2400–2404. [Google Scholar] [CrossRef][Green Version]
- Ashton, N.N.; Allyn, G.; Porter, S.T.; Haussener, T.J.; Sebahar, P.R.; Looper, R.E.; Williams, D.L. In vitro testing of a first-in-class tri-alkylnorspermidine-biaryl antibiotic in an anti-biofilm silicone coating. Acta Biomater. 2019, 93, 25–35. [Google Scholar] [CrossRef]
- Williams, D.L.; Epperson, R.T.; Ashton, N.N.; Taylor, N.B.; Kawaguchi, B.; Olsen, R.E.; Haussener, T.J.; Sebahar, P.R.; Allyn, G.; Looper, R.E. In vivo analysis of a first-in-class tri-alkyl norspermidine-biaryl antibiotic in an active release coating to reduce the risk of implant-related infection. Acta Biomater. 2019, 93, 36–49. [Google Scholar] [CrossRef]
- Ehrlich, G.D.; Stoodley, P.; Kathju, S.; Zhao, Y.; McLeod, B.R.; Balaban, N.; Ze Hu, F.; Sotereanos, N.G.; Costerton, J.W.; Stewart, P.S.; et al. Engineering approaches for the detection and control of orthopaedic biofilm infections. Clin. Orthop. Relat. Res. 2005, 437, 59–66. [Google Scholar] [CrossRef]
- Deng, W.; Shao, H.; Li, H.; Zhou, Y. Is surface modification effective to prevent periprosthetic joint infection? A systematic review of preclinical and clinical studies. Orthop. Traumatol. Surg. Res. 2019, 105, 967–974. [Google Scholar] [CrossRef]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Biel, M.A. Photodynamic therapy of bacterial and fungal biofilm infections. Methods Mol. Biol. 2010, 635, 175–194. [Google Scholar] [PubMed]
- Zeina, B.; Greenman, J.; Purcell, W.M.; Das, B. Killing of cutaneous microbial species by photodynamic therapy. Br. J. Dermatol. 2001, 144, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.N.; Teichert, M.C.; Biel, M.A. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 2001, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Golob, B.S.; Olivi, G.; Vrabec, M.; El Feghali, R.; Parker, S.; Benedicenti, S. Efficacy of Photon-induced Photoacoustic Streaming in the Reduction of Enterococcus faecalis within the Root Canal: Different Settings and Different Sodium Hypochlorite Concentrations. J. Endod. 2017, 43, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- Balic, M.; Lucic, R.; Mehadzic, K.; Bago, I.; Anic, I.; Jakovljevic, S.; Plecko, V. The efficacy of photon-initiated photoacoustic streaming and sonic-activated irrigation combined with QMiX solution or sodium hypochlorite against intracanal, E. faecalis biofilm. Lasers Med. Sci. 2016, 31, 335–342. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial Photodynamic Therapy to Control Clinically Relevant Biofilm Infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Seong, D.-Y.; Kim, Y.-J. Enhanced photodynamic therapy efficacy of methylene blue-loaded calcium phosphate nanoparticles. J. Photochem. Photobiol. B Biol. 2015, 146, 34–43. [Google Scholar] [CrossRef]
- Vassena, C.; Fenu, S.; Giuliani, F.; Fantetti, L.; Roncucci, G.; Simonutti, G.; Romano, C.L.; De Francesco, R.; Drago, L. Photodynamic antibacterial and antibiofilm activity of RLP068/Cl against Staphylococcus aureus and Pseudomonas aeruginosa forming biofilms on prosthetic material. Int. J. Antimicrob. Agents 2014, 44, 47–55. [Google Scholar] [CrossRef]
