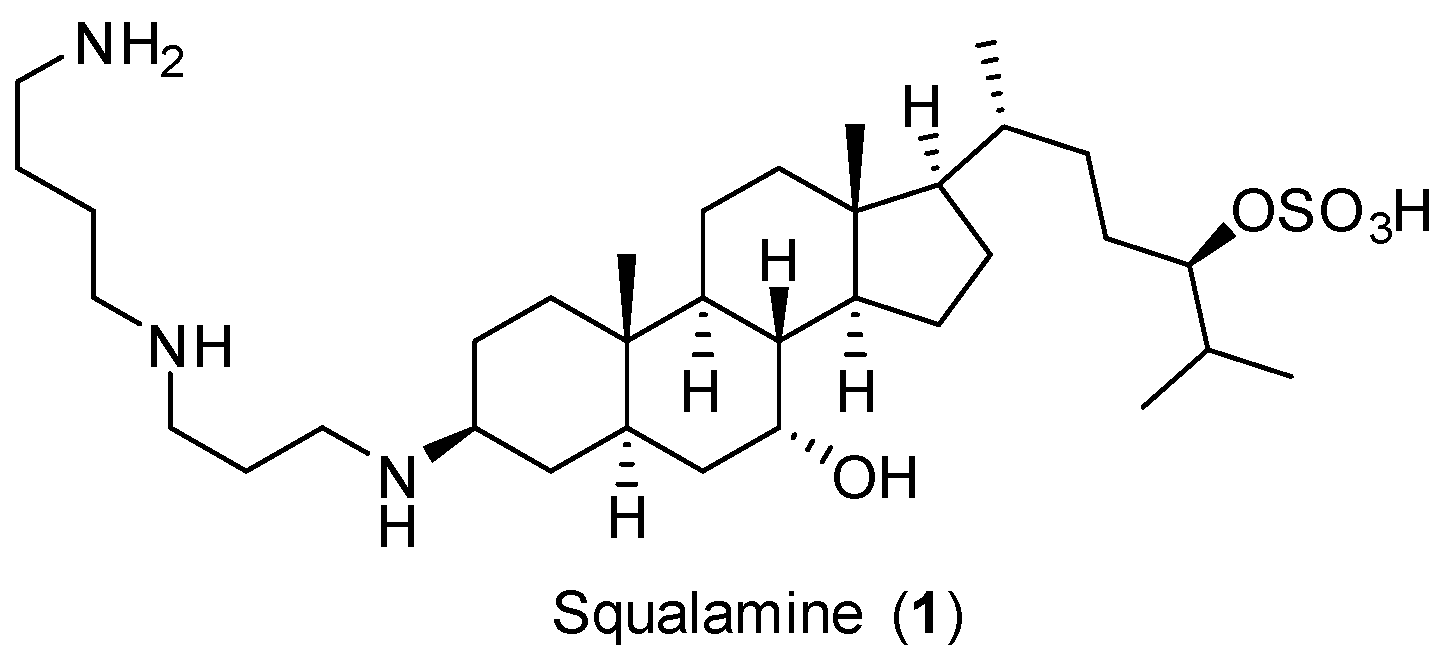
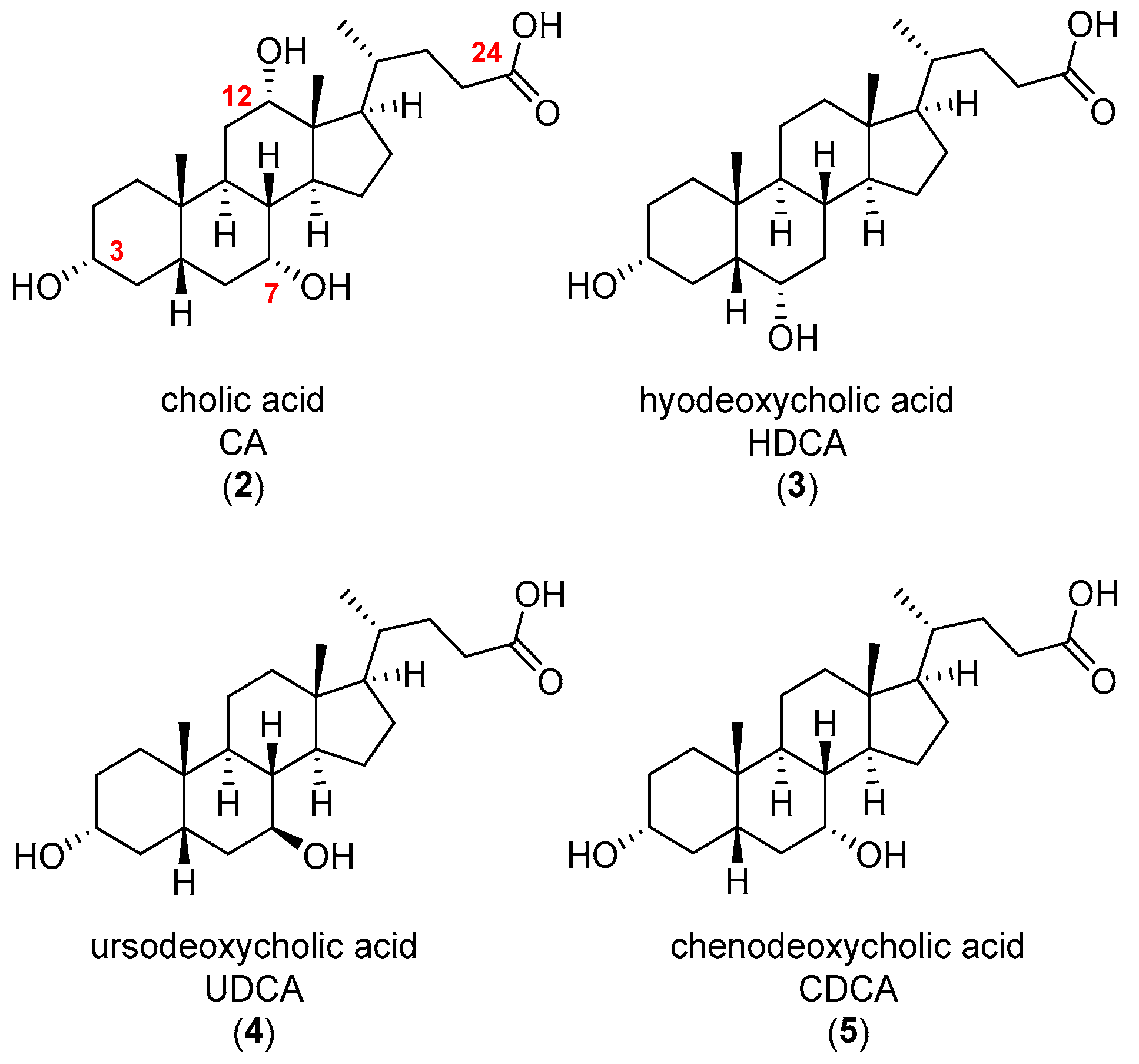
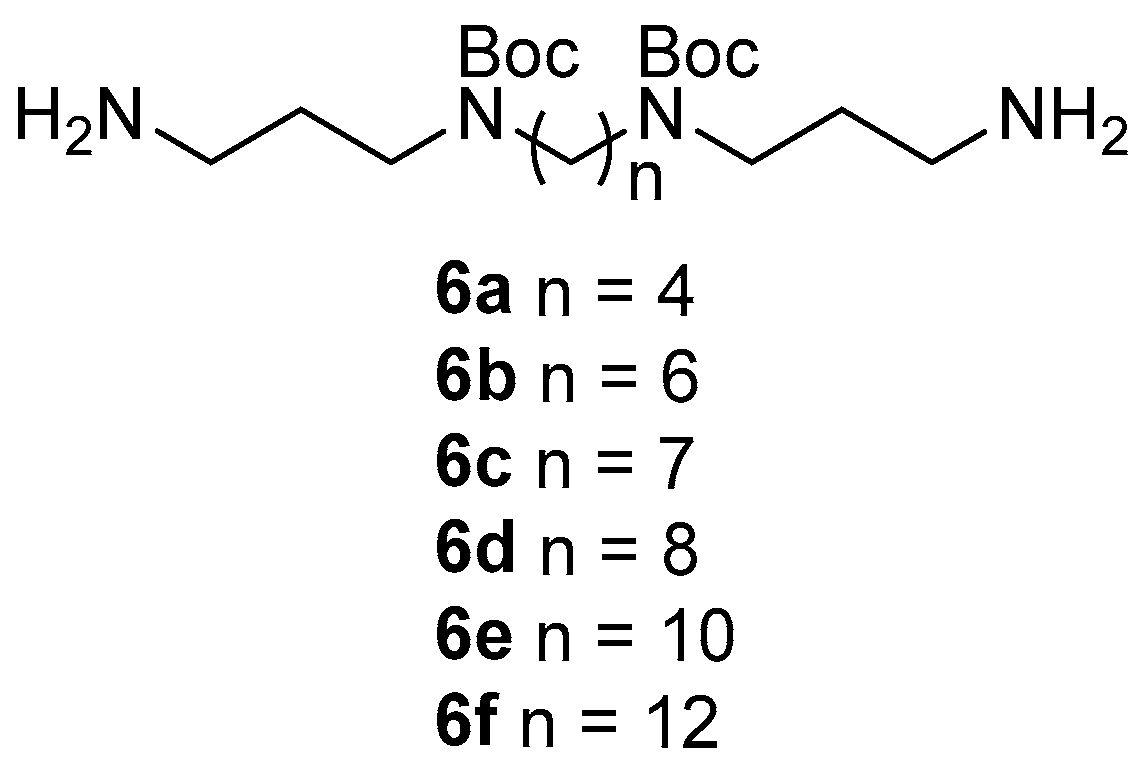
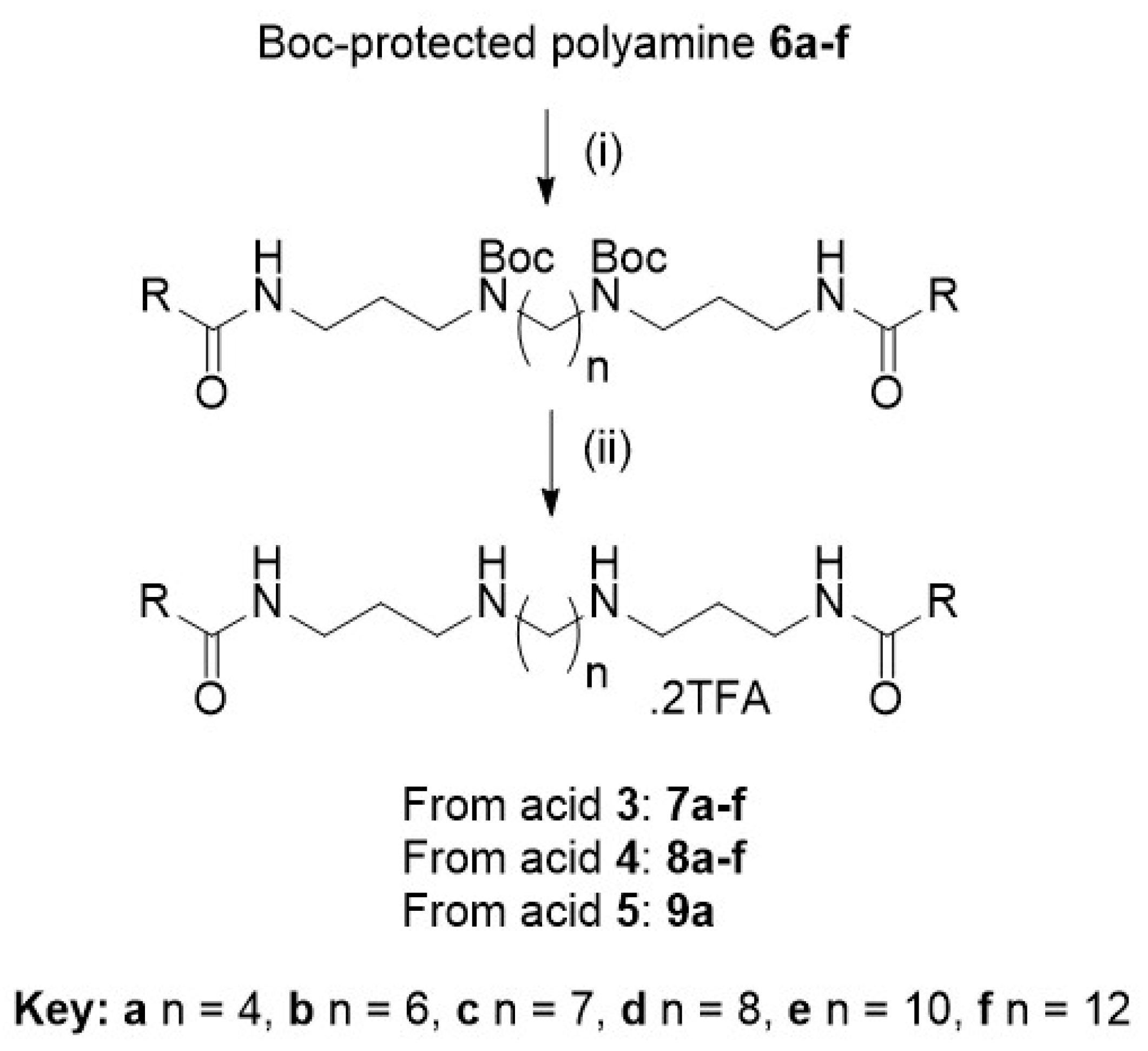
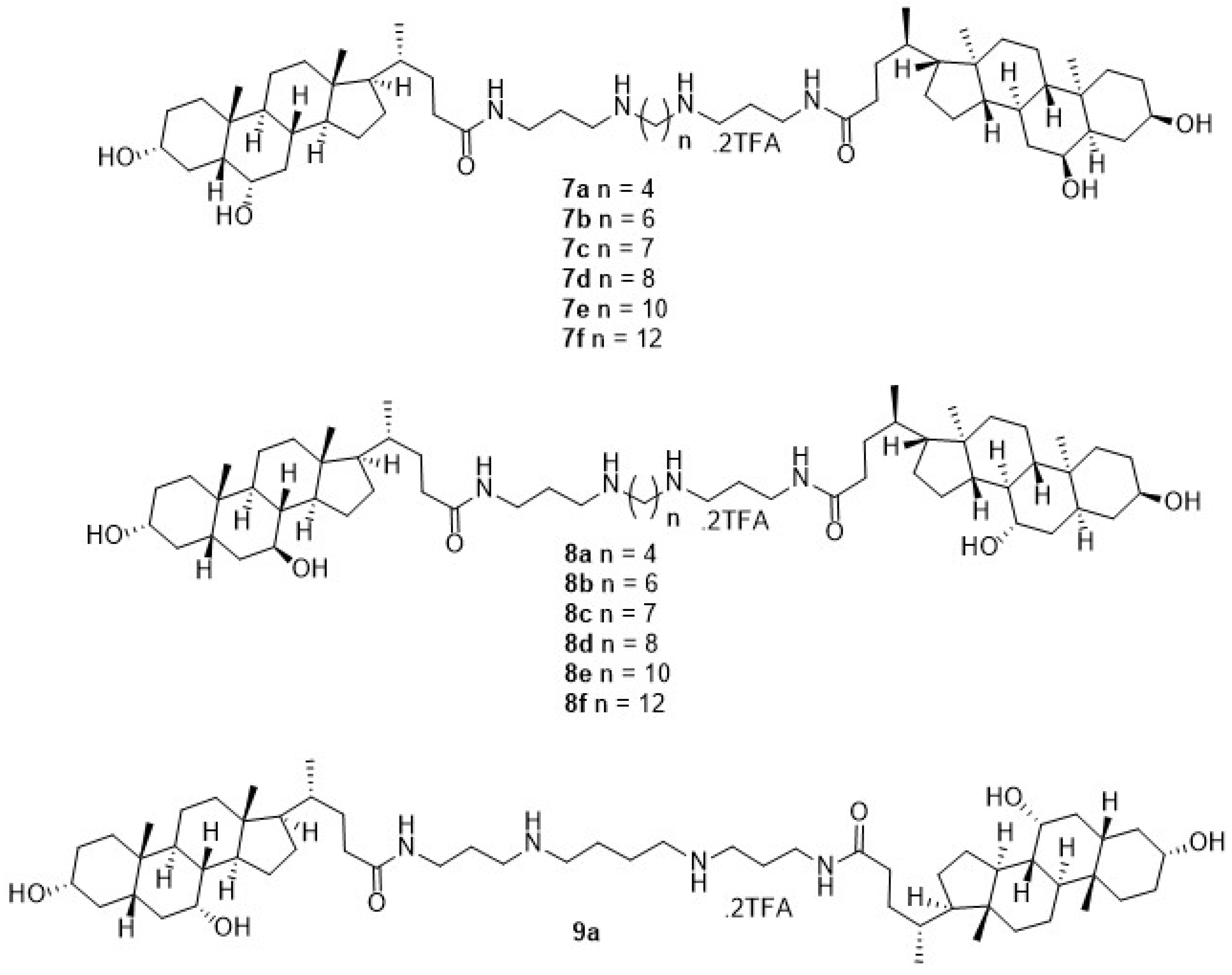
3.1. Chemical Synthesis General Methods
Mass spectra were recorded using a MicrOTOF-QII mass spectrometer (Bruker Daltonics, Bremen, Germany) coupled with a KD Scientific syringe pump, with analysis using Bruker Compass DataAnalysis v 4.1 software. Infrared spectra were recorded on a Perkin Elmer Spectrum 100 Fourier Transform infrared spectrometer (Waltham, MA, USA) equipped with a universal ATR accessory. Optical rotations were obtained with a Rudolph Analytical (Hackettstown, NJ, USA) Autopol IV automatic polarimeter using a 0.1 dm cell (concentration units of g/100 mL). All NMR spectra were recorded using a Bruker (Karlsruhe, Germany) Avance 400 spectrometer operating at 400.13 MHz for
1H nuclei and 100.62 MHz for
13C nuclei. Chemical shifts are expressed in parts per million (ppm) relative to the solvent peaks (DMSO-
d6:
1H 2.50,
13C 39.52 ppm). Assignments are based on 1- and 2-dimensional NMR experiments and analogue comparisons. Standard Bruker pulse sequences were utilized. Reversed-phase flash column chromatography was carried out using LiChroPrep RP-8 (40–63 μm) (Merck Millipore, Darmstadt, Germany). Analytical thin layer chromatography (TLC) was carried out on 0.2 mm thick plates of Merck DC Kieselgel 60 RP-18 F254S plates. All solvents were of analytical grade or better and/or purified according to standard procedures. Chemical reagents used were purchased from standard chemical suppliers and used as purchased. Protected polyamines di-
tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (
6a) di-
tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (
6b), di-
tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (
6c), di-
tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (
6d), di-
tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (
6e), and di-
tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (
6f) were synthesized using literature procedures [
27,
28,
29,
30].
