In Vitro and In Vivo Studies of Heraclenol as a Novel Bacterial Histidine Biosynthesis Inhibitor against Invasive and Biofilm-Forming Uropathogenic Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Challenge Organisms
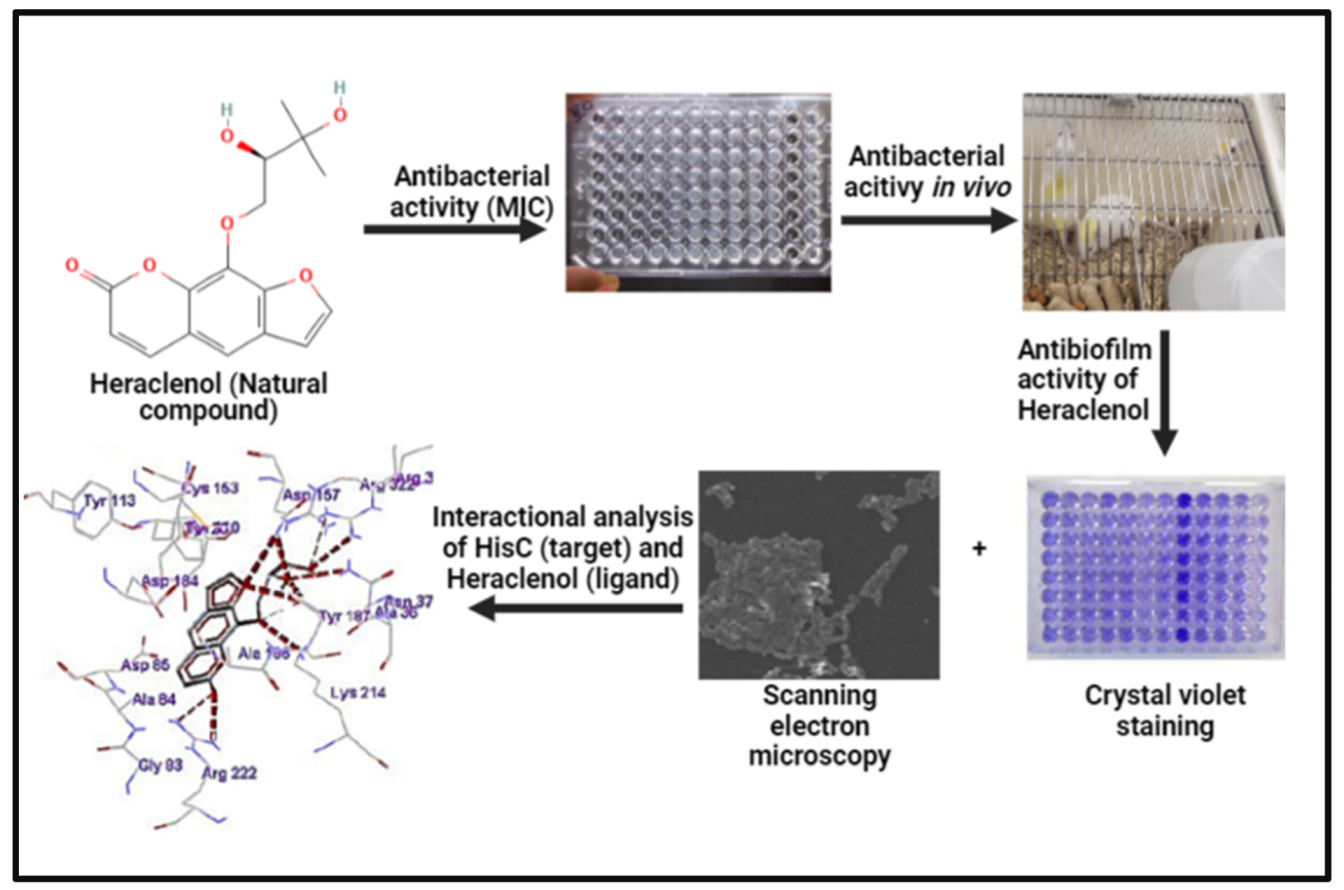
2.2. Minimum Inhibitory Concentration (MIC)
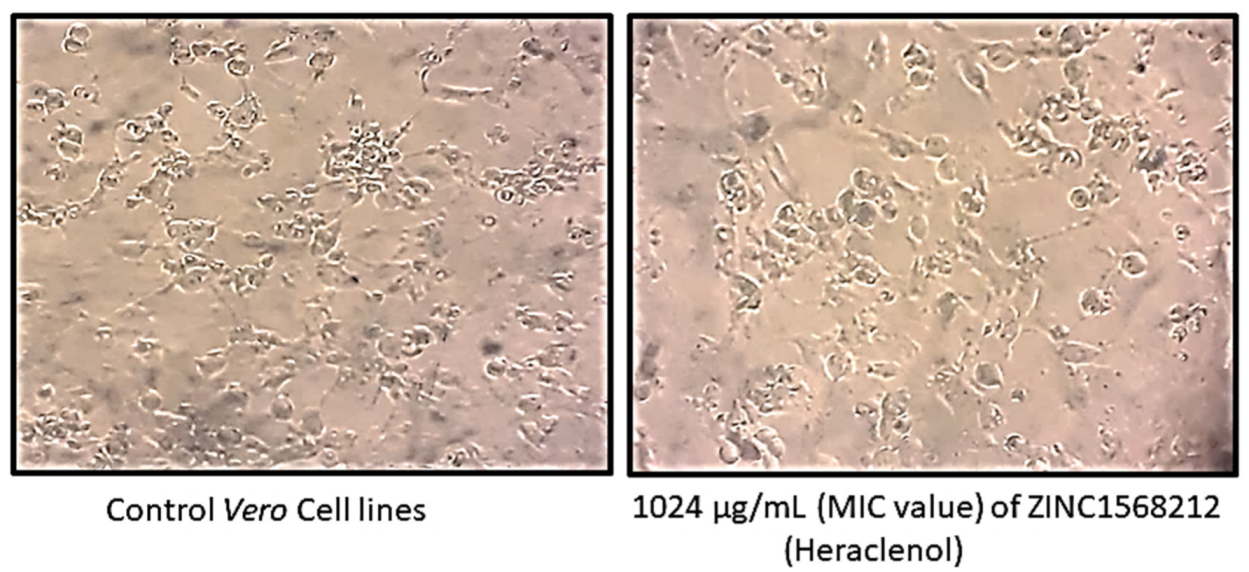
2.3. Cell Cytotoxicity Analysis Using MTT
2.4. In Vivo Assessment of the Efficacy of Heraclenol
2.5. Histopathological Analysis
2.6. Biofilm Formation and Treatment
2.7. Scanning Electron Microscopy (SEM)
2.8. Molecular Docking Studies for Analysis of Binding between Heraclenol and the HisC
2.9. Statistical Analysis
3. Results
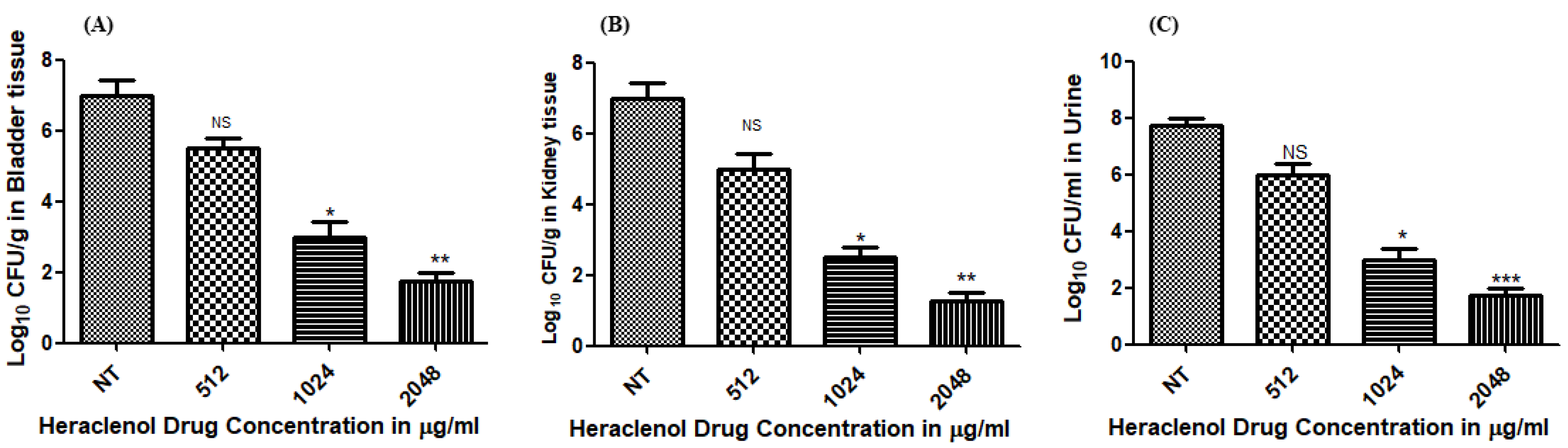
3.1. Antibacterial Activity of Heraclenol In Vivo
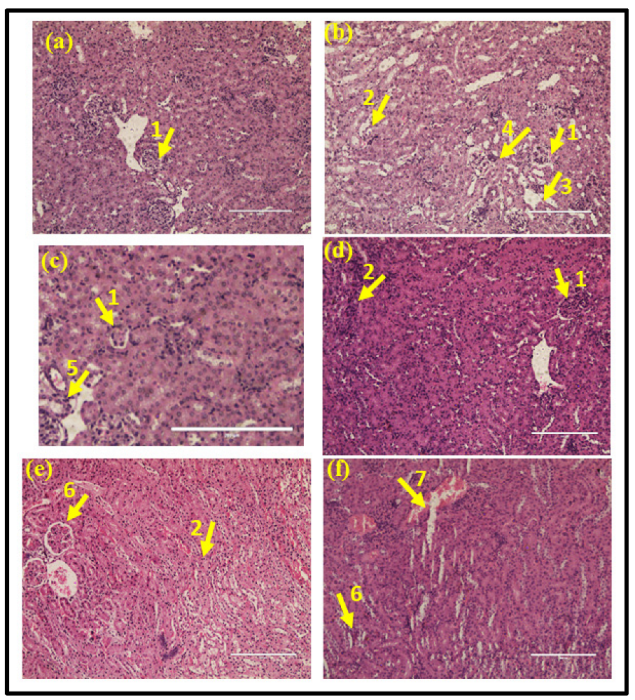
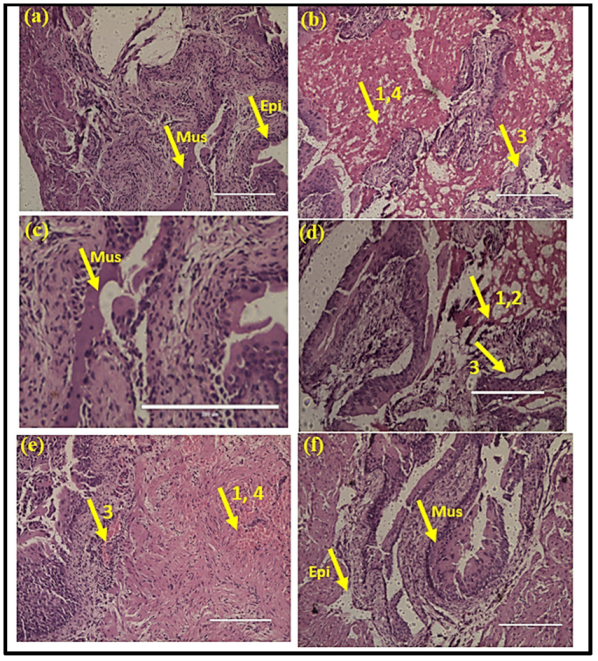
3.2. Histopathology Analysis of Kidney Tissue
3.3. Histopathology Analysis of the Bladder Tissue
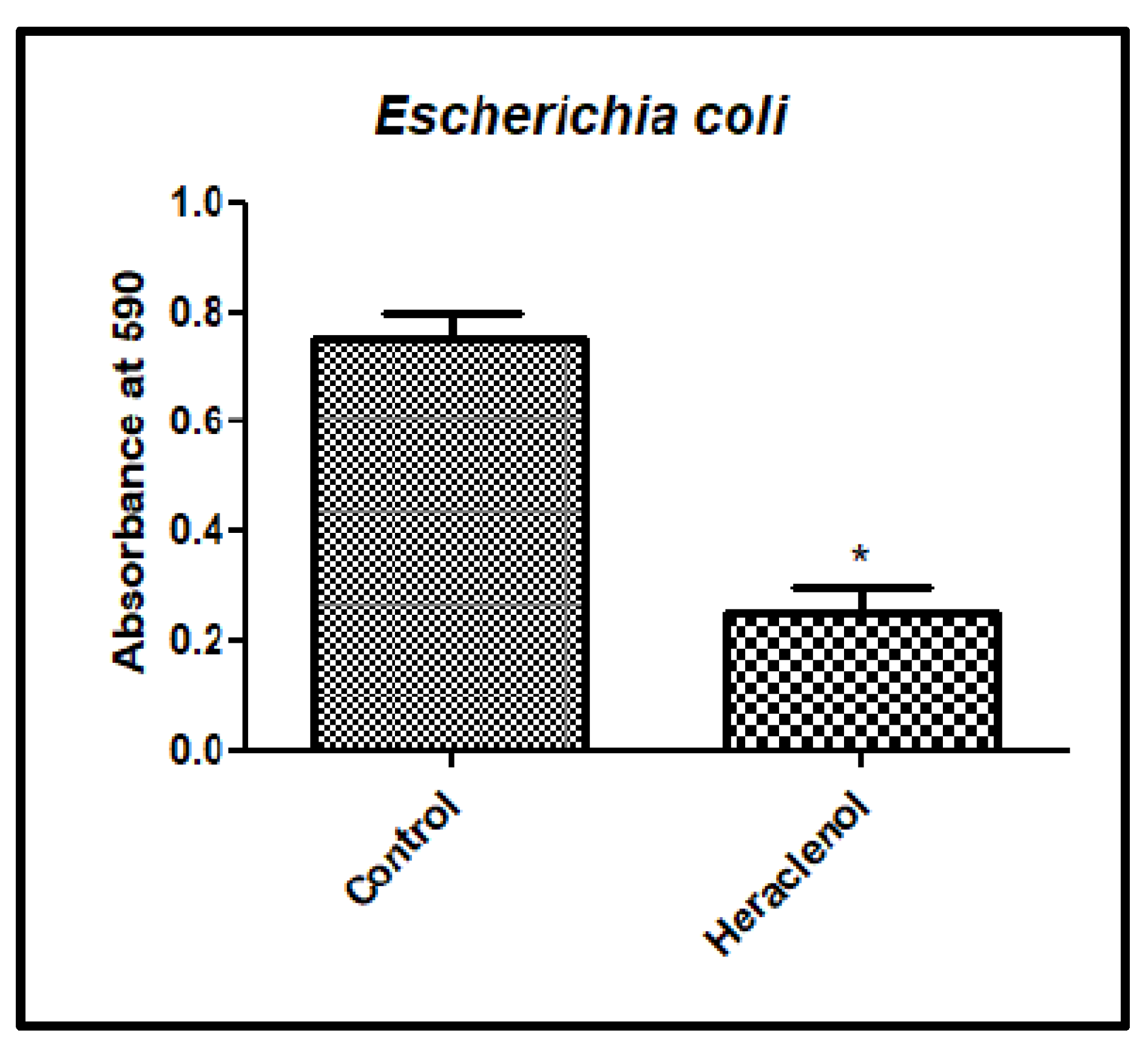
3.4. Antibiofilm Activity of Heraclenol
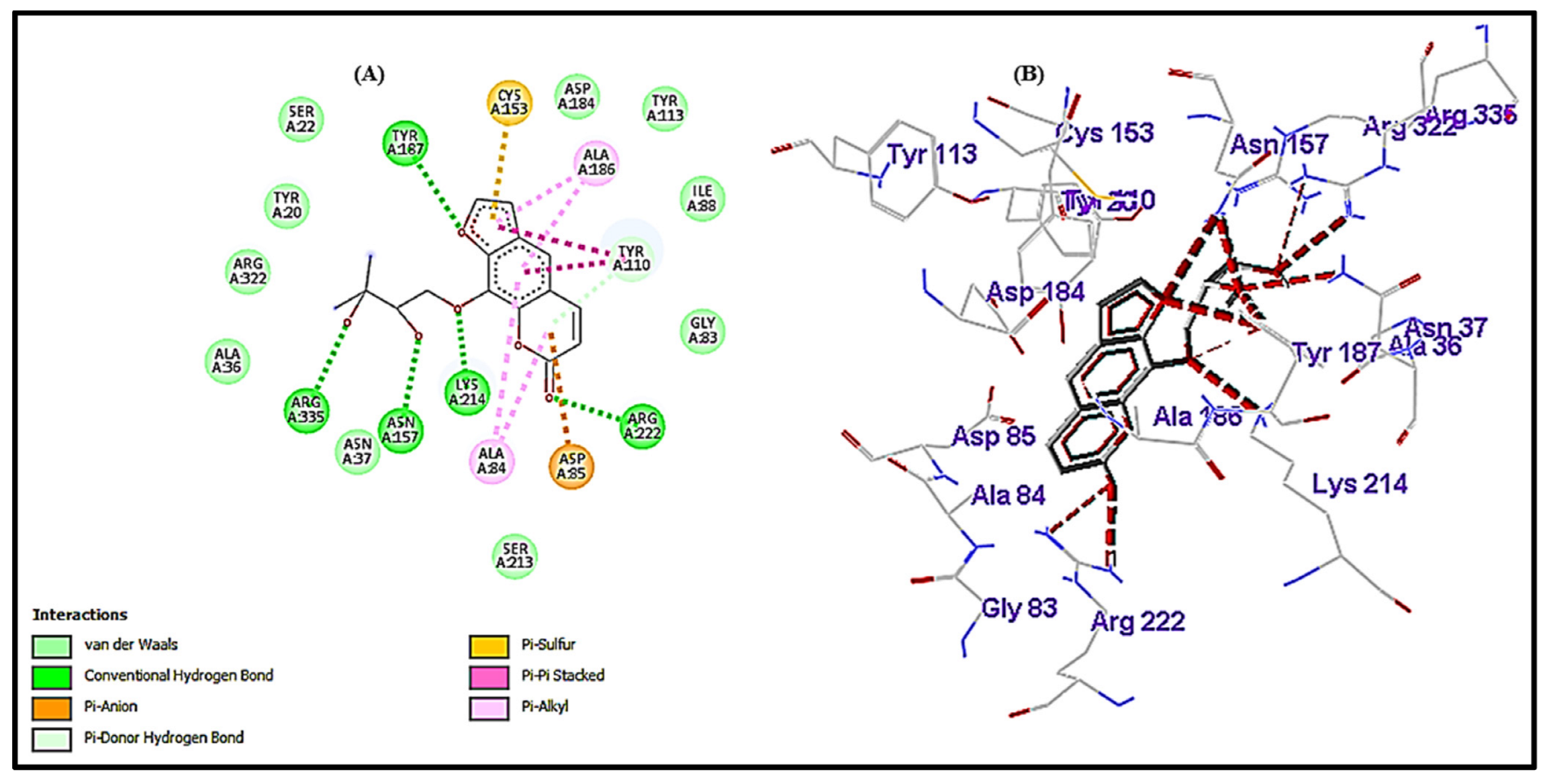