- Ashokkumar, M. The characterization of acoustic cavitation bubbles—An overview. Ultrason. Sonochem. 2011, 18, 864–872. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R.C. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Joyce, E.; Phull, S.S.; Lorimer, J.P.; Mason, T.J. The development and evaluation of ultrasound for the treatment of bacterial suspensions. A study of frequency, power and sonication time on cultured Bacillus species. Ultrason. Sonochem. 2003, 10, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Hameister, R.; Lim, C.T.; Lohmann, C.H.; Wang, W.; Singh, G. What Is the Role of Diagnostic and Therapeutic Sonication in Periprosthetic Joint Infections? J. Arthroplast. 2018, 33, 2575–2581. [Google Scholar] [CrossRef] [PubMed]
- Groen, M.H.A.; Slieker, F.J.B.; Vink, A.; de Borst, G.J.; Simons, M.V.; Ebbini, E.S.; Doevendans, P.A.; Hazenberg, C.E.V.B.; van Es, R. Safety and feasibility of arterial wall targeting with robot-assisted high intensity focused ultrasound: A preclinical study. Int. J. Hyperth. 2020, 37, 903–912. [Google Scholar] [CrossRef]
- Trampuz, A.; Piper, K.E.; Jacobson, M.J.; Hanssen, A.D.; Unni, K.K.; Osmon, D.R.; Mandrekar, J.N.; Cockerill, F.R.; Steckelberg, J.M.; Greenleaf, J.F.; et al. Sonication of Removed Hip and Knee Prostheses for Diagnosis of Infection. N. Engl. J. Med. 2007, 357, 654–663. [Google Scholar] [CrossRef]
- Nelson, C.L.; Jones, R.B.; Wingert, N.C.; Foltzer, M.; Bowen, T.R. Sonication of antibiotic spacers predicts failure during two-stage revision for prosthetic knee and hip infections. Clin. Orthop. Relat. Res. 2014, 472, 2208–2214. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Li, H.; Qin, A.; Liu, G.; Liu, X.; Wu, C.; Li, H.; Zhu, Z.; Qu, X.; Dai, K. Meta-analysis of sonication fluid samples from prosthetic components for diagnosis of infection after total joint arthroplasty. J. Clin. Microbiol. 2014, 52, 1730–1736. [Google Scholar] [CrossRef]
- Rothenberg, A.C.; Wilson, A.E.; Hayes, J.P.; O’Malley, M.J.; Klatt, B.A. Sonication of Arthroplasty Implants Improves Accuracy of Periprosthetic Joint Infection Cultures. Clin. Orthop. Relat. Res. 2017, 475, 1827–1836. [Google Scholar] [CrossRef]
- Ensing, G.T.; Neut, D.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. The combination of ultrasound with antibiotics released from bone cement decreases the viability of planktonic and biofilm bacteria: An in vitro study with clinical strains. J. Antimicrob. Chemother. 2006, 58, 1287–1290. [Google Scholar] [CrossRef]
- Singh, G.; Hameister, R.; Feuerstein, B.; Awiszus, F.; Meyer, H.; Lohmann, C.H. Low-frequency sonication may alter surface topography of endoprosthetic components and damage articular cartilage without eradicating biofilms completely. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1835–1846. [Google Scholar] [CrossRef]
- Gao, S.; Hemar, Y.; Ashokkumar, M.; Paturel, S.; Lewis, G.D. Inactivation of bacteria and yeast using high-frequency ultrasound treatment. Water Res. 2014, 60, 93–104. [Google Scholar] [CrossRef]
- Sesal, N.C.; Kekeç, Ö. Effects of pulsed ultrasound on Escherichia coli and Staphylococcus aureus. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bigelow, T.A.; Halverson, L.J.; Middendorf, J.M.; Rusk, B. Minimization of treatment time for in vitro 1.1 MHz destruction of Pseudomonas aeruginosa biofilms by high-intensity focused ultrasound. Ultrasonics 2012, 52, 668–675. [Google Scholar] [CrossRef]
- Bigelow, T.A.; Northagen, T.; Hill, T.M.; Sailer, F.C. The Destruction of Escherichia coli Biofilms Using High-Intensity Focused Ultrasound. Ultrasound Med. Biol. 2009, 35, 1026–1031. [Google Scholar] [CrossRef]
