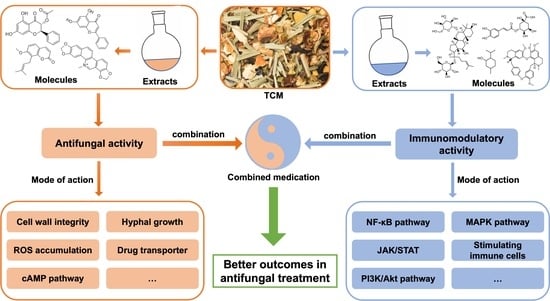
Antifungal and Immunomodulatory Ingredients from Traditional Chinese Medicine
Abstract
1. Introduction
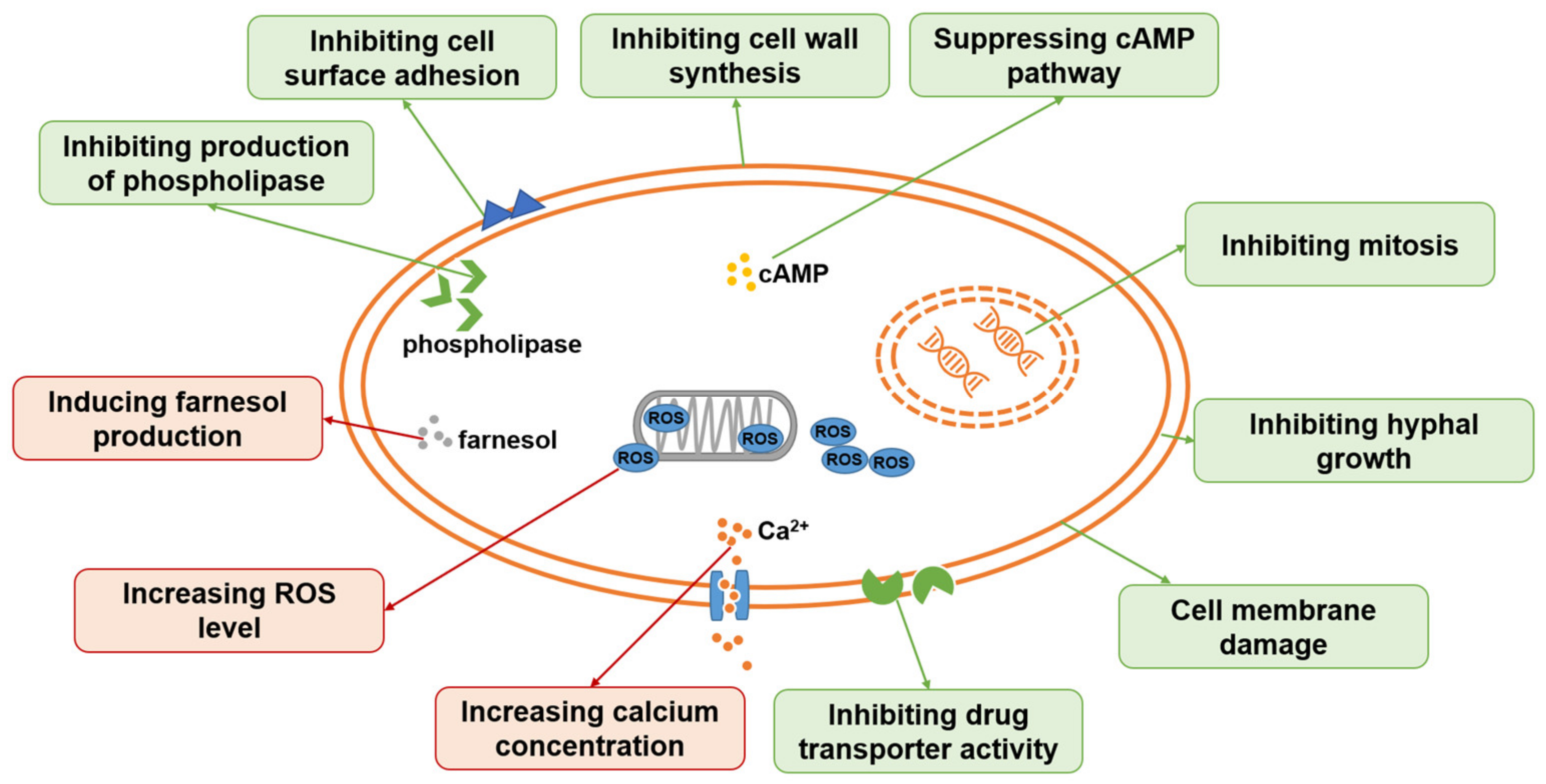
2. Antifungal Ingredients from TCM
2.1. Terpenoids
2.2. Volatile Oils
2.3. Flavonoids
2.4. Alkaloids
2.5. Quinones
2.6. Coumarin
2.7. Others
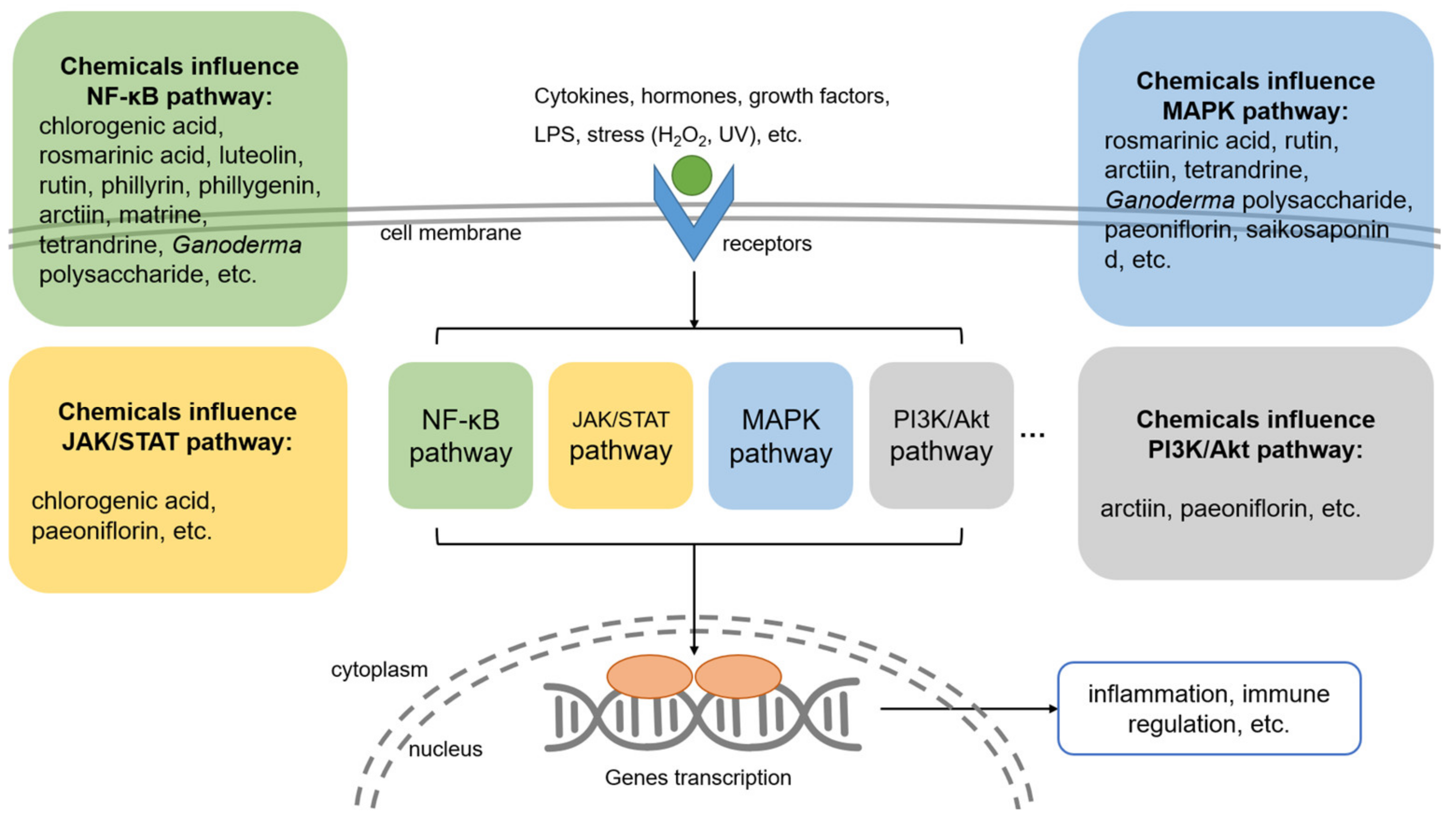
3. Immunomodulatory Ingredients from TCM
3.1. Phenolic Acids
3.2. Flavonoids
3.3. Volatile Oils
3.4. Lignans
3.5. Alkaloids
3.6. Polysaccharides
3.7. Glycosides
4. Discussion
4.1. Material Basis of TCM for Treating Fungal Infection Diseases
4.2. Mechanism of Chinese Medicine in Treating Fungal Infectious Diseases
4.3. Exploitation of TCM and Development of New Antifungal Drugs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ya-jing, X.; Yong-zhuo, W.; Jia-yin, L.; Rong-jie, Y.; Yun, G.; Dan-qi, D. Retrospective analysis of Chinese antifungal herbal medicines in China. J. Dermatol. Venereol. 2019, 10, 26–29. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Y.; Hong, N.; Yang, Y.; Lei, W.; Du, L.; Zhao, J.; Lei, X.; Xiong, L.; Cai, L.; et al. Epidemiology of fungal infections in China. Front. Med. 2018, 12, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Strickland, A.B.; Shi, M. Mechanisms of fungal dissemination. Cell. Mol. Life Sci. 2021, 78, 3219–3238. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi that Infect Humans. Microbiol. Spectr. 2017, 5, 813–843. [Google Scholar] [CrossRef]
- Li, L.P.; Wang, X.J.; Zhang, J.Y.; Zhang, L.L.; Cao, Y.B.; Gu, L.Q.; Yu, Y.Q.; Yang, Q.L.; Shen, C.Y.; Han, B.; et al. Antifungal activity of osthol in vitro and enhancement in vivo through Eudragit S100 nanocarriers. Virulence 2018, 9, 555–562. [Google Scholar] [CrossRef]
- Lou, L.; Zhou, J.; Liu, Y.; Wei, Y.I.; Zhao, J.; Deng, J.; Dong, B.; Zhu, L.; Wu, A.; Yang, Y.; et al. Chlorogenic acid induces apoptosis to inhibit inflammatory proliferation of IL-6-induced fibroblast-like synoviocytes through modulating the activation of JAK/STAT and NF-κB signaling pathways. Exp. Ther. Med. 2016, 11, 2054–2060. [Google Scholar] [CrossRef]
- Cen, L.; Xiao, R.; Li, M.; Wang, Z.; Chang, B. Antitumor and anti-inflammatory mechanisms of taraxacum: Research advances. J. Int. Pharm. Res. 2020, 47, 954–961. [Google Scholar] [CrossRef]
- Huang, X.; Yi, Y.; Yong, J.; Sun, J.; Song, Z.; Li, D.; Li, Y. Inhibitory effect of berberine hydrochloride against Candida albicans and the role of the HOG-MAPK pathway. J. Antibiot. 2021, 74, 807–816. [Google Scholar] [CrossRef]
- Wang, T.; Shi, G.; Shao, J.; Wu, D.; Yan, Y.; Zhang, M.; Cui, Y.; Wang, C. In vitro antifungal activity of baicalin against Candida albicans biofilms via apoptotic induction. Microb. Pathog. 2015, 87, 21–29. [Google Scholar] [CrossRef]
- Dovigo, L.N.; Carmello, J.C.; de Souza Costa, C.A.; Vergani, C.E.; Brunetti, I.L.; Bagnato, V.S.; Pavarina, A.C. Curcumin-mediated photodynamic inactivation of Candida albicans in a murine model of oral candidiasis. Med. Mycol. 2013, 51, 243–251. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, G.; Elewa, L.; Al-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; Abd-Elhakim, Y.M.; Prasad Devkota, H. Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [PubMed]
- Jia-jia, F.; Yuan-yong, T. Research progress on antifungal effect of traditional Chinese medicine. J. Pract. Dermatol. 2019, 12, 34–37. [Google Scholar]
- Yang, L.; Liu, X.; Zhong, L.; Sui, Y.; Quan, G.; Huang, Y.; Wang, F.; Ma, T. Dioscin Inhibits Virulence Factors of Candida albicans. Biomed. Res. Int. 2018, 2018, 4651726. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yin, L.; Xu, L.; Peng, J. Dioscin: A diverse acting natural compound with therapeutic potential in metabolic diseases, cancer, inflammation and infections. Pharmacol. Res. 2018, 137, 259–269. [Google Scholar] [CrossRef]
- Kim, T.H.; Hatano, T.; Okamoto, K.; Yoshida, T.; Kanzaki, H.; Arita, M.; Ito, H. Antifungal and Ichthyotoxic Sesquiterpenoids from Santalum album Heartwood. Molecules 2017, 22, 1139. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Quispe, C.; Turgumbayeva, A.; Mertdinç, Z.; Tütüncü, S.; Aydar, E.F.; Özçelik, B.; Anna, S.W.; Mariola, S.; Koziróg, A.; et al. Santalum Genus: Phytochemical constituents, biological activities and health promoting-effects. Z. Fur Naturforschung. C J. Biosci. 2022, 12, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Shang, Z.C.; Li, T.X.; Yang, M.H.; Kong, L.Y. In Vitro Antibiofilm Activity of Eucarobustol E against Candida albicans. Antimicrob. Agents Chemother. 2017, 61, e02707. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, C.; Zhao, X.; Wang, D.; Liu, Y.; Sun, S. Antifungal activity and potential mechanism of Asiatic acid alone and in combination with fluconazole against Candida albicans. Biomed. Pharmacother. 2021, 139, 111568. [Google Scholar] [CrossRef]