3.5. Interactional Analysis of HisC (Receptor) and Heraclenol (Ligand)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary Tract Infections: Epidemiology, Mechanisms of Infection and Treatment Options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Stamm, W.E.; Norrby, S.R. Urinary Tract Infections: Disease Panorama and Challenges. J. Infect. Dis. 2001, 183, S1–S4. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, P.; Nagappan, R. Extended Spectrum Beta -Lactamase, Biofilm-Producing Uropathogenic Pathogens and Their Antibiotic Susceptibility Patterns from Urinary Tract Infection- An Overview. Int. J. Microbiol. Res. 2013, 4, 101–118. [Google Scholar] [CrossRef]
- Emody, L.; Kerényi, M.; Nagy, G. Virulence Factors of Uropathogenic Escherichia Coli. Int. J. Antimicrob. Agents 2003, 22 (Suppl. 2), 29–33. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm Infections, Their Resilience to Therapy and Innovative Treatment Strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef]
- Zalewska-Piatek, B.M.; Wilkanowicz, S.I.; Piatek, R.J.; Kur, J.W. Biofilm Formation as a Virulence Determinant of Uropathogenic Escherichia Coli Dr+ Strains. Pol. J. Microbiol. 2009, 58, 223–229. [Google Scholar] [PubMed]