- Scherba, G.; Weigel, R.M.; O’Brien, W.D., Jr. Quantitative assessment of the germicidal efficacy of ultrasonic energy. Appl. Environ. Microbiol. 1991, 57, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.G.; Paff, M.; Friedman, G.; Fridman, G.; Fridman, A.; Brooks, A.D. Control of methicillin-resistant Staphylococcus aureus in planktonic form and biofilms: A biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am. J. Infect. Control. 2010, 38, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Sanaei, N.; Ayan, H. Bactericidal efficacy of dielectric barrier discharge plasma on methicillin-resistant staphylococcus aureus and Escherichia coli in planktonic phase and colonies in vitro. Plasma Med. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Gupta, T.T.; Ayan, H. Application of non-thermal plasma on biofilm: A review. Appl. Sci. 2019, 9, 3548. [Google Scholar] [CrossRef]
- Şen Karaman, D.; Ercan, U.K.; Bakay, E.; Topaloğlu, N.; Rosenholm, J.M. Evolving Technologies and Strategies for Combating Antibacterial Resistance in the Advent of the Postantibiotic Era. Adv. Funct. Mater. 2020, 30, 1908783. [Google Scholar] [CrossRef]
- Parkey, J.; Cross, J.; Hayes, R.; Parham, C.; Staack, D.; Sharma, A.C. A Battery Powered, Portable, and Self-Contained Non-Thermal Helium Plasma Jet Device for Point-of-Injury Burn Wound Treatment. Plasma Process. Polym. 2015, 12, 1244–1255. [Google Scholar] [CrossRef]
- Usta, Y.H.; Çukur, E.; Yıldırım, Ç.; Ercan, U.K. Design of a portable, battery-powered non-thermal atmospheric plasma device and characterization of its antibacterial efficacies. J. Electrost. 2019, 99, 1–8. [Google Scholar] [CrossRef]
- Del Pozo, J.L.; Rouse, M.S.; Patel, R. Bioelectric effect and bacterial biofilms. A systematic review. Int. J. Artif. Organs. 2008, 31, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G. Membrane electrostatics. Biochim. Biophys. Acta 1990, 1031, 311–382. [Google Scholar] [CrossRef]
- Sen, C.K.; Mathew-Steiner, S.S.; Das, A.; Sundaresan, V.B.; Roy, S. Electroceutical Management of Bacterial Biofilms and Surgical Infection. Antioxid. Redox Signal 2020, 33, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.P.; Wagle, N.; Anderson, M.D.; Warren, M.M. Bacterial and fungal killing by iontophoresis with long-lived electrodes. Antimicrob. Agents Chemother. 1991, 35, 2131–2134. [Google Scholar] [CrossRef]
- Pareilleux, A.; Sicard, N. Lethal effects of electric current on Escherichia coli. Appl. Microbiol. 1970, 19, 421–424. [Google Scholar] [CrossRef]
- Davis, C.P.; Shirtliff, M.E.; Trieff, N.M.; Hoskins, S.L.; Warren, M.M. Quantification, qualification, and microbial killing efficiencies of antimicrobial chlorine-based substances produced by iontophoresis. Antimicrob. Agents Chemother. 1994, 38, 2768–2774. [Google Scholar] [CrossRef]
- Brinkman, C.L.; Schmidt-Malan, S.M.; Karau, M.J.; Greenwood-Quaintance, K.; Hassett, D.J.; Mandrekar, J.N.; Patel, R. Exposure of Bacterial Biofilms to Electrical Current Leads to Cell Death Mediated in Part by Reactive Oxygen Species. PLoS ONE 2016, 11, e0168595. [Google Scholar] [CrossRef]
- Schmidt-Malan, S.M.; Karau, M.J.; Cede, J.; Greenwood-Quaintance, K.E.; Brinkman, C.L.; Mandrekar, J.N.; Patel, R. Antibiofilm Activity of Low-Amperage Continuous and Intermittent Direct Electrical Current. Antimicrob. Agents Chemother. 2015, 59, 4610–4615. [Google Scholar] [CrossRef]
- Costerton, J.W.; Ellis, B.; Lam, K.; Johnson, F.; Khoury, A.E. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob. Agents Chemother. 1994, 38, 2803–2809. [Google Scholar] [CrossRef]