- Doke, S.K.; Raut, J.S.; Dhawale, S.; Karuppayil, S.M. Sensitization of Candida albicans biofilms to fluconazole by terpenoids of plant origin. J. Gen. Appl. Microbiol. 2014, 60, 163–168. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, S.; Yang, Y.; Fan, L.; Su, F.; Ye, M. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2019, 33, 1924–1930. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and Thyme Essential Oil-New Insights into Selected Therapeutic Applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.P.C.; Nóbrega, R.O.; Lima, E.O.; Araújo, W.O.; Lima, I.O. Antifungal activity study of the monoterpene thymol against Cryptococcus neoformans. Nat. Prod. Res. 2020, 34, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Olea, A.F.; Bravo, A.; Martínez, R.; Thomas, M.; Sedan, C.; Espinoza, L.; Zambrano, E.; Carvajal, D.; Silva-Moreno, E.; Carrasco, H. Antifungal Activity of Eugenol Derivatives against Botrytis cinerea. Molecules 2019, 24, 1239. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Pavan, M.; Novello, G.; Massa, N.; Rocchetti, A.; Berta, G.; Gamalero, E. Sensitivity of Candida albicans to essential oils: Are they an alternative to antifungal agents? J. Appl. Microbiol. 2016, 121, 1530–1545. [Google Scholar] [CrossRef]
- Meccatti, V.M.; Oliveira, J.R.; Figueira, L.W.; Lagareiro Netto, A.A.; Zamarioli, L.S.; Marcucci, M.C.; Camargo, S.E.A.; Carvalho, C.A.T.; Oliveira, L.D. Rosmarinus officinalis L. (rosemary) extract has antibiofilm effect similar to the antifungal nystatin on Candida samples. An. Acad. Bras. Ciênc. 2021, 93, e20190366. [Google Scholar] [CrossRef]
- Mertas, A.; Garbusińska, A.; Szliszka, E.; Jureczko, A.; Kowalska, M.; Król, W. The influence of tea tree oil (Melaleuca alternifolia) on fluconazole activity against fluconazole-resistant Candida albicans strains. Biomed. Res. Int. 2015, 2015, 590470. [Google Scholar] [CrossRef]
- Roana, J.; Mandras, N.; Scalas, D.; Campagna, P.; Tullio, V. Antifungal Activity of Melaleuca alternifolia Essential Oil (TTO) and Its Synergy with Itraconazole or Ketoconazole against Trichophyton rubrum. Molecules 2021, 26, 461. [Google Scholar] [CrossRef]
- Pandey, K.P.; Mishra, R.K.; Kamran, A.; Mishra, P.; Bajaj, A.K.; Dikshit, A. Studies on antidermatophytic activity of waste leaves of Curcuma longa L. Physiol. Mol. Biol. Plants 2010, 16, 177–185. [Google Scholar] [CrossRef][Green Version]
- Jankasem, M.; Wuthi-Udomlert, M.; Gritsanapan, W. Antidermatophytic Properties of Ar-Turmerone, Turmeric Oil, and Curcuma longa Preparations. ISRN Dermatol. 2013, 2013, 250597. [Google Scholar] [CrossRef]
- Achimón, F.; Brito, V.D.; Pizzolitto, R.P.; Ramirez Sanchez, A.; Gómez, E.A.; Zygadlo, J.A. Chemical composition and antifungal properties of commercial essential oils against the maize phytopathogenic fungus Fusarium verticillioides. Rev. Argent. Microbiol. 2021, 53, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Shreaz, S.; Bhatia, R.; Ahmad, S.I.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 2012, 83, 434–440. [Google Scholar] [CrossRef]
- Dong, H.H.; Wang, Y.H.; Peng, X.M.; Zhou, H.Y.; Zhao, F.; Jiang, Y.Y.; Zhang, D.Z.; Jin, Y.S. Synergistic antifungal effects of curcumin derivatives as fungal biofilm inhibitors with fluconazole. Chem. Biol. Drug Des. 2021, 97, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.V.; da Silva, D.L.; Neres, A.T.; Magalhães, T.F.; Watanabe, G.A.; Modolo, L.V.; Sabino, A.A.; de Fátima, A.; de Resende, M.A. Curcumin as a promising antifungal of clinical interest. J. Antimicrob. Chemother. 2009, 63, 337–339. [Google Scholar] [CrossRef]
- Brochot, A.; Guilbot, A.; Haddioui, L.; Roques, C. Antibacterial, antifungal, and antiviral effects of three essential oil blends. Microbiologyopen 2017, 6, e00459. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, D.R.; Tintino, S.R.; Braga, M.F.; Boligon, A.A.; Athayde, M.L.; Coutinho, H.D.; de Menezes, I.R.; Fachinetto, R. In vitro antimicrobial and modulatory activity of the natural products silymarin and silibinin. Biomed. Res. Int. 2015, 2015, 292797. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Silibinin triggers yeast apoptosis related to mitochondrial Ca(2+) influx in Candida albicans. Int. J. Biochem. Cell Biol. 2016, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Mittal, S.; Aggarwal, D.; Parashar, G.; Parashar, N.C.; Upadhyay, S.K.; Barwal, T.S.; Jain, A.; Kaur, G.; Savla, R.; et al. Path of Silibinin from diet to medicine: A dietary polyphenolic flavonoid having potential anti-cancer therapeutic significance. Semin. Cancer Biol. 2021, 73, 196–218. [Google Scholar] [CrossRef]