3.1.1. General Procedure A: Amide Bond Formation for Hyodeoxycholic Acid Derivatives 7a–f
To a solution of hyodeoxycholic acid (0.050 g, 0.127 mmol) in dry DMF (1 mL) was added Boc-protected polyamine (0.063 mmol) in dry DMF (1 mL), PyBop (0.073 g, 0.140 mmol) and DIPEA (0.067 mL, 0.382 mmol). The solution was allowed to stir for 18 h at rt under N2 atmosphere. The resulting solution was added to EtOAc (20 mL) and was washed with H2O (5 × 20 mL), then dried under reduced pressure and the crude product was subjected to diol-bonded silica column chromatography (CH2Cl2/MeOH, 80:20→90:10) to yield the desired Boc-protected hyodeoxycholic acid derivative.
3.1.2. General Procedure B: Amide Bond Formation for Cholic Acid Derivatives 8a–f and 9a
To an ice-cold solution of the appropriate cholic acid (2 equiv.) in dry DMF (1 mL) was added Boc-protected polyamine (1 equiv.), HOBt (1 equiv.) and DIPEA (4 equiv.). The solution was stirred for 10 min at 0 °C under N2 atmosphere. EDC·HCl (3 equiv.) in dry DMF (1 mL) was added to the solution and the resulting mixture was left to stir for 18 h at rt under N2 atmosphere. The resulting solution was added to EtOAc (20 mL) and washed with H2O (2 × 20 mL). The organic layer was dried under reduced pressure and the crude product was purified with silica gel column chromatography (CH2Cl2/MeOH, 80:20→90:10) to afford the desired Boc-protected product.
3.1.3. General Procedure C: Boc Deprotection
A solution of the tert-butyl-carbamate derivative was stirred in CH2Cl2 (2 mL) with TFA (0.2 mL) at room temperature under N2 for 2 h, then dried under reduced pressure. The crude product was purified using C8 reversed-phase column chromatography (MeOH (+0.05% TFA):H2O (+0.05% TFA), 50:50→100:0) to afford the product as the di-TFA salt.
3.1.4. N1,N4-Bis(3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (7a)
Following general procedure A, reaction of hyodeoxycholic acid (0.050 g, 0.127 mmol) with di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (6a) (0.026 g, 0.0637 mmol), PyBop (0.073 g, 0.140 mmol) and DIPEA (0.067 mL, 0.382 mmol) in DMF (2 mL) afforded di-tert-butyl butane-1,4-diylbis((3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.056 g, 76%) as a clear colorless oil. Following general procedure C, a sub-sample of the product (0.047 g, 0.0408 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 7a (0.034 g, 71%) as a pale-yellow oil. [α = +3 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.17; IR (ATR) νmax 3339, 2941, 1635, 1455, 1202, 1139, 1027 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.63 (4H, br s, NH2-29), 8.01 (2H, t, J = 5.7 Hz, NH-25), 3.84–3.79 (2H, m, H-6), 3.35–3.27 (2H, m, H-3), 3.09 (4H, q, J = 6.3 Hz, H2-26), 2.90–2.87 (8H, m, H2-28, H2-30), 2.11–2.08 (2H, m, H2-23b), 1.99–-1.95 (2H, m, H2-23a), 1.92–0.93 (56H, m, H2-1, H2-2, H2-4, H-5, H2-7, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31), 0.87 (6H, d, J = 6.4 Hz, H3-21), 0.83 (6H, s, H3-19), 0.59 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.2 (C-24), 70.0 (C-3), 65.9 (C-6), 55.9 (C-14), 55.5 (C-17), 48.3 (C-5), 46.1 (C-30), 44.6 (C-28), 42.4 (C-13), 39.5 (C-9, obscured by solvent), 39.4 (C-12, obscured by solvent), 35.5, 35.4 (C-1, C-26), 35.0, 34.9 (C-7, C-8, C-10), 34.4 (C-20), 32.3 (C-23), 31.5 (C-22), 30.3 (C-4), 29.3 (C-2), 27.7 (C-16), 26.1 (C-27), 23.9 (C-15), 23.6 (C-19), 22.7 (C-31), 20.4 (C-11), 18.3 (C-21), 11.9 (C-18); (+)-HRESIMS [M + H]+ m/z 951.7873 (calcd for C58H103N4O6, 951.7872).
3.1.5. N1,N6-Bis(3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (7b)
Following general procedure A, reaction of hyodeoxycholic acid (0.050 g, 0.127 mmol) with di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (6b) (0.027 g, 0.0637 mmol), PyBop (0.073 g, 0.140 mmol) and DIPEA (0.067 mL, 0.382 mmol) in DMF (2 mL) afforded di-tert-butyl hexane-1,6-diylbis((3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.014 g, 19%) as a clear colorless oil. Following general procedure C, a sub-sample of the product (0.013 g, 0.0110 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 7b (0.011 g, 83%) as a clear colorless oil. [α = +4 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.14; IR (ATR) νmax 2938, 1676, 1204, 1137, 1055, 1033 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.42 (4H, br s, NH2-29), 7.98 (2H, t, J = 5.8 Hz, NH-25), 3.84–3.81 (2H, m, H-6), 3.32 (2H, obscured by H2O, H-3), 3.10 (4H, q, J = 6.3 Hz, H2-26), 2.86 (8H, br s, H2-28, H2-30), 2.11–2.07 (2H, m, H2-23b), 2.02–1.96 (2H, m, H2-23a), 1.94–0.91 (60H, m, H2-1, H2-2, H2-4, H-5, H2-7, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32), 0.88 (6H, d, J = 6.5 Hz, H3-21), 0.83 (6H, s, H3-19), 0.60 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 69.9 (C-3), 65.8 (C-6), 55.9 (C-14), 55.5 (C-17), 48.2 (C-5), 46.6 (C-30), 44.6 (C-28), 42.3 (C-13), 39.4 (C-12, obscured by solvent), 39.3 (C-9, obscured by solvent), 35.5, 35.4 (C-1, C-26), 34.9, 34.8 (C-7, C-8, C-10), 34.3 (C-20), 32.2 (C-23), 31.5 (C-22), 30.3 (C-4), 29.2 (C-2), 27.7 (C-16), 26.2 (C-27), 25.5 (C-31), 25.3 (C-32), 23.8 (C-15), 23.5 (C-19), 20.4 (C-11), 18.3 (C-21), 11.9 (C-18); (+)-HRESIMS [M + H]+ m/z 979.8185 (calcd for C60H107N4O6, 979.8185).