- McLeod, B.R.; Fortun, S.; Costerton, J.W.; Stewart, P.S. Enhanced bacterial biofilm control using electromagnetic fields in combination with antibiotics. Methods Enzymol. 1999, 310, 656–670. [Google Scholar] [PubMed]
- del Pozo, J.L.; Rouse, M.S.; Mandrekar, J.N.; Sampedro, M.F.; Steckelberg, J.M.; Patel, R. Effect of electrical current on the activities of antimicrobial agents against Pseudomonas aeruginosa, Staphylococcus aureus, and Staphylococcus epidermidis biofilms. Antimicrob. Agents Chemother. 2009, 53, 35–40. [Google Scholar] [CrossRef]
- Pickering, S.A.; Bayston, R.; Scammell, B.E. Electromagnetic augmentation of antibiotic efficacy in infection of orthopaedic implants. J. Bone Jt. Surg. Br. 2003, 85, 588–593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jass, J.; Lappin-Scott, H.M. The efficacy of antibiotics enhanced by electrical currents against Pseudomonas aeruginosa biofilms. J Antimicrob. Chemother. 1996, 38, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Caubet, R.; Pedarros-Caubet, F.; Chu, M.; Freye, E.; de Belém Rodrigues, M.; Moreau, J.M.; Ellison, W.J. A radio frequency electric current enhances antibiotic efficacy against bacterial biofilms. Antimicrob. Agents Chemother. 2004, 48, 4662–4664. [Google Scholar] [CrossRef] [PubMed]
- Poortinga, A.T.; Smit, J.; van der Mei, H.C.; Busscher, H.J. Electric field induced desorption of bacteria from a conditioning film covered substratum. Biotechnol. Bioeng. 2001, 76, 395–399. [Google Scholar] [CrossRef] [PubMed]
- van der Borden, A.J.; van der Werf, H.; van der Mei, H.C.; Busscher, H.J. Electric current-induced detachment of Staphylococcus epidermidis biofilms from surgical stainless steel. Appl. Environ. Microbiol. 2004, 70, 6871–6874. [Google Scholar] [CrossRef]
- Ehrensberger, M.T.; Tobias, M.E.; Nodzo, S.R.; Hansen, L.A.; Luke-Marshall, N.R.; Cole, R.F.; Wild, L.M.; Campagnari, A.A. Cathodic voltage-controlled electrical stimulation of titanium implants as treatment for methicillin-resistant Staphylococcus aureus periprosthetic infections. Biomaterials 2015, 41, 97–105. [Google Scholar] [CrossRef]
- Nodzo, S.; Tobias, M.; Hansen, L.; Luke-Marshall, N.R.; Cole, R.; Wild, L.; Campagnari, A.A.; Ehrensberger, M.T. Cathodic Electrical Stimulation Combined With Vancomycin Enhances Treatment of Methicillin-resistant Staphylococcus aureus Implant-associated Infections. Clin. Orthop. Relat. Res. 2015, 473, 2856–2864. [Google Scholar] [CrossRef]
- Nodzo, S.R.; Tobias, M.; Ahn, R.; Hansen, L.; Luke-Marshall, N.R.; Howard, C.; Wild, L.; Campagnari, A.A.; Ehrensberg, M.T. Cathodic Voltage-controlled Electrical Stimulation Plus Prolonged Vancomycin Reduce Bacterial Burden of a Titanium Implant-associated Infection in a Rodent Model. Clin. Orthop. Relat. Res. 2016, 474, 1668–1675. [Google Scholar] [CrossRef]
- Canty, M.; Luke-Marshall, N.; Campagnari, A.; Ehrensberger, M. Cathodic voltage-controlled electrical stimulation of titanium for prevention of methicillin-resistant Staphylococcus aureus and Acinetobacter baumannii biofilm infections. Acta Biomater. 2017, 48, 451–460. [Google Scholar] [CrossRef]