- Jingwen, T.; Siyu, L.; Yiyang, W.; Zhiqin, G.; Hong, Y.; Lianjuan, Y. Flavonoids constitutes and in vitro anti-Candida albicans activity in propolis. World Clin. Drugs 2021, 42, 957–961. [Google Scholar]
- Seleem, D.; Benso, B.; Noguti, J.; Pardi, V.; Murata, R.M. In Vitro and In Vivo Antifungal Activity of Lichochalcone-A against Candida albicans Biofilms. PLoS ONE 2016, 11, e0157188. [Google Scholar] [CrossRef]
- Wong, K.S.; Tsang, W.K. In vitro antifungal activity of the aqueous extract of Scutellaria baicalensis Georgi root against Candida albicans. Int. J. Antimicrob. Agents 2009, 34, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cao, Y.Y.; Dai, B.D.; Sun, X.R.; Zhu, Z.Y.; Cao, Y.B.; Wang, Y.; Gao, P.H.; Jiang, Y.Y. In vitro synergism of fluconazole and baicalein against clinical isolates of Candida albicans resistant to fluconazole. Biol. Pharm. Bull. 2008, 31, 2234–2236. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, X.; Zhou, P. In vitro Antifungal Effects of Berberine against Candida spp. In Planktonic and Biofilm Conditions. Drug Des. Dev. Ther. 2020, 14, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Cao, Y.Y.; Xu, Z.; Zhao, J.X.; Gao, P.H.; Qin, X.F.; Jiang, Y.Y. Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob. Agents Chemother. 2006, 50, 1096–1099. [Google Scholar] [CrossRef]
- Qian, W.; Yang, M.; Li, X.; Sun, Z.; Li, Y.; Wang, X.; Wang, T. Anti-microbial and anti-biofilm activities of combined chelerythrine-sanguinarine and mode of action against Candida albicans and Cryptococcus neoformans in vitro. Colloids Surf. B Biointerfaces 2020, 191, 111003. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, H.; Hu, Z.; Zhong, X.; Guan, Y.; Zhao, Y.; Wang, L.; Ye, L.; Ming, L.; Riaz Rajoka, M.S.; et al. Sanguinarine, Isolated From Macleaya cordata, Exhibits Potent Antifungal Efficacy Against Candida albicans Through Inhibiting Ergosterol Synthesis. Front. Microbiol. 2022, 13, 908461. [Google Scholar] [CrossRef]
- Gong, Y.; Li, S.; Wang, W.; Li, Y.; Ma, W.; Sun, S. In vitro and in vivo activity of chelerythrine against Candida albicans and underlying mechanisms. Future Microbiol. 2019, 14, 1545–1557. [Google Scholar] [CrossRef]
- de Paiva, S.R.; Figueiredo, M.R.; Aragão, T.V.; Kaplan, M.A. Antimicrobial activity in vitro of plumbagin isolated from Plumbago species. Mem. Inst. Oswaldo Cruz 2003, 98, 959–961. [Google Scholar] [CrossRef]
- Qian, W.; Wang, W.; Zhang, J.; Fu, Y.; Liu, Q.; Li, X.; Wang, T.; Zhang, Q. Exploitation of the antifungal and antibiofilm activities of plumbagin against Cryptococcus neoformans. Biofouling 2022, 38, 558–574. [Google Scholar] [CrossRef]
- Elansary, H.O.; Yessoufou, K.; Mahmoud, E.A.; Skalicka-Woźniak, K. In vitro Antioxidant and Antimicrobial Effects of Ceratostigma plumbaginoides. Nat. Prod. Commun. 2016, 11, 1455–1458. [Google Scholar] [CrossRef]
- Sasaki, K.; Abe, H.; Yoshizaki, F. In vitro antifungal activity of naphthoquinone derivatives. Biol. Pharm. Bull. 2002, 25, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Tan, F.; Miao, H.; Wang, H.; Cao, Y. Effect of Shikonin Against Candida albicans Biofilms. Front. Microbiol. 2019, 10, 1085. [Google Scholar] [CrossRef] [PubMed]
- Li, D.D.; Chai, D.; Huang, X.W.; Guan, S.X.; Du, J.; Zhang, H.Y.; Sun, Y.; Jiang, Y.Y. Potent In Vitro Synergism of Fluconazole and Osthole against Fluconazole-Resistant Candida albicans. Antimicrob. Agents Chemother. 2017, 61, e00436-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pan, M.; Xiao, N.; Wu, J.; Wang, Q.; Cheng, T.; Yan, G.; Wu, D.; Li, N.; Shao, J. In vitro and in vivo analysis of monotherapy and dual therapy with ethyl caffeate and fluconazole on virulence factors of Candida albicans and systemic candidiasis. J. Glob. Antimicrob. Resist. 2021, 27, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Hamaamin Hussen, N.; Hameed Hasan, A.; Jamalis, J.; Shakya, S.; Chander, S.; Kharkwal, H.; Murugesan, S.; Ajit Bastikar, V.; Pyarelal Gupta, P. Potential inhibitory activity of phytoconstituents against black fungus: In silico ADMET, molecular docking and MD simulation studies. Comput. Toxicol. 2022, 24, 100247. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Lau, K.M.; Wu, Y.O.; Cheng, L.; Wong, C.W.; Yew, D.T.; Leung, P.C.; Fung, K.P.; Hui, M.; Ng, T.B.; et al. Antifungal mode of action of macrocarpal C extracted from Eucalyptus globulus Labill (Lan An) towards the dermatophyte Trichophyton mentagrophytes. Chin. Med. 2015, 10, 34. [Google Scholar] [CrossRef]