3.1.6. N1,N7-Bis(3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (7c)
Following general procedure A, reaction of hyodeoxycholic acid (0.050 g, 0.127 mmol) with di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (6c) (0.028 g, 0.0637 mmol), PyBop (0.073 g, 0.140 mmol) and DIPEA (0.067 mL, 0.382 mmol) in DMF (2 mL) afforded di-tert-butyl heptane-1,7-diylbis((3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.061 g, 80%) as a clear oil. Following general procedure C, a sub-sample of the product (0.045 g, 0.0377 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 7c (0.041, 89%) as a pale-yellow oil. [α= +2 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.13; IR (ATR) νmax 3361, 2931, 1675, 1202, 1135, 1038, 1026, 993 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.52 (4H, br s, NH2-29), 8.00 (2H, t, J = 5.8 Hz, NH-25), 3.83–3.80 (2H, m, H-6), 3.35–3.25 (4H, m, H-3, H-7), 3.10 (4H, q, J = 6.3 Hz, H2-26), 2.89–2.82 (8H, m, H2-28, H2-30), 2.15–2.07 (2H, m, H2-23b), 2.02–1.97 (2H, m, H-23a), 1.94–0.92 (62H, m, H2-1, H2-2, H2-4, H-5, H2-7, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33), 0.89 (6H, d, J = 6.5 Hz, H3-21), 0.87 (6H, s, H3-19), 0.59 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.2 (C-24), 70.0 (C-3), 65.9 (C-6), 55.9 (C-14), 55.5 (C-17), 48.3 (C-5), 46.7 (C-30), 44.6 (C-28), 42.4 (C-13), 39.4 (C-9, obscured by solvent), 39.3 (C-12, obscured by solvent), 35.6, 35.5 (C-1, C-26), 35.0 (C-7), 34.9 (C-8, C-10), 34.4 (C-20), 32.3 (C-23), 31.5 (C-22), 30.3 (C-4), 29.3 (C-2), 28.0 (C-33), 27.7 (C-16), 26.1 (C-27), 25.8 (C-32), 25.4 (C-31), 23.9 (C-15), 23.6 (C-19), 20.4 (C-11), 18.3 (C-21), 11.9 (C-18); (+)-HRESIMS [M + H]+ m/z 993.8342 (calcd for C61H109N4O6, 993.8342).
3.1.7. N1,N8-Bis(3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (7d)
Following general procedure A, reaction of hyodeoxycholic acid (0.050 g, 0.127 mmol) with di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (6d) (0.029 g, 0.0637 mmol), PyBop (0.073 g, 0.140 mmol) and DIPEA (0.067 mL, 0.382 mmol) in DMF (2 mL) afforded di-tert-butyl octane-1,8-diylbis((3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.074 g, 96%) as a clear colorless oil. Following general procedure C, the product (0.074 g, 0.0613 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 7d (0.063 g, 83%) as a pale-yellow oil. [α = +5 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.13; IR (ATR) νmax 3343, 2938, 2865, 1635, 1456, 1377, 1201, 1136, 1028, 800, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.55 (4H, br s, NH2-29), 8.01 (2H, t, J = 5.5 Hz, NH-25), 3.84–3.79 (2H, m, H-6), 3.35–3.28 (2H, m, H-3), 3.09 (4H, q, J = 6.0 Hz, H2-26), 2.85 (8H, br s, H2-28, H2-30), 2.14–2.07 (2H, m, H2-23b), 2.01–1.96 (2H, m, H2-23a), 1.94–0.91 (64H, m, H2-1, H2-2, H2-4, H-5, H2-7, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33), 0.87 (6H, d, J = 6.2 Hz, H3-21), 0.83 (6H, s, H3-19), 0.59 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.4 (C-24), 70.1 (C-3), 66.0 (C-6), 56.0 (C-14), 55.6 (C-17), 48.3 (C-5), 46.8 (C-30), 44.7 (C-28), 42.4 (C-13), 39.5 (C-12, obscured by solvent), 39.4 (C-9, obscured by solvent), 35.6, 35.5 (C-1, C-26), 35.0, 34.9 (C-7, C-8, C-10), 34.4 (C-20), 32.3 (C-23), 31.6 (C-22), 30.4 (C-4), 29.3 (C-2), 28.4 (C-33), 27.8 (C-16), 26.2 (C-27), 25.9 (C-32), 25.5 (C-31), 23.9 (C-15), 23.6 (C-19), 20.5 (C-11), 18.3 (C-21), 11.9 (C-18); (+)-HRESIMS [M + 2H]2+ m/z 504.4286 (calcd for C62H112N4O6, 504.4285).
3.1.8. N1,N10-Bis(3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (7e)
Following general procedure A, reaction of hyodeoxycholic acid (0.050 g, 0.127 mmol) with di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (6e) (0.031 g, 0.0637 mmol), PyBop (0.073 g, 0.140 mmol) and DIPEA (0.067 mL, 0.382 mmol) in DMF (2 mL) afforded di-tert-butyl decane-1,10-diylbis((3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.065 g, 83%) as a clear colorless oil. Following general procedure C, a sub-sample of the product (0.013 g, 0.0105 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 7e (0.011 g, 83%) as a pale-yellow oil. [α = +4 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.10; IR (ATR) νmax 3333, 2935, 2864, 1674, 1456, 1202, 1137, 1028, 721 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.40 (4H, br s, NH2-29), 8.00 (2H, t, J = 5.5 Hz, NH-25), 3.84–3.80 (2H, m, H-6), 3.34–3.29 (2H, m, H-3), 3.10 (4H, q, J = 6.1 Hz, H2-26), 2.85 (8H, br s, H2-28, H2-30), 2.14–2.07 (2H, m, H2-23b), 2.02–1.97 (2H, m, H2-23a), 1.94–0.92 (68H, m, H2-1, H2-2, H2-4, H-5, H2-7, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33, H2-34), 0.88 (6H, d, J = 6.1 Hz, H3-21), 0.83 (6H, s, H3-19), 0.60 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 70.0 (C-3), 65.9 (C-6), 55.9 (C-14), 55.5 (C-17), 48.2 (C-5), 46.8 (C-30), 44.5 (C-28), 42.4 (C-13), 39.5 (C-12 and C-9, obscured by solvent), 35.5, 35.4 (C-1, C-26), 35.0, 34.9 (C-7, C-8, C-10), 34.3 (C-20), 32.3 (C-23), 31.5 (C-22), 30.3 (C-4), 29.3 (C-2), 28.8 (C-34), 28.6 (C-33), 27.7 (C-16), 26.2 (C-27), 25.9 (C-32), 25.5 (C-31), 23.9 (C-15), 23.5 (C-19), 20.4 (C-11), 18.3 (C-21), 11.9 (C-18); (+)-HRESIMS [M + 2H]2+ m/z 518.4441 (calcd for C64H116N4O6, 518.4442).
