Sorting Out the Risks and Benefits of the #797 Recommended Intrapartum Vancomycin Dosing Approach
Abstract
1. Introduction
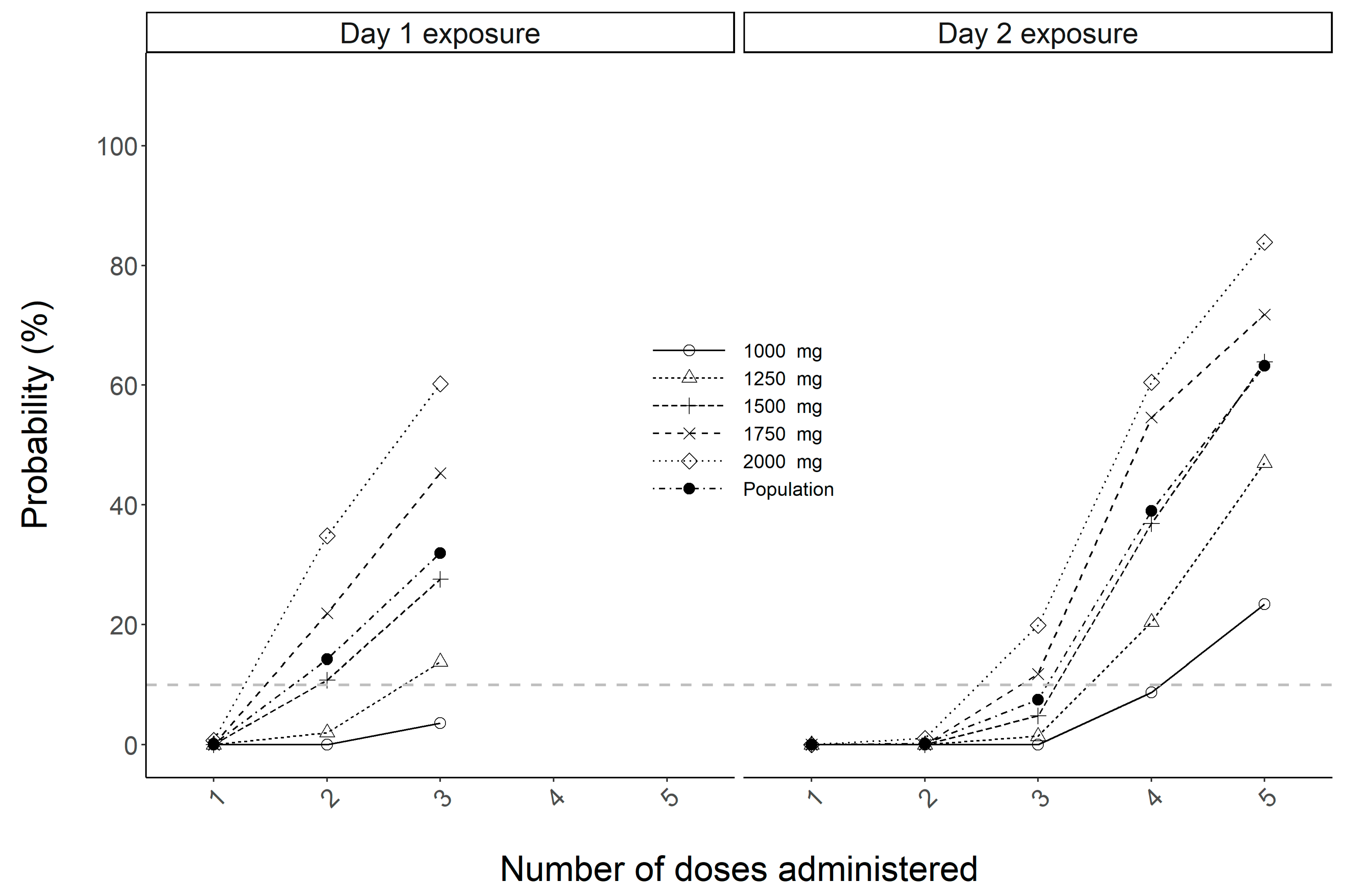
2. Results
| Parameter | Median (%CV) |
|---|---|
| V, maternal (L) | 39.66 (49.9) |
| V, fetal (L) | 2.07 (39.5) |
| CL, maternal (L/h) | 4.78 (43.6) |
| Kmf (h−1) | 0.51 (31.7) |
| Kfm (h−1) | 0.36 (34.1) |
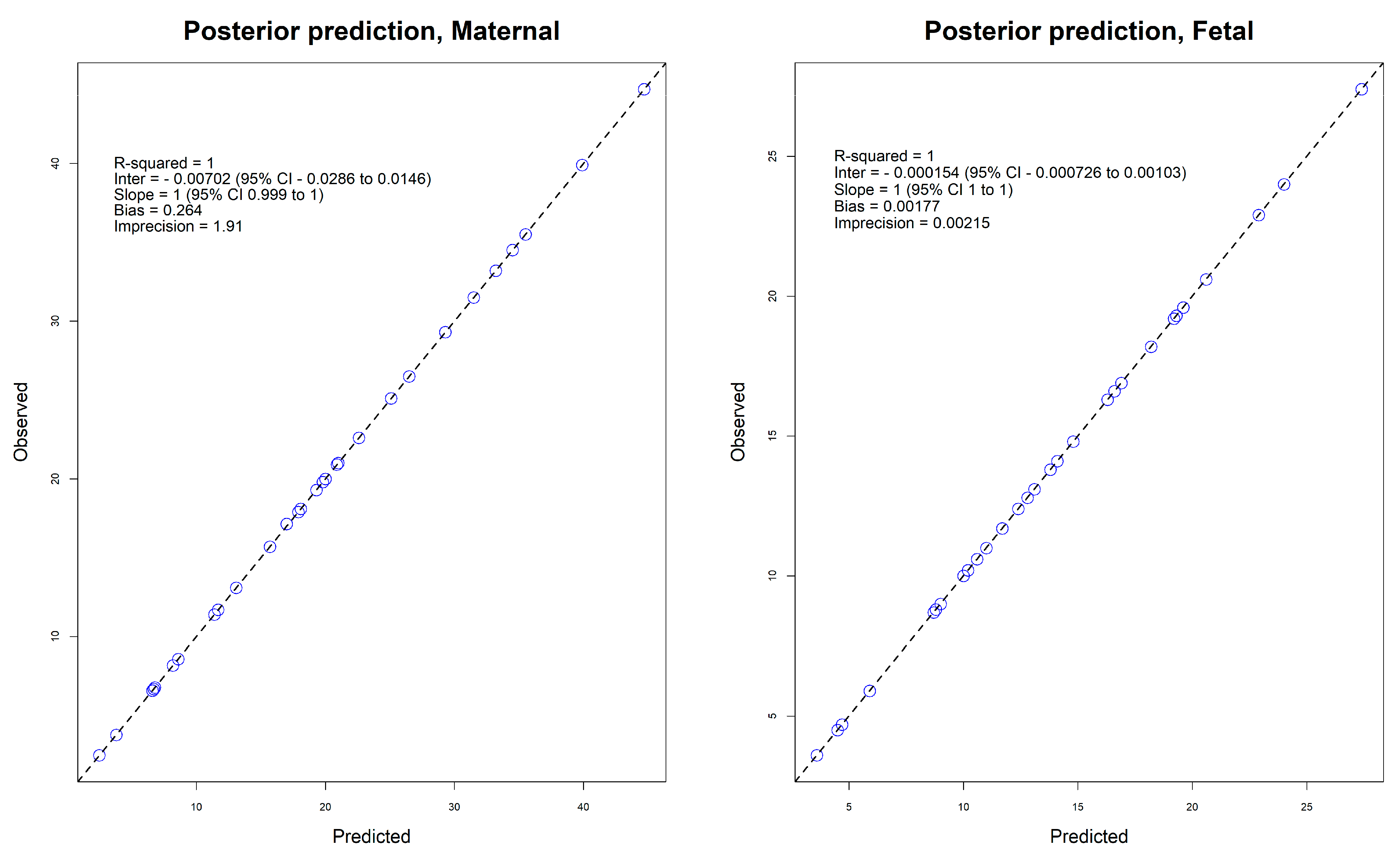
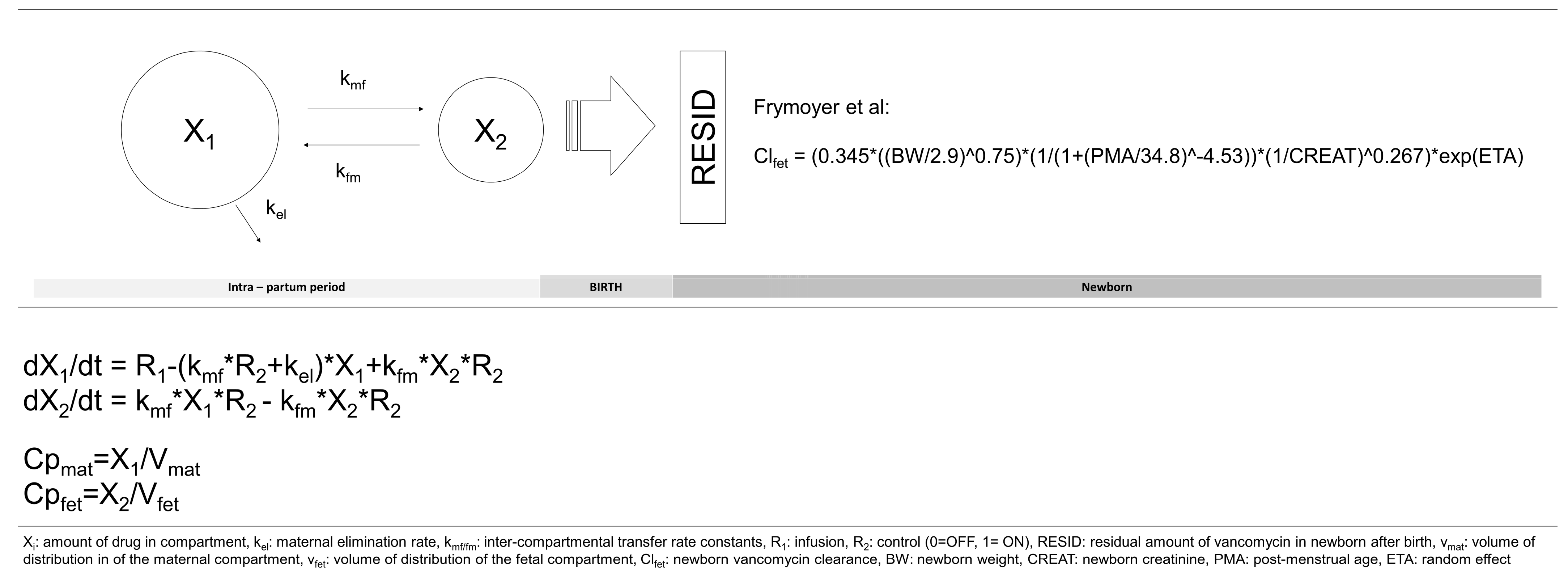
3. Discussion
4. Methods
4.1. Study Design
4.2. Pharmacokinetic Analysis
4.3. Monte Carlo Simulation
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prevention of group B streptococcal early-onset disease in newborns. ACOG Committee Opinion No. 797. American College of Obstetricians and Gynecologists. Obstet. Gynecol. 2020, 135, e51–e72. [Google Scholar] [CrossRef]
- Onwuchuruba, C.N.; Towers, C.V.; Howard, B.C.; Hennessy, M.D.; Wolfe, L.; Brown, M.S. Transplacental passage of vancomycin from mother to neonate. Am. J. Obstet. Gynecol. 2014, 210, 352.e1–352.e4. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.V.; Weitz, B. Transplacental passage of vancomycin. J. Matern. Fetal Neonatal Med. 2018, 31, 1021–1024. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.; Wong, S.C.; Sridhar, S. Vancomycin dosage for group B streptococcus prophylaxis. Am. J. Obstet. Gynecol. 2014, 211, 573–574. [Google Scholar] [CrossRef]