- Bharate, S.B.; Khan, S.I.; Yunus, N.A.; Chauthe, S.K.; Jacob, M.R.; Tekwani, B.L.; Khan, I.A.; Singh, I.P. Antiprotozoal and antimicrobial activities of O-alkylated and formylated acylphloroglucinols. Bioorganic Med. Chem. 2007, 15, 87–96. [Google Scholar] [CrossRef]
- Qu, C.; Li, Z.; Wang, X. UHPLC-HRMS-Based Untargeted Lipidomics Reveal Mechanism of Antifungal Activity of Carvacrol against Aspergillus flavus. Foods 2021, 11, 93. [Google Scholar] [CrossRef]
- Jung, K.W.; Chung, M.S.; Bai, H.W.; Chung, B.Y.; Lee, S. Investigation of Antifungal Mechanisms of Thymol in the Human Fungal Pathogen, Cryptococcus neoformans. Molecules 2021, 26, 3476. [Google Scholar] [CrossRef]
- Guo-hui, Y.; Jin-ping, C.; Li-li, W.; Xiao-wen, H. Analysis of Chemical Compositions and Antifungal Activity of Plant Essential Oils Against Candida spp. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 215–227. [Google Scholar]
- Abers, M.; Schroeder, S.; Goelz, L.; Sulser, A.; St Rose, T.; Puchalski, K.; Langland, J. Antimicrobial activity of the volatile substances from essential oils. BMC Complement. Med. Ther. 2021, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, L.; Xia, L.; Jiang, S.; Kong, Y.; Chen, X.; Wang, H. The kinetics and release behaviour of curcumin loaded pH-responsive PLGA/chitosan fibers with antitumor activity against HT-29 cells. Carbohydr. Polym. 2021, 265, 118077. [Google Scholar] [CrossRef]
- Pontes-Quero, G.M.; Benito-Garzón, L.; Pérez Cano, J.; Aguilar, M.R.; Vázquez-Lasa, B. Amphiphilic polymeric nanoparticles encapsulating curcumin: Antioxidant, anti-inflammatory and biocompatibility studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 121, 111793. [Google Scholar] [CrossRef]
- Fakhrullina, G.; Khakimova, E.; Akhatova, F.; Lazzara, G.; Parisi, F.; Fakhrullin, R. Selective Antimicrobial Effects of Curcumin@Halloysite Nanoformulation: A Caenorhabditis elegans Study. ACS Appl. Mater. Interfaces 2019, 11, 23050–23064. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Assessment of silibinin as a potential antifungal agent and investigation of its mechanism of action. IUBMB Life 2017, 69, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pérez, M.L.; Vadillo-Rodríguez, V.; Fernández-Babiano, I.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. Antimicrobial activity of a novel Spanish propolis against planktonic and sessile oral Streptococcus spp. Sci. Rep. 2021, 11, 23860. [Google Scholar] [CrossRef] [PubMed]
- Nani, B.D.; Sardi, J.C.O.; Lazarini, J.G.; Silva, D.R.; Massariolli, A.P.; Cunha, T.M.; de Alencar, S.M.; Franchin, M.; Rosalen, P.L. Anti-inflammatory and anti-Candida Effects of Brazilian Organic Propolis, a Promising Source of Bioactive Molecules and Functional Food. J. Agric. Food Chem. 2020, 68, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef]
- Yanshan, L.; Wenjun, P.; Yongguang, B. Ultrasonic extraction of flavonoids from propolis. Tech. Acoust. 2020, 39, 190–194. [Google Scholar] [CrossRef]
- Zulhendri, F.; Chandrasekaran, K.; Kowacz, M.; Ravalia, M.; Kripal, K.; Fearnley, J.; Perera, C.O. Antiviral, Antibacterial, Antifungal, and Antiparasitic Properties of Propolis: A Review. Foods 2021, 10, 1360. [Google Scholar] [CrossRef]
- Su, H.; Song, S.; Yan, X.; Fang, L.; Zeng, B.; Zhu, Y. Endogenous salicylic acid shows different correlation with baicalin and baicalein in the medicinal plant Scutellaria baicalensis Georgi subjected to stress and exogenous salicylic acid. PLoS ONE 2018, 13, e0192114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Zhou, H.-Y.; Yang, N.; Zhao, F.; Jin, Y.-S. Synthesis and synergistic antifungal effects of flavonoids against drug resistant Candida albicans. Bioorganic Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef]
- Fu, Z.; Lu, H.; Zhu, Z.; Yan, L.; Jiang, Y.; Cao, Y. Combination of baicalein and Amphotericin B accelerates Candida albicans apoptosis. Biol. Pharm. Bull. 2011, 34, 214–218. [Google Scholar] [CrossRef]
- Li, D.D.; Xu, Y.; Zhang, D.Z.; Quan, H.; Mylonakis, E.; Hu, D.D.; Li, M.B.; Zhao, L.X.; Zhu, L.H.; Wang, Y.; et al. Fluconazole assists berberine to kill fluconazole-resistant Candida albicans. Antimicrob. Agents Chemother. 2013, 57, 6016–6027. [Google Scholar] [CrossRef]
- Xu, Y.; Quan, H.; Wang, Y.; Zhong, H.; Sun, J.; Xu, J.; Jia, N.; Jiang, Y. Requirement for Ergosterol in Berberine Tolerance Underlies Synergism of Fluconazole and Berberine against Fluconazole-Resistant Candida albicans Isolates. Front. Cell. Infect. Microbiol. 2017, 7, 491. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lee, J.H. Berberine synergy with amphotericin B against disseminated candidiasis in mice. Biol. Pharm. Bull. 2005, 28, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.; Kok, S.H.; Lee, K.K.; Lam, K.H.; Hau, D.K.; Wong, W.Y.; Bian, Z.; Gambari, R.; Chui, C.H. Sensitization of Candida albicans to terbinafine by berberine and berberrubine. Biomed. Rep. 2016, 4, 449–452. [Google Scholar] [CrossRef]