- Nahar, S.; Lim, H.; Kim, Y.; Ha, A.J.; Kumar, P.; Hong, S.; Rahaman, F.; Ha, S. Inhibitory Effects of Flavourzyme on Biofilm Formation, Quorum Sensing, and Virulence Genes of Foodborne Pathogens Salmonella Typhimurium and Escherichia Coli. Food Res. Int. 2021, 147, 110461. [Google Scholar] [CrossRef] [PubMed]
- Kaza, P.; Mahindroo, J.; Veeraraghavan, B.; Mavuduru, R.S.; Mohan, B.; Taneja, N. Evaluation of Risk Factors for Colistin Resistance among Uropathogenic Isolates of Escherichia Coli and Klebsiella Pneumoniae: A Case–Control Study. J. Med. Microbiol. 2019, 68, 837–847. [Google Scholar] [CrossRef]
- Kaur, H.; Kalia, M.; Taneja, N. Identification of Novel Non-Homologous Drug Targets against Acinetobacter Baumannii Using Subtractive Genomics and Comparative Metabolic Pathway Analysis. Microb. Pathog. 2021, 152, 104608. [Google Scholar] [CrossRef]
- Kaur, H.; Kalia, M.; Singh, V.; Modgil, V.; Mohan, B.; Taneja, N. In Silico Identification and Characterization of Promising Drug Targets in Highly Virulent Uropathogenic Escherichia Coli Strain CFT073 by Protein-Protein Interaction Network Analysis. Inform. Med. Unlocked 2021, 25, 100704. [Google Scholar] [CrossRef]
- Erdogan, I.; Akcelik, N.; Akcelik, M. Comparative Proteomic Analysis of Salmonella Typhimurium LT2 and Its HisG Gene Inactivated Mutant. Mol. Genet. Microbiol. Virol. 2015, 30, 48–56. [Google Scholar] [CrossRef]