3.1.9. N1,N12-Bis(3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (7f)
Following general procedure A, reaction of hyodeoxycholic acid (0.050 g, 0.127 mmol) with di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (6f) (0.033 g, 0.0637 mmol), PyBop (0.073 g, 0.140 mmol) and DIPEA (0.067 mL, 0.382 mmol) in DMF (2 mL) afforded di-tert-butyl dodecane-1,12-diylbis((3-((R)-4-((3R,5R,6S,8S,9S,10R,13R,14S,17R)-3,6-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.010 g, 12%) as a clear colorless oil. Following general procedure C, the product (0.010 g, 0.00791 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 7f (0.008 g, 78%) as a pale-yellow oil. [α= +5 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.09; IR (ATR) νmax 3340, 2932, 1643, 1203, 1138, 1039, 1026, 988 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.33 (4H, br s, NH2-29), 8.00 (2H, t, J = 5.8 Hz, NH-25), 3.84–3.79 (2H, m, H-6), 3.35–3.29 (2H, m, H-3), 3.10 (4H, q, J = 6.3 Hz, H2-26), 2.85 (8H, br s, H2-28, H2-30), 2.15–2.08 (2H, m, H2-23b), 2.02–1.97 (2H, m, H2-23a), 1.95–0.95 (72H, m, H2-1, H2-2, H2-4, H-5, H2-7, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33, H2-34, H2-35), 0.88 (6H, d, J = 6.5 Hz, H3-21), 0.83 (6H, s, H3-19), 0.60 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 70.0 (C-3), 65.9 (C-6), 55.9 (C-14), 55.5 (C-17), 48.2 (C-5), 46.8 (C-30), 44.5 (C-28), 42.4 (C-13), 39.6 (C-12, obscured by solvent), 39.2 (C-9, obscured by solvent), 35.5, 35.4 (C-1, C-26), 34.94, 34.86 (C-7, C-8, C-10), 34.3 (C-20), 32.3 (C-23), 31.5 (C-22), 30.3 (C-4), 29.2 (C-2), 29.0 (C-35), 28.9 (C-34), 28.6 (C-33), 27.7 (C-16), 26.2 (C-27), 25.9 (C-32), 25.5 (C-31), 23.9 (C-15), 23.5 (C-19), 20.4 (C-11), 18.3 (C-21), 11.9 (C-18); (+)-HRESIMS [M + H]+ m/z 1063.9127 (calcd for C66H119N4O6, 1063.9124).
3.1.10. N1,N4-Bis(3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (8a)
Following general procedure B, ursodeoxycholic acid (0.045 g, 0.115 mmol) was reacted with di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (6a) (0.023 g, 0.0573 mmol), EDC·HCl (0.033 g, 0.172 mmol), HOBt (0.008 g, 0.0573 mmol) and DIPEA (0.040 mL, 0.229 mmol) to afford di-tert-butyl butane-1,4-diylbis((3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.061 g, 92%) as a clear colorless oil. Following general procedure C, a sub-sample of the product (0.005 g, 0.00434 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 8a (0.005 g, 98%) as a pale-yellow oil. [α = +24 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.26; IR (ATR) νmax 3328, 1638, 1202, 1038, 1027 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.42 (4H, br s, NH2-29), 7.99 (2H, t, J = 5.5 Hz, NH-25), 3.39–3.28 (4H, m, H-3, H-7), 3.11 (4H, q, J = 6.2 Hz, H2-26), 2.92–2.88 (8H, m, H2-28, H2-30), 2.09–0.83 (72H, m, H2-1, H2-2, H2-4, H-5, H2-6, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H3-19, H-20, H3-21, H2-22, H2-23, H2-27, H2-31), 0.61 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 69.7 (C-3), 69.5 (C-7), 55.9 (C-14), 54.7 (C-17), 46.1 (C-30), 44.6 (C-28), 43.1, 43.0 (C-8, C-13), 42.2 (C-5), 39.2 (C-12, obscured by solvent), 38.8 (C-10), 37.7 (C-4), 37.3 (C-6), 35.6 (C-26), 35.0, 34.9 (C-9, C-15, C-20), 33.8 (C-1), 32.4 (C-23), 31.7 (C-22), 30.2 (C-2), 28.2 (C-16), 26.1 (C-27), 23.3 (C-19), 22.7 (C-31), 20.9 (C-11), 18.4 (C-21), 12.0 (C-18); (+)-HRESIMS [M + H]+ m/z 951.7873 (calcd for C58H103N4O6, 951.7872).
3.1.11. N1,N6-Bis(3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)hexane-1,6-diaminium 2,2,2-trifluoroacetate (8b)
Following general procedure B, ursodeoxycholic acid (0.050 g, 0.127 mmol) was reacted with di-tert-butyl hexane-1,6-diylbis((3-aminopropyl)carbamate) (6b) (0.027 g, 0.0637 mmol), EDC·HCl (0.037 g, 0.191 mmol), HOBt (0.009 g, 0.0637 mmol) and DIPEA (0.044 mL, 0.255 mmol) affording di-tert-butyl hexane-1,6-diylbis((3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.061 g, 81%) as a clear colorless oil. Following general procedure C, a sub-sample of the product (0.043 g, 0.0364 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 8b (0.030 g, 68%) as a pale-yellow oil. [α = +20 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.23; IR (ATR) νmax 3308, 2935, 2866, 1637, 1456, 1202, 1138, 1050 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.45 (4H, br s, NH2-29), 7.98 (2H, t, J = 5.7 Hz, NH-25), 4.46 (2H, br s, OH-3 or OH-7), 3.87 (2H, d, J = 6.3 Hz, OH-3 or OH-7), 3.32 (4H, obscured by water, H-3, H-7), 3.10 (4H, q, J = 6.3 Hz, H2-26), 2.86 (8H, br s, H2-28, H2-30), 2.15–2.07 (2H, m, H2-23b), 2.02–1.97 (2H, m, H2-23a), 1.96–0.91 (60H, m, H2-1, H2-2, H2-4, H-5, H2-6, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32), 0.89 (6H, d, J = 6.5 Hz, H3-21), 0.87 (6H, s, H3-19), 0.61 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 69.7 (C-3), 69.5 (C-7), 55.9 (C-14), 54.7 (C-17), 46.6 (C-30), 44.6 (C-28), 43.1, 43.0 (C-8, C-13), 42.1 (C-5), 39.2 (C-12, obscured by solvent), 38.7 (C-10), 37.7 (C-4), 37.2 (C-6), 35.5 (C-26), 35.0 (C-15), 34.9, 34.8 (C-9, C-20), 33.7 (C-1), 32.3 (C-23), 31.6 (C-22), 30.2 (C-2), 28.2 (C-16), 26.2 (C-27), 25.5 (C-32), 25.3 (C-31), 23.3 (C-19), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); (+)-HRESIMS [M + 2H]2+ m/z 490.4129 (calcd for C60H108N4O6, 490.4129).