- Zhong, H.; Hu, D.D.; Hu, G.H.; Su, J.; Bi, S.; Zhang, Z.E.; Wang, Z.; Zhang, R.L.; Xu, Z.; Jiang, Y.Y.; et al. Activity of Sanguinarine against Candida albicans Biofilms. Antimicrob. Agents Chemother. 2017, 61, e02259-16. [Google Scholar] [CrossRef]
- Ghosh, K.; Bhattacharya, T.K. Chemical constituents of Piper betle Linn. (Piperaceae) roots. Molecules 2005, 10, 798–802. [Google Scholar] [CrossRef]
- Tang, G.H.; Chen, D.M.; Qiu, B.Y.; Sheng, L.; Wang, Y.H.; Hu, G.W.; Zhao, F.W.; Ma, L.J.; Wang, H.; Huang, Q.Q.; et al. Cytotoxic amide alkaloids from Piper boehmeriaefolium. J. Nat. Prod. 2011, 74, 45–49. [Google Scholar] [CrossRef]
- Prasetya, F.; Salam, S.; Rahmadani, A.; Haikal, K.; Febrina, L.; Anshory, H.; Arifuddin, M.; Siregar, V.O.; Narsa, A.C.; Herman, H.; et al. Novel Amides Derivative with Antimicrobial Activity of Piper betle var. nigra Leaves from Indonesia. Molecules 2021, 26, 335. [Google Scholar] [CrossRef]
- Futuro, D.O.; Ferreira, P.G.; Nicoletti, C.D.; Borba-Santos, L.P.; Silva, F.C.D.; Rozental, S.; Ferreira, V.F. The Antifungal Activity of Naphthoquinones: An Integrative Review. An. Acad. Bras. Ciências 2018, 90, 1187–1214. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Zhu, Z.; Li, L.; Wang, L.; Wang, H.; Jiang, Y.; Cao, Y. Metabonomics on Candida albicans indicate the excessive H3K56ac is involved in the antifungal activity of Shikonin. Emerg. Microbes Infect. 2019, 8, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhi, X. Xiangdousu Zaheti Jiqi Kangzhenjun Huoxing [Coumarin derivatives and their antifungal activities]. Guowai Yiyao Kangshengsu Fence 2018, 39, 327–335. [Google Scholar]
- Guo, Y.; Li, M.; Chen, P.; Wu, Q.; Gao, C.; Lu, Y.; Zhang, L.; Yuan, D.; Fu, H. A pair of new elemanolide sesquiterpene lactones from Elephantopus scaber L. Magn. Reson. Chem. 2017, 55, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.T.; Liu, C.J.; Yeh, C.C. Protective and immunomodulatory effect of flos Lonicerae japonicae by augmenting IL-10 expression in a murine model of acute lung inflammation. J. Ethnopharmacol. 2015, 168, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Rocha da Silva, C.; Sá, L.; Dos Santos, E.V.; Ferreira, T.L.; Coutinho, T.; Moreira, L.E.A.; de Sousa Campos, R.; de Andrade, C.R.; Barbosa da Silva, W.M.; de Sá Carneiro, I.; et al. Evaluation of the antifungal effect of chlorogenic acid against strains of Candida spp. resistant to fluconazole: Apoptosis induction and in silico analysis of the possible mechanisms of action. J. Med. Microbiol. 2022, 71, 001526. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Xuanhao, C.; Jin, L.; Jun, H.; Yanxu, C. Research development of Menthae Haplocalycis Herba on chemical composition and pharmacological activity. J. Tianjin Univ. Tradit. Chin. Med. 2022, 41, 4–13. [Google Scholar]
- Wang, S.J.; Wang, X.H.; Dai, Y.Y.; Ma, M.H.; Rahman, K.; Nian, H.; Zhang, H. Prunella vulgaris: A Comprehensive Review of Chemical Constituents, Pharmacological Effects and Clinical Applications. Curr. Pharm. Des. 2019, 25, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Xiangyang, C. Study on Chemical Constituents and Anti-Inflammatory Activity of Phenolic Fraction of Menthae Haplocalycis Herba. Ph.D. Thesis, Beijing University of Chinese Medicine, Beijing, China, 2016. [Google Scholar]
- Kikuchi, T.; Tanaka, A.; Uriuda, M.; Yamada, T.; Tanaka, R. Three Novel Triterpenoids from Taraxacum officinale Roots. Molecules 2016, 21, 1121. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.J.; Cha, D.S.; Ko, J.S.; Park, H.J.; Choi, H.D. Anti-inflammatory effect of Taraxacum officinale leaves on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells. J. Med. Food 2010, 13, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Park, J.Y.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Kotanidou, A.; Xagorari, A.; Bagli, E.; Kitsanta, P.; Fotsis, T.; Papapetropoulos, A.; Roussos, C. Luteolin reduces lipopolysaccharide-induced lethal toxicity and expression of proinflammatory molecules in mice. Am. J. Respir. Crit. Care Med. 2002, 165, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.N.; Han, C.; Song, P.Y.; Zhao, X.H.; Zhong, X.H. Anti-inflammatory Effects of Luteolin and Quercetin in Vitro. Prog. Vet. Med. 2017, 38, 56–61. [Google Scholar] [CrossRef]
- Shen, P.; Lin, W.; Deng, X.; Ba, X.; Han, L.; Chen, Z.; Qin, K.; Huang, Y.; Tu, S. Potential Implications of Quercetin in Autoimmune Diseases. Front. Immunol. 2021, 12, 689044. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, N.; Wu, Y.; Yan, N. Research Progress on Anti-inflammatory Active Components of Lianqiao (Fructus forsythiae) and its Action Mechanism. Chin. Arch. Tradit. Chin. Med. 2022, 40, 115–120. [Google Scholar] [CrossRef]