- Pilatz, S.; Breitbach, K.; Hein, N.; Fehlhaber, B.; Schulze, J.; Brenneke, B.; Eberl, L.; Steinmetz, I. Identification of Burkholderia Pseudomallei Genes Required for the Intracellular Life Cycle and In Vivo Virulence. Infect. Immun. 2006, 74, 3576–3586. [Google Scholar] [CrossRef] [PubMed]
- Abdo, M.R.; Joseph, P.; Mortier, J.; Turtaut, F.; Montero, J.L.; Masereel, B.; Köhler, S.; Winum, J.Y. Anti-Virulence Strategy against Brucella Suis: Synthesis, Biological Evaluation and Molecular Modeling of Selective Histidinol Dehydrogenase Inhibitors. Org. Biomol. Chem. 2011, 9, 3681–3690. [Google Scholar] [CrossRef]
- Kaur, H.; Kalia, M.; Chaudhary, N.; Singh, V.; Kumar, V.; Modgil, V.; Kant, V.; Mohan, B.; Bhatia, A.; Taneja, N. Repurposing of FDA Approved Drugs against Uropathogenic Escherichia Coli: In Silico, in Vitro, and in Vivo Analysis. Microb. Pathog. 2022, 169, 105665. [Google Scholar] [CrossRef]
- Jha, B.; Kumar, D.; Sharma, A.; Dwivedy, A.; Singh, R.; Biswal, B.K. Identification and Structural Characterization of a Histidinol Phosphate Phosphatase from Mycobacterium Tuberculosis. J. Biol. Chem. 2018, 293, 10102–10118. [Google Scholar] [CrossRef]