3.1.12. N1,N7-Bis(3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)heptane-1,7-diaminium 2,2,2-trifluoroacetate (8c)
Following general procedure B, ursodeoxycholic acid (0.050 g, 0.127 mmol) was reacted with di-tert-butyl heptane-1,7-diylbis((3-aminopropyl)carbamate) (6c) (0.028 g, 0.0637 mmol), EDC·HCl (0.037 g, 0.191 mmol), HOBt (0.009 g, 0.0637 mmol) and DIPEA (0.044 mL, 0.255 mmol) affording di-tert-butyl heptane-1,7-diylbis((3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.054 g, 71%) as a white foam. Following general procedure C, a sub-sample of the product (0.020 g, 0.0168 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 8c (0.012 g, 59%) as a pale-yellow oil. [α = +36 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.23; IR (ATR) νmax 3345, 2937, 2867, 1635, 1455, 1202, 1138, 1052, 1033, 1013 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.69 (4H, br s, NH2-29), 8.01 (2H, t, J = 5.7 Hz, NH-25), 3.31–3.25 (4H, m, H-3, H-7), 3.10 (4H, q, J = 6.3 Hz, H2-26), 2.84 (8H, br s, H2-28, H2-30), 2.15–2.07 (2H, m, H2-23b), 2.02–1.97 (2H, m, H2-23a), 1.96–0.91 (62H, m, H2-1, H2-2, H2-4, H-5, H2-6, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33), 0.89 (6H, d, J = 6.5 Hz, H3-21), 0.87 (6H, s, H3-19), 0.61 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.1 (C-24), 69.7 (C-3), 69.4 (C-7), 55.9 (C-14), 54.7 (C-17), 46.6 (C-30), 44.5 (C-28), 43.1, 43.0 (C-8, C-13), 42.1 (C-5), 39.2 (C-12, obscured by solvent), 38.7 (C-10), 37.7 (C-4), 37.2 (C-6), 35.5 (C-26), 35.0, 34.8 (C-9, C-15, C-20), 33.7 (C-1), 32.3 (C-23), 31.6 (C-22), 30.2 (C-2), 28.2 (C-16), 27.9 (C-33), 26.0 (C-27), 25.7 (C-32), 25.3 (C-31), 23.3 (C-19), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); (+)-HRESIMS [M + H]+ m/z 993.8342 (calcd for C61H109N4O6, 993.8342).
3.1.13. N1,N8-Bis(3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)octane-1,8-diaminium 2,2,2-trifluoroacetate (8d)
Following general procedure B, ursodeoxycholic acid (0.050 g, 0.127 mmol) was reacted with di-tert-butyl octane-1,8-diylbis((3-aminopropyl)carbamate) (6d) (0.029 g, 0.0637 mmol), EDC·HCl (0.037 g, 0.191 mmol), HOBt (0.009 g, 0.0637 mmol) and DIPEA (0.044 mL, 0.255 mmol) to afford di-tert-butyl octane-1,8-diylbis((3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.067 g, 87%) as a white foam. Following general procedure C, a sub-sample of the product (0.026 g, 0.0215 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 8d (0.025 g, 94%) as a pale-yellow oil. [α = +20 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.23; IR (ATR) νmax 3310, 2933, 2865, 1635, 1454, 1202, 1137, 1015 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.48 (4H, br s, NH2-29), 7.89 (2H, t, J = 5.7 Hz, NH-25), 3.35–3.25 (4H, m, H-3, H-7), 3.10 (4H, q, J = 6.3 Hz, H2-26), 2.86 (8H, br s, H2-28, H2-30), 2.15–2.07 (2H, m, H2-23b), 2.02–1.95 (2H, m, H2-23a), 1.92–0.91 (64H, m, H2-1, H2-2, H2-4, H-5, H2-6, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33), 0.88 (6H, d, J = 6.5 Hz, H3-21), 0.87 (6H, s, H3-19), 0.61 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 69.7 (C-3), 69.5 (C-7), 55.9 (C-14), 54.7 (C-17), 46.8 (C-30), 44.6 (C-28), 43.1, 43.0 (C-8, C-13), 42.1 (C-5), 39.4 (C-12, obscured by solvent), 38.7 (C-10), 37.7 (C-4), 37.2 (C-6), 35.5 (C-26), 35.0, 34.8 (C-9, C-15, C-20), 33.7 (C-1), 32.3 (C-23), 31.6 (C-22), 30.2 (C-2), 28.3 (C-33), 28.2 (C-16), 26.2 (C-27), 25.8 (C-32), 25.4 (C-31), 23.3 (C-19), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); (+)-HRESIMS [M + 2H]2+ m/z 504.4260 (calcd for C62H112N4O6, 504.4285).
3.1.14. N1,N10-Bis(3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)decane-1,10-diaminium 2,2,2-trifluoroacetate (8e)
Following general procedure B, ursodeoxycholic acid (0.034 g, 0.0886 mmol) was reacted with di-tert-butyl decane-1,10-diylbis((3-aminopropyl)carbamate) (6e) (0.021 g, 0.0443 mmol), EDC·HCl (0.025 g, 0.130 mmol), HOBt (0.006 g, 0.0443 mmol) and DIPEA (0.030 mL, 0.173 mmol) to afford di-tert-butyl decane-1,10-diylbis((3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a] phenanthren-17-yl)pentanamido)propyl) carbamate) as a clear colorless oil (0.044 g, 82%). Following general procedure C, a sub-sample of the product (0.009 g, 0.00728 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 8e (0.006 g, 65%) as a pale-yellow oil. [α = +33 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.19; IR (ATR) νmax 3363, 2931, 2861, 1676, 1452, 1202, 1136, 1016, 953, 801, 722 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.38 (4H, br s, NH2-29), 7.98 (2H, t, J = 5.7 Hz, NH-25), 3.36–3.23 (4H, m, H-3, H-7), 3.10 (4H, q, J = 6.3 Hz, H2-26), 2.85 (8H, br s, H2-28, H2-30), 2.15–2.08 (2H, m, H2-23b), 2.02–1.96 (2H, m, H2-23a), 1.94–0.91 (68H, m, H2-1, H2-2, H2-4, H-5, H2-6, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33, H2-34), 0.89 (6H, d, J = 6.6 Hz, H3-21), 0.87 (6H, s, H3-19), 0.61 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 69.7 (C-3), 69.4 (C-7), 55.9 (C-14), 54.7 (C-17), 46.8 (C-30), 44.5 (C-28), 43.1, 43.0 (C-8, C-13), 42.1 (C-5), 39.4 (C-12, obscured by solvent), 38.7 (C-10), 37.7 (C-4), 37.2 (C-6), 35.4 (C-26), 35.0, 34.8 (C-9, C-15, C-20), 33.7 (C-1), 32.3 (C-23), 31.6 (C-22), 30.2 (C-2), 28.8 (C-34), 28.5 (C-33), 28.2 (C-16), 26.2 (C-27), 25.9 (C-32), 25.5 (C-31), 23.3 (C-19), 20.8 (C-11), 18.4 (C-21), 12.0 (C-18); (+)-HRESIMS [M + 2H]2+ m/z 518.4441 (calcd for C64H116N4O6, 518.4442).