- Canty, M.K.; Hansen, L.A.; Tobias, M.; Spencer, S.; Henry, T.; Luke-Marshall, N.R.; Campagnari, A.A.; Ehrensberger, M.T. Antibiotics Enhance Prevention and Eradication Efficacy of Cathodic-Voltage-Controlled Electrical Stimulation against Titanium-Associated Methicillin-Resistant Staphylococcus aureus and Pseudomonas aeruginosa Biofilms. mSphere 2019, 4, e00178-19. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.; Keshvari, J.; Lappalainen, R. Interaction of radio frequency electromagnetic fields and passive metallic implants—A brief review. Bioelectromagnetics 2006, 27, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.; Nguyen, D.; Mogarala, S.; Osiñski, M.; Smyth, H.D. Magnetic fields suppress Pseudomonas aeruginosa biofilms and enhance ciprofloxacin activity. Biofouling 2015, 31, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Rudnev, V.; Cook, R.; Loveless, D. Handbook of Induction Heating, 2nd ed.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2017. [Google Scholar]
- Coffel, J.; Nuxoll, E. Magnetic nanoparticle/polymer composites for medical implant infection control. J. Mater. Chem. B 2015, 3, 7538–7545. [Google Scholar] [CrossRef] [PubMed]
- Merola, M.; Affatato, S. Materials for Hip Prostheses: A Review of Wear and Loading Considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Pijls, B.G.; Sanders, I.M.J.G.; Kuijper, E.J.; Nelissen, R.G.H.H. Segmental induction heating of orthopaedic metal implants. Bone Jt. Res. 2018, 7, 609–619. [Google Scholar] [CrossRef]
- Müller, C.W.; ElKashef, T.; Pfeifer, R.; Decker, S.; Neunaber, C.; Meier, K.; Fehr, M.; Wesling, V.; Gosling, T.; Hurschler, C.; et al. Transcutaneous electromagnetic induction heating of an intramedullary nickel-titanium shape memory implant. Int. Orthop. 2014, 38, 2551–2557. [Google Scholar] [CrossRef]
- Fang, C.H.; Tsai, P.I.; Huang, S.W.; Sun, J.S.; Chang, J.Z.; Shen, H.H.; Chen, S.Y.; Lin, F.H.; Hsu, L.T.; Chen, Y.C. Magnetic hyperthermia enhance the treatment efficacy of peri-implant osteomyelitis. BMC Infect. Dis. 2017, 17, 516. [Google Scholar] [CrossRef]
- Pijls, B.G.; Sanders, I.; Kujiper, E.J.; Nelissen, R. Induction heating for eradicating Staphylococcus epidermidis from biofilm. Bone Jt. Res. 2020, 9, 192–199. [Google Scholar] [CrossRef]
- Pijls, B.G.; Sanders, I.; Kuijper, E.J.; Nelissen, R. Synergy between induction heating, antibiotics, and N-acetylcysteine eradicates Staphylococcus aureus from biofilm. Int. J. Hyperth. 2020, 37, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Chopra, R.; Shaikh, S.; Chatzinoff, Y.; Munaweera, I.; Cheng, B.; Daly, S.M.; Xi, Y.; Bing, C.; Burns, D.; Greenberg, D.E. Employing high-frequency alternating magnetic fields for the non-invasive treatment of prosthetic joint infections. Sci. Rep. 2017, 7, 7520. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Chatzinoff, Y.; Szczepanski, D.; Bing, C.; Shaikh, S.; Wyman, O.; Perry, C.E.; Richardson, J.A.; Burns, D.K.; Evers, B.M.; et al. Remote acoustic sensing as a safety mechanism during exposure of metal implants to alternating magnetic fields. PLoS ONE 2018, 13, e0197380. [Google Scholar] [CrossRef] [PubMed]
- Munaweera, I.; Shaikh, S.; Maples, D.; Nigatu, A.S.; Sethuraman, S.N.; Ranjan, A.; Greenberg, D.E.; Chopra, R. Temperature-sensitive liposomal ciprofloxacin for the treatment of biofilm on infected metal implants using alternating magnetic fields. Int. J. Hyperth. Off. J. Eur. Soc. Hyperthermic Oncol. N. Am. Hyperth. Group 2018, 34, 189–200. [Google Scholar] [CrossRef]

| Method | Site of Implant Modification | Strategy/Approach (Biological, Chemical, Physical) | Advantages | Progress towards Clinical Use/Disadvantages | Refs. |
|---|---|---|---|---|---|
| Bioactive glass and biocomposites | Material | Drug loaded, biodegradable | Evidence of clinical efficacy lacking | [24] | |