- Stan, D.; Enciu, A.M.; Mateescu, A.L.; Ion, A.C.; Brezeanu, A.C.; Stan, D.; Tanase, C. Natural Compounds With Antimicrobial and Antiviral Effect and Nanocarriers Used for Their Transportation. Front. Pharmacol. 2021, 12, 2405. [Google Scholar] [CrossRef]
- Roy, P.K.; Song, M.G.; Park, S.Y. The Inhibitory Effect of Quercetin on Biofilm Formation of Listeria Monocytogenes Mixed Culture and Repression of Virulence. Antioxidants 2022, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Widelski, J.; Popova, M.; Graikou, K.; Glowniak, K.; Chinou, I. Coumarins from Angelica Lucida L.—Antibacterial Activities. Molecules 2009, 14, 2729–2734. [Google Scholar] [CrossRef]
- Kaur, H.; Kalia, M.; Singh, V.; Taneja, N. Identification of Novel Inhibitors against Escherichia Coli Utilizing HisC as a Target from Histidine Biosynthesis Pathway Identification of Novel Inhibitors against Escherichia Coli Utilizing HisC as a Target from Histidine Biosynthesis Pathway. J. Biomol. Struct. Dyn. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; CLSI Document M07-A9; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume 32, p. 18. [Google Scholar]