- Frymoyer, A.; Hersh, A.L.; El-Komy, M.H.; Gaskari, S.; Su, F.; Drover, D.R.; Van Meurs, K. Association between vancomycin trough concentration and area under the concentration-time curve in neonates. Antimicrob. Agents Chemother. 2014, 58, 6454–6461. [Google Scholar] [CrossRef]
- Morrison, A.P.; Melanson, S.E.; Carty, M.G.; Bates, D.W.; Szumita, P.M.; Tanasijevic, M.J. What proportion of vancomycin trough levels are drawn too early?: Frequency and impact on clinical actions. Am. J. Clin. Pathol. 2012, 137, 472–478. [Google Scholar] [CrossRef]
- Wallace, M.R.; Mascola, J.R.; Oldfield, E.C., 3rd. Red man syndrome: Incidence, etiology, and prophylaxis. J. Infect. Dis. 1991, 164, 1180–1185. [Google Scholar] [CrossRef]
- Le, J.; Ny, P.; Capparelli, E.; Lane, J.; Ngu, B.; Muus, R.; Romanowski, G.; Vo, T.; Bradley, J. Pharmacodynamic Characteristics of Nephrotoxicity Associated With Vancomycin Use in Children. J. Pediatr. Infect. Dis. Soc. 2015, 4, e109–e116. [Google Scholar] [CrossRef]
- Bhargava, V.; Malloy, M.; Fonseca, R. The association between vancomycin trough concentrations and acute kidney injury in the neonatal intensive care unit. BMC Pediatr. 2017, 17, 50. [Google Scholar] [CrossRef]