- Javadi, F.; Ahmadzadeh, A.; Eghtesadi, S.; Aryaeian, N.; Zabihiyeganeh, M.; Rahimi Foroushani, A.; Jazayeri, S. The Effect of Quercetin on Inflammatory Factors and Clinical Symptoms in Women with Rheumatoid Arthritis: A Double-Blind, Randomized Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 9–15. [Google Scholar] [CrossRef]
- Carullo, G.; Cappello, A.R.; Frattaruolo, L.; Badolato, M.; Armentano, B.; Aiello, F. Quercetin and derivatives: Useful tools in inflammation and pain management. Future Med. Chem. 2017, 9, 79–93. [Google Scholar] [CrossRef]
- Nikfarjam, B.A.; Adineh, M.; Hajiali, F.; Nassiri-Asl, M. Treatment with Rutin—A Therapeutic Strategy for Neutrophil-Mediated Inflammatory and Autoimmune Diseases: Anti-inflammatory Effects of Rutin on Neutrophils. J. Pharmacopunct. 2017, 20, 52–56. [Google Scholar] [CrossRef]
- Chen, X.; Yu, M.; Xu, W.; Zou, L.; Ye, J.; Liu, Y.; Xiao, Y.; Luo, J. Rutin inhibited the advanced glycation end products-stimulated inflammatory response and extra-cellular matrix degeneration via targeting TRAF-6 and BCL-2 proteins in mouse model of osteoarthritis. Aging 2021, 13, 22134–22147. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Pirbaluti, M.; Motaghi, E.; Bozorgi, H. The effect of menthol on acute experimental colitis in rats. Eur. J. Pharmacol. 2017, 805, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wen, T.; Xu, F.; Sang, W.; Chen, R.; Nan, Z. The protective effects of menthone on endotoxin-induced infalmmation in mice. Chin. Pharmacol. Bull. 2017, 33, 227–234. [Google Scholar]
- Yang, L.; Zhou, X.; Huang, W.; Fang, Q.; Hu, J.; Yu, L.; Ma, N.; Zhang, W. Protective Effect of Phillyrin on Lethal LPS-Induced Neutrophil Inflammation in Zebrafish. Cell. Physiol. Biochem. 2017, 43, 2074–2087. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, R.; Pan, W.; Huang, W.; Liu, B.; Xie, Y.; Wang, Z.; Li, C.; Jiang, H.; Huang, J.; et al. Phillyrin (KD-1) exerts anti-viral and anti-inflammatory activities against novel coronavirus (SARS-CoV-2) and human coronavirus 229E (HCoV-229E) by suppressing the nuclear factor kappa B (NF-κB) signaling pathway. Phytomedicine 2020, 78, 153296. [Google Scholar] [CrossRef]
- Hu, N.; Wang, C.; Dai, X.; Zhou, M.; Gong, L.; Yu, L.; Peng, C.; Li, Y. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-κB signaling pathway. J. Ethnopharmacol. 2020, 248, 112361. [Google Scholar] [CrossRef]
- Zhou, M.; Tang, Y.; Liao, L.; Liu, M.; Deng, Y.; Zhao, X.; Li, Y. Phillygenin inhibited LPS-induced RAW 264.7 cell inflammation by NF-κB pathway. Eur. J. Pharmacol. 2021, 899, 174043. [Google Scholar] [CrossRef]
- Lee, S.; Shin, S.; Kim, H.; Han, S.; Kim, K.; Kwon, J.; Kwak, J.H.; Lee, C.K.; Ha, N.J.; Yim, D.; et al. Anti-inflammatory function of arctiin by inhibiting COX-2 expression via NF-κB pathways. J. Inflamm. 2011, 8, 16. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, L.; Liang, Y.; Li, J.; Pan, X. Arctiin suppresses H9N2 avian influenza virus-mediated inflammation via activation of Nrf2/HO-1 signaling. BMC Complement. Med. Ther. 2021, 21, 289. [Google Scholar] [CrossRef]
- Zhou, B.; Weng, G.; Huang, Z.; Liu, T.; Dai, F. Arctiin Prevents LPS-Induced Acute Lung Injury via Inhibition of PI3K/AKT Signaling Pathway in Mice. Inflammation 2018, 41, 2129–2135. [Google Scholar] [CrossRef]
- Niu, Y.; Dong, Q.; Li, R. Matrine regulates Th1/Th2 cytokine responses in rheumatoid arthritis by attenuating the NF-κB signaling. Cell Biol. Int. 2017, 41, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.J.; Juan, T.Y.; Chao, P.; Wu, W.L.; Chang, D.M.; Chang, S.Y.; Lai, J.H. Plant alkaloid tetrandrine downregulates IkappaBalpha kinases-IkappaBalpha-NF-kappaB signaling pathway in human peripheral blood T cell. Br. J. Pharmacol. 2004, 143, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kusano, J.; Chen, S.; Yamamoto, R.; Matsuda, H.; Hara, Y.; Fujii, Y.; Hayashi, S.; Tanaka, S.; Sugiyama, K.; et al. Absolute configuration of tetrandrine and isotetrandrine influences their anti-proliferation effects in human T cells via different regulation of NF-κB. Z. Fur Naturforschung. C J. Biosci. 2021, 76, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Zhang, T. Immunomodulatory activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021, 340, 127933. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef]
- Lee, J.H.; Han, Y. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int. Immunopharmacol. 2006, 6, 1424–1430. [Google Scholar] [CrossRef]