3.1.15. N1,N12-Bis(3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)dodecane-1,12-diaminium 2,2,2-trifluoroacetate (8f)
Following general procedure B, ursodeoxycholic acid (0.050 g, 0.127 mmol) was reacted with di-tert-butyl dodecane-1,12-diylbis((3-aminopropyl)carbamate) (6f) (0.033 g, 0.0637 mmol), EDC·HCl (0.037 g, 0.191 mmol), HOBt (0.009 g, 0.0637 mmol) and DIPEA (0.044 mL, 0.255 mmol) to afford di-tert-butyl dodecane-1,12-diylbis((3-((R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.066 g, 82%) as a white foam. Following general procedure C, a sub-sample of the product (0.009 g, 0.00712 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 8f (0.007 g, 76%) as a pale-yellow oil. = +27 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.17; IR (ATR) νmax 3309, 2930, 2852, 1636, 1449, 1202, 1038, 1026 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 9.00 (4H, br s, NH2-29), 8.11 (2H, br s, NH-25), 3.34–3.23 (4H, m, H-3, H-7), 3.08 (4H, q, J = 6.2 Hz, H2-26), 2.80 (8H, br s, H2-28, H2-30), 2.14–2.07 (2H, m, H2-23b), 2.02–1.96 (2H, m, H2-23a), 1.93–0.93 (72H, m, H2-1, H2-2, H2-4, H-5, H2-6, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31, H2-32, H2-33, H2-34, H2-35), 0.87 (6H, d, J = 6.5 Hz, H3-21), 0.85 (6H, s, H3-19), 0.59 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 69.8 (C-3), 69.5 (C-7), 55.9 (C-14), 54.8 (C-17), 46.8 (C-30), 44.5 (C-28), 43.2, 43.1 (C-8, C-13), 42.2 (C-5), 39.8 (C-10, obscured by solvent), 39.2 (C-12, obscured by solvent), 38.8 (C-10), 37.8 (C-4), 37.3 (C-6), 35.7 (C-26), 35.1, 34.9 (C-9, C-15, C-20), 33.8 (C-1), 32.4 (C-23), 31.7 (C-22), 30.3 (C-2), 29.0 (C-35), 28.9 (C-34), 28.6 (C-33), 28.3 (C-16), 26.1 (C-27), 25.9 (C-32), 25.4 (C-31), 23.4 (C-19), 20.9 (C-11), 18.5 (C-21), 12.1 (C-18); (+)-HRESIMS [M + H]+ m/z 1063.9124 (calcd for C66H119N4O6, 1063.9124).
3.1.16. N1,N4-Bis(3-((R)-4-((3R,5S,7R,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)butane-1,4-diaminium 2,2,2-trifluoroacetate (9a)
Following general procedure B, chenodeoxycholic acid (0.050 g, 0.127 mmol) was reacted with di-tert-butyl butane-1,4-diylbis((3-aminopropyl)carbamate) (6a) (0.026 g, 0.0637 mmol), EDC·HCl (0.037 g, 0.191 mmol), HOBt (0.009 g, 0.0637 mmol) and DIPEA (0.044 mL, 0.255 mmol) to afford di-tert-butyl butane-1,4-diylbis((3-((R)-4-((3R,5S,7R,8R,9S,10S,13R,14S,17R)-3,7-dihydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanamido)propyl)carbamate) (0.060 g, 82%) as a clear colorless oil. Following general procedure C, a sub-sample of the product (0.030 g, 0.0260 mmol) was reacted with TFA in CH2Cl2 to afford, after chromatography, the di-TFA salt 9a (0.018 g, 59%) as a clear, colorless oil. [α]D = +8 (c = 0.1, MeOH); Rf (RP-18, 10% aq. HCl:MeOH 1:3) 0.11; IR (ATR) νmax 3308, 2961, 2838, 1645, 1410, 1259, 1092, 1038, 1015, 798 cm−1; 1H NMR (DMSO-d6, 400 MHz) δ 8.46 (4H, br s, NH2-29), 7.98 (2H, t, J = 5.7 Hz, NH-25), 4.01 (2H, br s, OH-7), 3.62 (2H, br s, H-7), 3.22–3.15 (2H, m, H-3), 3.10 (8H, q, J = 6.3 Hz, H2-28, H2-30), 2.92–2.83 (4H, m, H2-26), 2.23–2.14 (2H, m, H2-4a), 2.12–2.08 (2H, m, H2-23b), 2.02–1.96 (2H, m, H2-23a), 1.95–0.96 (54H, m, H2-1, H2-2, H2-4b, H-5, H2-6, H-8, H-9, H2-11, H2-12, H-14, H2-15, H2-16, H-17, H-20, H2-22, H2-27, H2-31), 0.89 (6H, d, J = 6.4 Hz, H3-21), 0.83 (6H, s, H3-19), 0.60 (6H, s, H3-18); 13C NMR (DMSO-d6, 100 MHz) δ 173.3 (C-24), 70.3 (C-3), 66.1 (C-7), 55.5 (C-17), 50.0 (C-14), 46.1 (C-30), 44.6 (C-28), 41.9 (C-8), 41.4 (C-5), 40.5 (C-13), 39.8 (C-4, obscured by solvent), 39.2 (C-9 and C-12, obscured by solvent), 35.5 (C-26), 35.3 (C-6), 35.1, 34.8 (C-10, C-16), 34.7 (C-20), 32.3 (C-1, C-23), 31.5 (C-22), 30.5 (C-2), 26.2 (C-27), 23.1 (C-15), 22.7 (C-19, C-31), 20.2 (C-11), 18.3 (C-21), 11.6 (C-18); (+)-HRESIMS [M + H]+ m/z 951.7874 (calcd for C58H103N4O6, 951.7872).