- Dawoud, T.H.; Khan, N.; Afzal, U.; Varghese, N.; Rahmani, A.; Abu-Sa’da, O. Assessment of initial vancomycin trough levels and risk factors of vancomycin-induced nephrotoxicity in neonates. Eur. J. Hosp. Pharm. 2022, 29, 44–49. [Google Scholar] [CrossRef]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Sakoulas, G.; Gold, H.S.; Cohen, R.A.; Venkataraman, L.; Moellering, R.C.; Eliopoulos, G.M. Effects of prolonged vancomycin administration on methicillin-resistant Staphylococcus aureus (MRSA) in a patient with recurrent bacteraemia. J. Antimicrob. Chemother. 2006, 57, 699–704. [Google Scholar] [CrossRef]
- Howden, B.P.; Ward, P.B.; Charles, P.G.; Korman, T.M.; Fuller, A.; du Cros, P.; Grabsch, E.A.; Roberts, S.A.; Robson, J.; Read, K.; et al. Treatment outcomes for serious infections caused by methicillin-resistant Staphylococcus aureus with reduced vancomycin susceptibility. Clin. Infect. Dis. 2004, 38, 521–528. [Google Scholar] [CrossRef]
- Charles, P.G.; Ward, P.B.; Johnson, P.D.; Howden, B.P.; Grayson, M.L. Clinical features associated with bacteremia due to heterogeneous vancomycin-intermediate Staphylococcus aureus. Clin. Infect. Dis. 2004, 38, 448–451. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Moghadam, M.T.; van Belkum, A.; Yaslianifard, S.; Darban-Sarokhalil, D. Global prevalence and distribution of vancomycin resistant, vancomycin intermediate and heterogeneously vancomycin intermediate Staphylococcus aureus clinical isolates: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 12689. [Google Scholar] [CrossRef]