- Qi, X.; Fan, M.; Huang, N.; Zhang, X.; Liu, J.; Li, X.; Sun, R. Saikosaponin d contributed to cancer chemotherapy induced neutropenia therapy by promoting neutrophil differentiation via activation CBL-dependent ERK pathway. Pharmacol. Res. 2020, 160, 105149. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of Shikonin and Derivatives: Isolation, Chemistry, Biosynthesis, Pharmacology and Toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar] [CrossRef]
- Valipour, M.; Zarghi, A.; Ebrahimzadeh, M.A.; Irannejad, H. Therapeutic potential of chelerythrine as a multi-purpose adjuvant for the treatment of COVID-19. Cell Cycle 2021, 20, 2321–2336. [Google Scholar] [CrossRef]
- Figat, R.; Zgadzaj, A.; Geschke, S.; Sieczka, P.; Pietrosiuk, A.; Sommer, S.; Skrzypczak, A. Cytotoxicity and antigenotoxicity evaluation of acetylshikonin and shikonin. Drug Chem. Toxicol. 2021, 44, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. The Effectiveness Study of Polysaccharides and Its Componets from Ginseng on Alzheimer’s Disease. Ph.D. Thesis, Jilin University, Changchun, China, 2014. [Google Scholar]
- Nan, M.; Zhao, Y.; Lv, N.; He, Y.; Si, X.; Zhao, Q. Advances in Studies on the Chemical Structure and Hypoglycemic Activity of Ginseng Polysaccharide. China Pharm. 2014, 25, 4506–4508. [Google Scholar] [CrossRef]
- Phu, H.T.; Thuan, D.T.B.; Nguyen, T.H.D.; Posadino, A.M.; Eid, A.H.; Pintus, G. Herbal Medicine for Slowing Aging and Aging-associated Conditions: Efficacy, Mechanisms and Safety. Curr. Vasc. Pharmacol. 2020, 18, 369–393. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, F.; Wu, L.; Xu, Y.; Du, Q. Matrine Exerts Pharmacological Effects Through Multiple Signaling Pathways: A Comprehensive Review. Drug Des. Dev. Ther. 2022, 16, 533–569. [Google Scholar] [CrossRef]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Müller, P.; Kuttenkeuler, D.; Gesellchen, V.; Zeidler, M.P.; Boutros, M. Identification of JAK/STAT signalling components by genome-wide RNA interference. Nature 2005, 436, 871–875. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef]
- Fu, R.; Li, J.; Yu, H.; Zhang, Y.; Xu, Z.; Martin, C. The Yin and Yang of traditional Chinese and Western medicine. Med. Res. Rev. 2021, 41, 3182–3200. [Google Scholar] [CrossRef]

| Natural Product | Source | Target Fungi | MIC (μg/mL) a | FICI a | References |
|---|---|---|---|---|---|
dioscin | Dioscoreaceae family | C. albicans (2) b | 4 | - | [14,15] |
| C. glabrata | 2 | - | |||
| C. parapsilosis | 4 | - | |||
α-santalol | Santalum family | T. rubrum | 12.5 (μg/disc c) | - | [16,17] |
eucarobustol E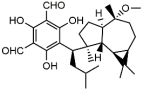 | Eucalyptus robusta | fluconazole-susceptible C. albicans (10) | 4–16 | - | [18] |
| fluconazole-resistant C. albicans (10) | 32–128 | - | [18] | ||
asiatic acid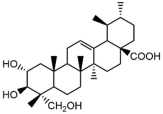 | Centella asiatica | fluconazole-susceptible C. albicans (4) | 64 | 0.75–1.00 | [19] |
| fluconazole-resistant C. albicans (4) | 64–128 | 0.25- | [19] | ||
carvacrol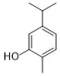 | Oreganum family | C. albicans | 250 | 0.374 | [20,21] |
thymol | Oreganum family | C. albicans | 500 | 1.062 | [20,22] |
| C. neoformans (10) | 20–51 | - | [23] | ||
eugenol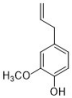 | Oreganum family | C. albicans | 1000 | 0.312 | [20,24] |
| laurel essential oil | Laurus nobilis | C. albicans (2) | >4 (% v/v) d | - | [25] |
| anise essential oil | Pimpinella anisum | C. albicans (6) | 4 or >4 (% v/v) | - | [25] |
| oregano essential oil | Thymus capitatus | C. albicans (31) | 0.0039–1, or <0.0019 (% v/v) | - | [25] |
| basil essential oil | Ocimum basilicum | C. albicans (11) | 4 or >4 (% v/v) | - | [25] |
| lavender essential oil | Lavandula latifolia | C. albicans (15) | 0.25–4 (% v/v) | - | [25] |
| mint essential oil | Mentha spicata | C. albicans (25) | 2–4 (% v/v) | - | [25] |
| rosemary essential oil | Rosmarinus officinalis | C. albicans (2) | 4 (% v/v) | - | [25] |
| rosemary extract | Rosmarinus officinalis L. | C. albicans | 50,000 | - | [26] |
| C. dubliniensis | 50,000 | - | [26] | ||
| C. glabrata | 50,000 | - | [26] | ||
| C. krusei | 50,000 | - | [26] | ||
| C. tropicalis | 50,000 | - | [26] | ||
| tea tree oil | Melaleuca alternifolia | C. albicans (44) | 0.06–4 (% v/v) | 0.25–1.25 | [25,27] |
terpinen-4-ol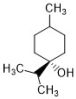 | Melaleuca alternifolia | C. albicans (33) | 0.06–0.25 | 0.250-0.252 | [27,28] |
| grapefruit essential oil | Citrus paradisi | C. albicans (12) | 0.0039–1 (% v/v) | - | [25] |
| turmeric essential oil | Curcuma longa L. | M.gypseum (2) | 0.25 (% v/v), 6.25 | - | [29,30] |
| T. mentagrophytes (2) | 0.25 (% v/v), 6.25 | - | [29,30] | ||
| T. rubrum | 1.56 | - | [30] | ||
| E. floccosum | 1.56 | - | [30] | ||