- Hoorn, C.; Paxton, C.G.M.; Crampton, W.G.R.; Burgess, P.; Marshall, L.G.; Lundberg, J.G.; Räsänen, M.E.; Linna, A.M. Miocene Deposits in the Amazonian Foreland Basin. Science 1996, 273, 122. [Google Scholar] [CrossRef]
- Mandal, S.M.; Migliolo, L.; Silva, O.N.; Fensterseifer, I.C.M.; Faria-Junior, C.; Dias, S.C.; Basak, A.; Hazra, T.K.; Franco, O.L. Controlling Resistant Bacteria with a Novel Class of β-Lactamase Inhibitor Peptides: From Rational Design to in Vivo Analyses. Sci. Rep. 2014, 4, 6015. [Google Scholar] [CrossRef]
- Chockalingam, A.; Stewart, S.; Xu, L.; Gandhi, A.; Matta, M.K.; Patel, V.; Sacks, L.; Rouse, R. Evaluation of Immunocompetent Urinary Tract Infected Balb/C Mouse Model For the Study of Antibiotic Resistance Development Using Escherichia Coli CFT073 Infection. Antibiotics 2019, 8, 170. [Google Scholar] [CrossRef]
- Vaara, M.; Vaara, T.; Vingsbo Lundberg, C. Polymyxin Derivatives NAB739 and NAB815 Are More Effective than Polymyxin B in Murine Escherichia Coli Pyelonephritis. J. Antimicrob. Chemother. 