- Spaetgens, R.; DeBella, K.; Ma, D.; Robertson, S.; Mucenski, M.; Davies, H.D. Perinatal antibiotic usage and changes in colonization and resistance rates of group B streptococcus and other pathogens. Obstet. Gynecol. 2002, 100, 525–533. [Google Scholar] [CrossRef]
- Ashkenazi-Hoffnung, L.; Melamed, N.; Ben-Haroush, A.; Livni, G.; Amir, J.; Bilavsky, E. The association of intrapartum antibiotic exposure with the incidence and antibiotic resistance of infantile late-onset serious bacterial infections. Clin. Pediatr. 2011, 50, 827–833. [Google Scholar] [CrossRef]
- Jauréguy, F.; Carton, M.; Panel, P.; Foucaud, P.; Butel, M.J.; Doucet-Populaire, F. Effects of intrapartum penicillin prophylaxis on intestinal bacterial colonization in infants. J. Clin. Microbiol. 2004, 42, 5184–5188. [Google Scholar] [CrossRef][Green Version]
- Neely, M.N.; van Guilder, M.G.; Yamada, W.M.; Schumitzky, A.; Jelliffe, R.W. Accurate detection of outliers and subpopulations with Pmetrics, a nonparametric and parametric pharmacometric modeling and simulation package for R. Ther. Drug Monit. 2012, 34, 467–476. [Google Scholar] [CrossRef]
- Optimum Dosing Strategies. Available online: https://www.optimum-dosing-strategies.org/ (accessed on 10 October 2022).