| Mixing process modified bone cement (PMMA) | Material | Drug loaded reservoir | Improved elution profiles for better delivery of reservoir of antibiotic | Can create drug resistant bacteria; randomized control trials ongoing | [24] |
| Calcium sulfate loaded radiopaque beads | Surface | Drug loaded reservoir | Improved elution profiles to PMMA | Cannot reduce already formed biofilms; can induce hypersensitivity reaction | [40] |
| TNTs with 2 phase release | Surface modification/nanoparticle coating | Drug carrier for local delivery | Release of ROS increased antimicrobial activity | In vitro study | [42,43] |
| Mg on Ti | Surface coating | Release of Mg ions created bactericidal alkaline environment | Use of ROS without harm to nearby osteoblasts | In vitro study, limited to 7 days of culture | [52] |
| Ag nanoparticle coating | Coating | Bactericidal through release of biologically active ions, creation of ROS, interaction with sulfhydryl groups | Can be incorporated into a number of materials | Some in vivo work completed | [44,45,46,47,48,49,50,51] |
| Poly-cyclodextrin in situ antibiotic treatment | Implant coating or drug delivery device | Polymer with drug affinity for loading and release | Refillable; can be both preventative and therapeutic | In vitro study in hernia mesh; not yet explored specifically for PJI | [55,56,57,58,59] |
| LbL drug loading | Coating | High drug loading, encourage bone growth, repair. Timed multidrug release. | Encourages bone growth and repair | Studies in rats; would require one stage revision | [60] |
| PDMS with CSA-13 | Synthetic analog peptide coating | Drug loaded, cationic interaction with neg charged bacteria. | Avoids protease degradation. Both preventative and therapeutic | Studies in sheep with recent emergency use in ET tubes | [61,62,63,64,65,66,67] |
| Polymers, hydrogels, cyclodextrin, and hydroxyapatite | Material/coating | Drug delivery | Some materials have extended release properites | Cyclodextrin coated meshes have progressed to in vivo animal studies | [24] |
| “Smart” implant through monitoring of quorum sensing activity | Built in MEMS biosensor | Exploits quorum sensing, antibiotic release, telemetric control | Antimicrobial properties built into implant, would not require additional revisions | Needs substantial support from manufacturer to prototype for practical use | [65] |
| Method/Strategy | Physical Effect | Bactericidal Effect | Advantages | Progress toward Clinical Use/Disadvantages | Refs. |
|---|---|---|---|---|---|
| Photodynamic Therapy | |||||
| Laser excitation of PS | Energy trans photosensitizer → O2 | ROS generation | Acts directly on bacterial biofilms | Not yet advanced to in vivo trials; invasive procedure needed to access implant | [71,72,73,74,75,76,77,78] |
| Sonication | |||||
| Cavitation oscillation-driven rectified gas diffusion, micro-streaming, bubble collapse, ROS formation | bacterial cell wall fatigue, micro-streaming induced intra-cellular shear forces, ROS attack | Acts directly on bacteria and in synergy with antibiotics | In vitro studies; some evidence of cartilage damage from sonication, lack of consensus results | [88,89,90,91,92,93,94] | |
| Plasma Treatment | |||||
| Dielectric barrier discharge plasma: ms-high V pulsed cold plasma bt quartz and sample | Generation of bactericidal species: ozone, nitric oxide, superoxide, hydrogen peroxide, singlet oxygen, OH radicals, ultraviolet radiation, electrons | Rapid sterilization, but varies by strain; can sterilize large surface areas | Not advanced to clinical stage; invasive procedure needed to access implant with embedded surfaces potentially inaccessible | [95,96,97,98,99,100,101] | |