2018, 73, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Mohankumar, R.; Kannappan, A.; Karthick Raja, V.; Archunan, G.; Karutha Pandian, S.; Ruckmani, K.; Veera Ravi, A. Exploring the Anti-Quorum Sensing and Antibiofilm Efficacy of Phytol against Serratia Marcescens Associated Acute Pyelonephritis Infection in Wistar Rats. Front. Cell. Infect. Microbiol. 2017, 7, 498. [Google Scholar] [CrossRef]
- Kerekes, E.-B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-Biofilm Forming and Anti-Quorum Sensing Activity of Selected Essential Oils and Their Main Components on Food-Related Micro-Organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef]
- Steffen, C.; Thomas, K.; Huniar, U.; Hellweg, A.; Rubner, O.; Schroer, A. Autodock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexiblity. J. Comput. Chem. 2010, 31, 2967–2970. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Sivaraman, J.; Li, Y.; Larocque, R.; Schrag, J.D.; Cygler, M.; Matte, A. Crystal Structure of Histidinol Phosphate Aminotransferase (HisC) from Escherichia Coli, and Its Covalent Complex with Pyridoxal-5′-Phosphate and L-Histidinol Phosphate. J. Mol. Biol. 2001, 311, 761–776. [Google Scholar] [CrossRef]
- ProteinDataBank RCSB Protein Data Bank—RCSB PDB RCSB Protein Data Bank—RCSB PDB. Hum. Serum Albumin 2013. Available online: https://www.rcsb.org/ (accessed on 8 December 2022).