- Kramer, M.S.; Platt, R.W.; Wen, S.W.; Joseph, K.S.; Allen, A.; Abrahamowicz, M.; Blondel, B.; Bréart, G.; Fetal/Infant Health Study Group of the Canadian Perinatal Surveillance System. A new and improved population-based Canadian reference for birth weight for gestational age. Pediatrics 2001, 108, E35. [Google Scholar] [CrossRef]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2020, 77, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Murakami, Y.; Andes, D.R.; Craig, W.A. Inoculum effects of ceftobiprole, daptomycin, linezolid, and vancomycin with Staphylococcus aureus and Streptococcus pneumoniae at inocula of 10(5) and 10(7) CFU injected into opposite thighs of neutropenic mice. Antimicrob. Agents Chemother. 2013, 57, 1434–1441. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, P.G.; Bhavnani, S.M.; Rubino, C.M.; Louie, A.; Gumbo, T.; Forrest, A.; Drusano, G.L. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: It’s not just for mice anymore. Clin. Infect. Dis. 2007, 44, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Kontnick, C.M.; Edberg, S.C. Direct detection of group B streptococci from vaginal specimens compared with quantitative culture. J. Clin. Microbiol. 1990, 28, 336–339. [Google Scholar] [CrossRef]
- Sapico, F.L.; Witte, J.L.; Canawati, H.N.; Montgomerie, J.Z.; Bessman, A.N. The infected foot of the diabetic patient: Quantitative microbiology and analysis of clinical features. Rev. Infect. Dis. 1984, 6 (Suppl. S1), S171–S176. [Google Scholar] [CrossRef]
- McNanley, A.R.; Glantz, J.C.; Hardy, D.J.; Vicino, D. The effect of intrapartum penicillin on vaginal group B streptococcus colony counts. Am. J. Obstet. Gynecol. 2007, 197, 583.e1–583.e4. [Google Scholar] [CrossRef]
- Knight, K.M.; Thornburg, L.L.; McNanley, A.R.; Hardy, D.J.; Vicino, D.; Glantz, J.C. The effect of intrapartum clindamycin on vaginal group B streptococcus colony counts. J. Matern. Fetal Neonatal Med. 2012, 25, 747–749. [Google Scholar] [CrossRef]
- Hamel, M.S.; Has, P.; Datkhaeva, I.; Delacy, K.; Ciolfi, D.; Hughes, B. The Effect of Intrapartum Vancomycin on Vaginal Group B Streptococcus Colony Counts. Am. J. Perinatol. 2019, 36, 555–560. [Google Scholar] [CrossRef]
- Lodise, T.P.; Drusano, G.L. Use of pharmacokinetic/pharmacodynamic systems analyses to inform dose selection of tedizolid phosphate. Clin. Infect.Dis. 2014, 58 (Suppl. S1), S28–S34. [Google Scholar] [CrossRef]
- Performance Standards for Antimicrobial Susceptibility Testing: Twenty-Third Informational Supplement. CLSI Document M100-S23; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017.
- Vancomycin. NeoFax Online. IBM Micromedex [Database Online]. Truven Health Analytics/IBM Watson Health. 2020. Available online: https://www.micromedexsolutions.com (accessed on 18 January 2022).