| Electric Fields and Currents | |||||
| Electroceuticals | Formation of toxic substances due to electrolysis | Disruption of internal bioelectric milieu | Can help activate host immune system | FDA approved, clinical trials underway for wound care w mixed results | [102,103,104,105,106,107] |
| DC current | Bioelectric effect: Reduces biofilm resistance to antibiotics | Electricidal effect of ROS rather than detachment | Bactericidal on its own | In vitro studies with varied results; requires invasive one-stage revision, matrix remains on implant | [108,109,110,111,112,113,114] |
| AC current | Alternating polarity may increase fluidity of antibiotics | Utilizes electroosmotic properties of matrix to detach biofilm | Easier penetration of antibiotics into biofilm | In vitro studies; requires invasive one-stage revision, only works in concert with antibiotics | [115,116,117] |
| CVCES | Modeled as capacitor, excess neg charge at interface | Repulsion; creation of alkaline environment | Combined with antibiotics can effectively treat and prevent biofilms and planktonic bacteria | In vivo rodent models; does require minimally invasive procedure | [118,119,120,121,122] |
| Electromagnetic Fields | |||||
| Conductive object in magnetic field | Dependent on orientation, size, shape, location | Metabolic, biomass reduction on exposure to static 1-sided, static switched, oscillating, & combined MFs | Non-invasive, works synergistically with antibiotics and NAC | In vivo studies; Non-uniform objects leads to non-uniform heating, requiring heat sinks or segmental heating | [123,124,128,129,130,131,132] |
| Conductive object in AMF | Eddy current generated induction heating | Heat source in direct contact with biofilm | Non-invasive; AMF uses skin effect and restricts heating to surface | In vitro studies; non-conductive surfaces (plastic, ceramic) untreated | [125,127] |
| Conductive coating in AMF | Heat generated by magnetic nanoparticles in AMF | Heat source in direct contact with biofilm | Non-invasive; AMF uses skin effect and restricts heating to surface | In vitro study; implant must be pre-treated with coating | [126] |
| High frequency (continuous or pulsed/intermitent) | Skin effect | Heat source in direct contact with biofilm | Non-invasive, save, effective, synergy with liposomal antibiotics | In vivo animal studies | [133,134,135] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciarolla, A.A.; Lapin, N.; Williams, D.; Chopra, R.; Greenberg, D.E. Physical Approaches to Prevent and Treat Bacterial Biofilm. Antibiotics 2023, 12, 54. https://doi.org/10.3390/antibiotics12010054
Ciarolla AA, Lapin N, Williams D, Chopra R, Greenberg DE. Physical Approaches to Prevent and Treat Bacterial Biofilm. Antibiotics. 2023; 12(1):54. https://doi.org/10.3390/antibiotics12010054
Chicago/Turabian StyleCiarolla, Alexa A., Norman Lapin, Dustin Williams, Rajiv Chopra, and David E. Greenberg. 2023. "Physical Approaches to Prevent and Treat Bacterial Biofilm" Antibiotics 12, no. 1: 54. https://doi.org/10.3390/antibiotics12010054
APA StyleCiarolla, A. A., Lapin, N., Williams, D., Chopra, R., & Greenberg, D. E. (2023). Physical Approaches to Prevent and Treat Bacterial Biofilm. Antibiotics, 12(1), 54. https://doi.org/10.3390/antibiotics12010054