- Luo, C.; Hu, G.Q.; Zhu, H. Genome Reannotation of Escherichia Coli CFT073 with New Insights into Virulence. BMC Genom. 2009, 10, 552. [Google Scholar] [CrossRef]
- Dars, C. Improving the Genome Reference Assembly for E. coli CFT073 and in Silico Detection of Potential Antibiotic Resistance Genes. Genome 2020, 21, 105861. [Google Scholar]
- de Souza, S.M.; Monache, F.D.; Smânia, A. Antibacterial Activity of Coumarins. Z. Naturforsch. C 2005, 60, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D.H. The Naturally Occurring Coumarins. Progress in the Chemistry of Organic Natural Products; Springer: Berlin/Heidelberg, Germany, 2002; Volume 83, pp. 1–619. [Google Scholar]
- Kalia, M.; Yadav, V.K.; Singh, P.K.; Sharma, D.; Narvi, S.S.; Agarwal, V. Exploring the Impact of Parthenolide as Anti-Quorum Sensing and Anti-Biofilm Agent against Pseudomonas Aeruginosa. Life Sci. 2018, 199, 96–103. [Google Scholar] [CrossRef]
- Girennavar, B.; Cepeda, M.L.; Soni, K.A.; Vikram, A.; Jesudhasan, P.; Jayaprakasha, G.K.; Pillai, S.D.; Patil, B.S. Grapefruit Juice and Its Furocoumarins Inhibits Autoinducer Signaling and Biofilm Formation in Bacteria. Int. J. Food Microbiol. 2008, 125, 204–208. [Google Scholar] [CrossRef]
- Shailesh Kumar, M.C. Role of CSE1034 in Escherichia Coli Biofilm Destruction. J. Microb. Biochem. Technol. 2013, 5, 54–58. [Google Scholar] [CrossRef]
- Mottaghipisheh, J.; Nové, M.; Spengler, G.; Kúsz, N.; Hohmann, J.; Csupor, D. Antiproliferative and Cytotoxic Activities of Furocoumarins of Ducrosia Anethifolia. Pharm. Biol. 2018, 56, 658–664. [Google Scholar] [CrossRef] [PubMed]

| Sr. No. | Concentration (µg/mL) | Absorbance 595 nm | % Cell Viability |
|---|---|---|---|
| 1 | 4096 | 0.7681 | 87.67 |
| 2 | 2048 | 0.7603 | 86.78 |
| 3 | 1024 | 0.7675 | 87.60 |
| 4 | 512 | 0.8253 | 94.20 |
| 5 | Control Cell | 0.8761 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, H.; Chaudhary, N.; Modgil, V.; Kalia, M.; Kant, V.; Mohan, B.; Bhatia, A.; Taneja, N. In Vitro and In Vivo Studies of Heraclenol as a Novel Bacterial Histidine Biosynthesis Inhibitor against Invasive and Biofilm-Forming Uropathogenic Escherichia coli. Antibiotics 2023, 12, 110. https://doi.org/10.3390/antibiotics12010110
Kaur H, Chaudhary N, Modgil V, Kalia M, Kant V, Mohan B, Bhatia A, Taneja N. In Vitro and In Vivo Studies of Heraclenol as a Novel Bacterial Histidine Biosynthesis Inhibitor against Invasive and Biofilm-Forming Uropathogenic Escherichia coli. Antibiotics. 2023; 12(1):110. https://doi.org/10.3390/antibiotics12010110
Chicago/Turabian StyleKaur, Harpreet, Naveen Chaudhary, Vinay Modgil, Manmohit Kalia, Vishal Kant, Balvinder Mohan, Alka Bhatia, and Neelam Taneja. 2023. "In Vitro and In Vivo Studies of Heraclenol as a Novel Bacterial Histidine Biosynthesis Inhibitor against Invasive and Biofilm-Forming Uropathogenic Escherichia coli" Antibiotics 12, no. 1: 110. https://doi.org/10.3390/antibiotics12010110
APA StyleKaur, H., Chaudhary, N., Modgil, V., Kalia, M., Kant, V., Mohan, B., Bhatia, A., & Taneja, N. (2023). In Vitro and In Vivo Studies of Heraclenol as a Novel Bacterial Histidine Biosynthesis Inhibitor against Invasive and Biofilm-Forming Uropathogenic Escherichia coli. Antibiotics, 12(1), 110. https://doi.org/10.3390/antibiotics12010110