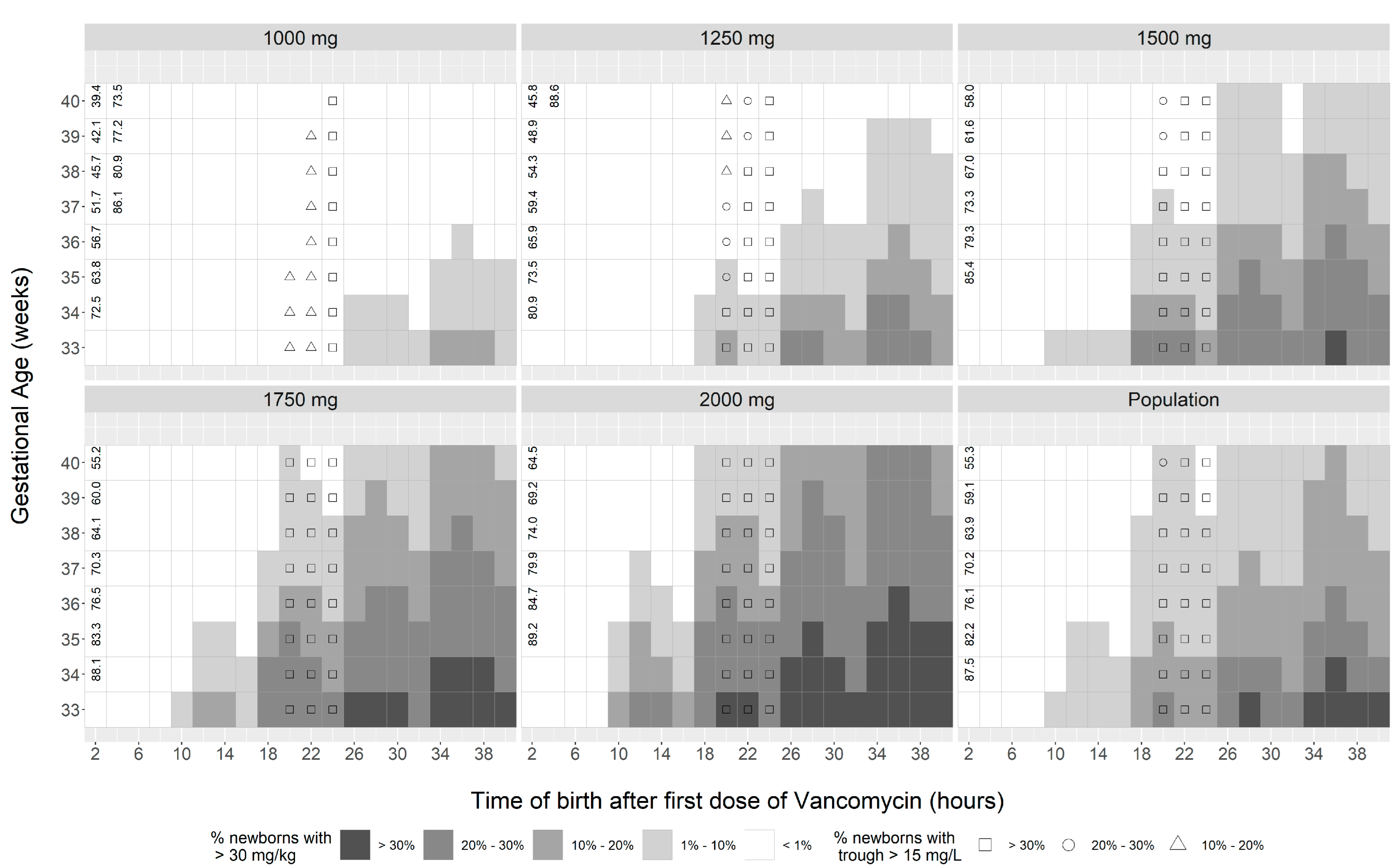
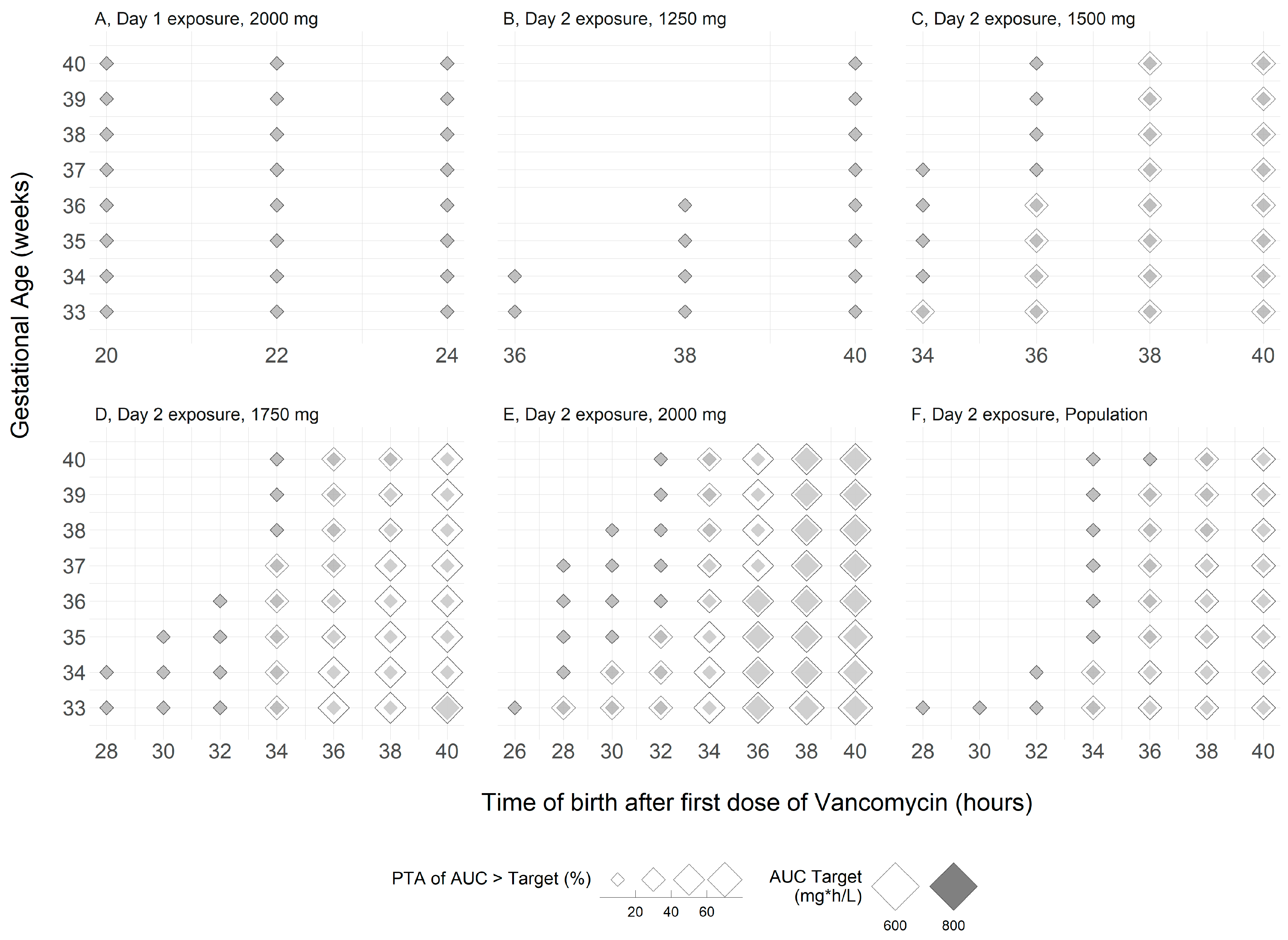
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, A.; Yassin, A. Sorting Out the Risks and Benefits of the #797 Recommended Intrapartum Vancomycin Dosing Approach. Antibiotics 2023, 12, 32. https://doi.org/10.3390/antibiotics12010032
Farkas A, Yassin A. Sorting Out the Risks and Benefits of the #797 Recommended Intrapartum Vancomycin Dosing Approach. Antibiotics. 2023; 12(1):32. https://doi.org/10.3390/antibiotics12010032
Chicago/Turabian StyleFarkas, Andras, and Arsheena Yassin. 2023. "Sorting Out the Risks and Benefits of the #797 Recommended Intrapartum Vancomycin Dosing Approach" Antibiotics 12, no. 1: 32. https://doi.org/10.3390/antibiotics12010032
APA StyleFarkas, A., & Yassin, A. (2023). Sorting Out the Risks and Benefits of the #797 Recommended Intrapartum Vancomycin Dosing Approach. Antibiotics, 12(1), 32. https://doi.org/10.3390/antibiotics12010032