Ar-turmerone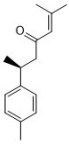 | Curcuma longa L. | M.gypseum | 7.81 | - | [30,31] |
| T. mentagrophytes | 7.81 | - | [30] | ||
| T. rubrum | 3.90 | - | [30] | ||
| E. floccosum | 3.90 | - | [30] | ||
curcumin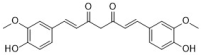 | Curcuma longa L. | fluconazole-susceptible Candida species (27) | 250–650 | - | [32,33] |
| fluconazole-resistant Candida species (11) | 250–500 | - | [32] | ||
| C. albicans | 64 | - | [34] | ||
| C. tropicalis | 256 | - | [34] | ||
| C. krusei | 256 | - | [34] | ||
| C. parapsilosis | >256 | - | [34] | ||
| C. glabrata | >256 | - | [34] | ||
| C. dubliniensis (2) | 32 | - | [34] | ||
| C. neoformans | 32 | - | [34] | ||
| S. schenckii | 32 | - | [34] | ||
| P. brasiliensis (7) | 0.5–32 | - | [34] | ||
| A. fumigatus (2) | >256 | - | [34] | ||
| A. nomius | >256 | - | [34] | ||
| A. flavus | >256 | - | [34] | ||
| A. tamarii | >256 | - | [34] | ||
| A. terreus | >256 | - | [34] | ||
| A. clavatus | >256 | - | [34] | ||
| a blend of essential oils (3.53% of cinnamaldehyde, 3.53% of eugenol, 3.53% of carvacol, 1.04% of carotol, and 88.35% of Camelina sativa oil) | Cinnamomum zeylanicum, Syzygium aromaticum, Origanum vulgare, Daucus carota, and Camelina sativa | C. albicans (4) | 0.02 (% v/v) | - | [35] |
| C. glabrata | 0.05 (% v/v) | - | [35] | ||
| C. tropicalis | 0.01 (% v/v) | - | [35] | ||
silibinin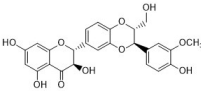 | Silybum marianum | C. albicans (2) | 19.3, 1024 | - | [36,37,38] |
| C. krusei | 1024 | - | [36] | ||
| C. tropicalis | 1024 | - | [36] | ||
| A. flavus | 9.6 | - | [37] | ||
| C. parapsilosis | 9.6 | - | [37] | ||
| Malassezia Furfur | 19.3 | - | [37] | ||
| Trichosporon species | 19.3 | - | [37] | ||
pinobanksin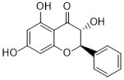 | Chinese propolis | C. albicans | 100 | - | [39] |
tectochrysin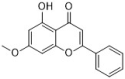 | Chinese propolis | C. albicans | 25 | - | [39] |
chrysin | Chinese propolis | C. albicans | 100 | - | [39] |
3-O-acetylpinobanksin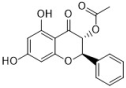 | Chinese propolis | C. albicans | 50 | - | [39] |
licochalcone A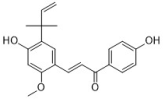 | Glycyrrhiza family | C. albicans (4) | 16.92–50.76 | - | [40] |
baicalein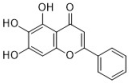 | Scutellaria baicalensis | C. albicans | 25 | 0.039 | [41,42] |
berberine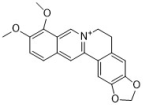 | many herbs such as Coptis chinensis and Mahonia aquifolium | C. albicans (4) | 80–160 | 0.017–0.127 | [43,44] |
| C. krusei (3) | 10–20 | - | [43] | ||
| C. glabrata (3) | 20–160 | - | [43] | ||
| C. dubliniensis | 40 | - | [43] | ||
sanguinarine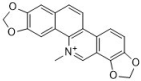 | Papaveraceae family | C. albicans (11) | 4, 37.5–50 | - | [45,46] |
| C. neoformans | 64 | - | [45] | ||
chelerythrine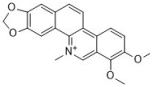 | Papaveraceae family | C. albicans (3) | 2–4 | - | [45,47] |
| C. glabrata (2) | 16 | [47] | |||
| C. krusei (2) | 16 | [47] | |||
| C. tropicalis (2) | 8 | [47] | |||
| C. neoformans | 64 | - | [45] | ||
plumbagin | Plumbago scandens | C. albicans | 0.78 | - | [48] |
| C. neoformans | 8 | - | [49] | ||
bis-naphthoquinone | Ceratostigma plumbaginoides | C. albicans | 0.09 | - | [50] |
shikonin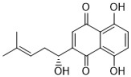 | Lithospermum erythrorhizon | C. albicans (12) | 2-8, >64 | - | [51,52] |
| C. krusei | 4 | - | [51] | ||
| C. glabrata | 8 | - | [51] | ||
| C. tropicalis | 8 | - | [51] | ||
| C. parapsilosis | 16 | - | [51] | ||
| Saccharomyces cerevisiae | 4 | - | [51] | ||
| C. neoformans | 8 | - | [51] | ||
| T. cutaneum | 8 | - | [51] | ||
| A. fumigatus | >64 | - | [51] | ||
osthole | Cnidii fructus | C. albicans (52) | 8–64, >64 | 0.04–0.37 | [6,53] |
ethyl caffeate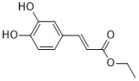 | Elephantopus scaber L. | C. albicans (26) | 64–256 | 0.047–0.375 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, H.; Han, L.; Lu, R.-Y.; Wang, Y. Antifungal and Immunomodulatory Ingredients from Traditional Chinese Medicine. Antibiotics 2023, 12, 48. https://doi.org/10.3390/antibiotics12010048
Zhong H, Han L, Lu R-Y, Wang Y. Antifungal and Immunomodulatory Ingredients from Traditional Chinese Medicine. Antibiotics. 2023; 12(1):48. https://doi.org/10.3390/antibiotics12010048
Chicago/Turabian StyleZhong, Hua, Lei Han, Ren-Yi Lu, and Yan Wang. 2023. "Antifungal and Immunomodulatory Ingredients from Traditional Chinese Medicine" Antibiotics 12, no. 1: 48. https://doi.org/10.3390/antibiotics12010048
APA StyleZhong, H., Han, L., Lu, R.-Y., & Wang, Y. (2023). Antifungal and Immunomodulatory Ingredients from Traditional Chinese Medicine. Antibiotics, 12(1), 48. https://doi.org/10.3390/antibiotics12010048