Minocycline and the SPR741 Adjuvant Are an Efficacious Antibacterial Combination for Acinetobacter baumannii Infections
Abstract
1. Introduction
2. Results
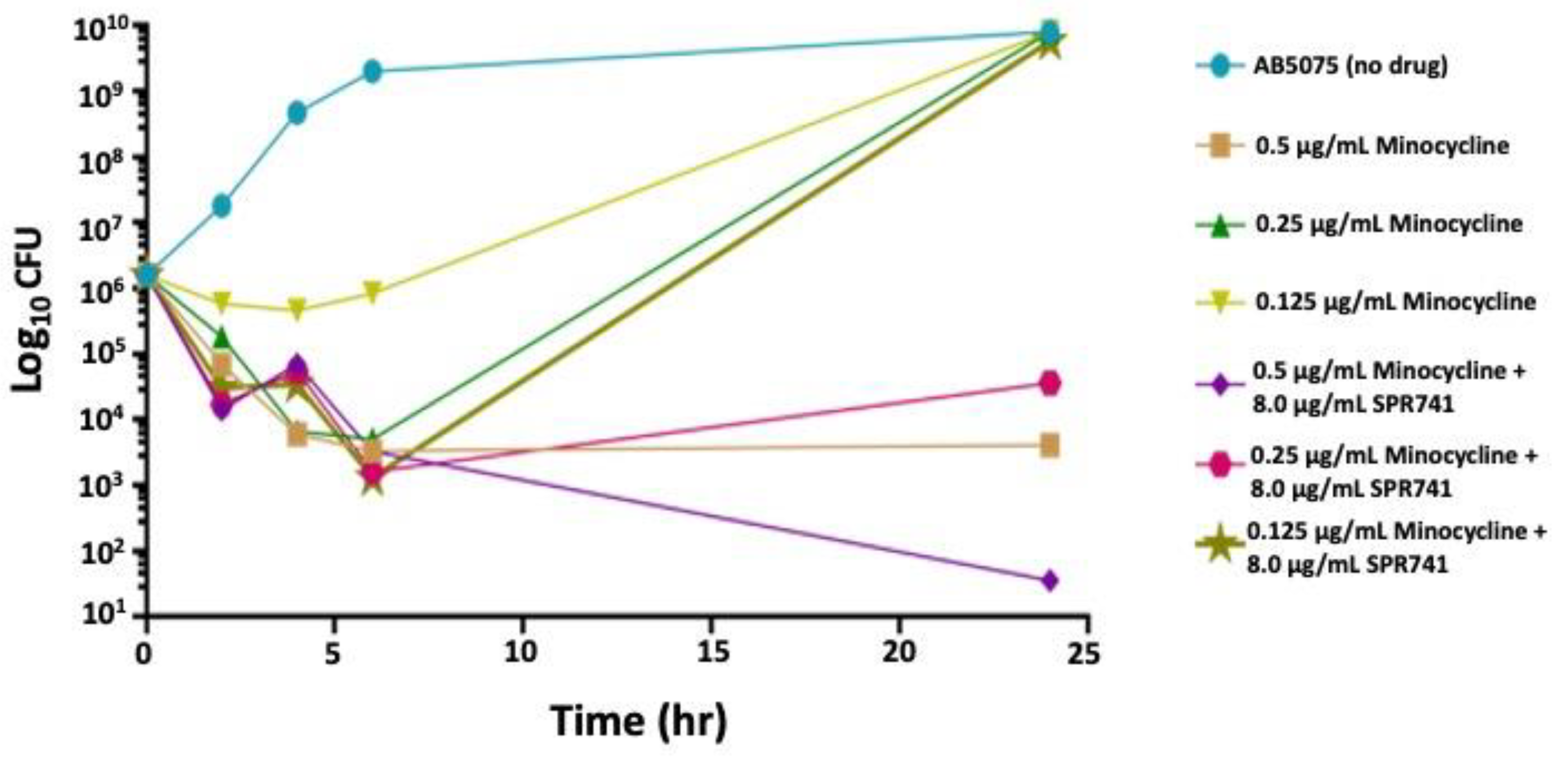
2.1. In Vitro Analysis
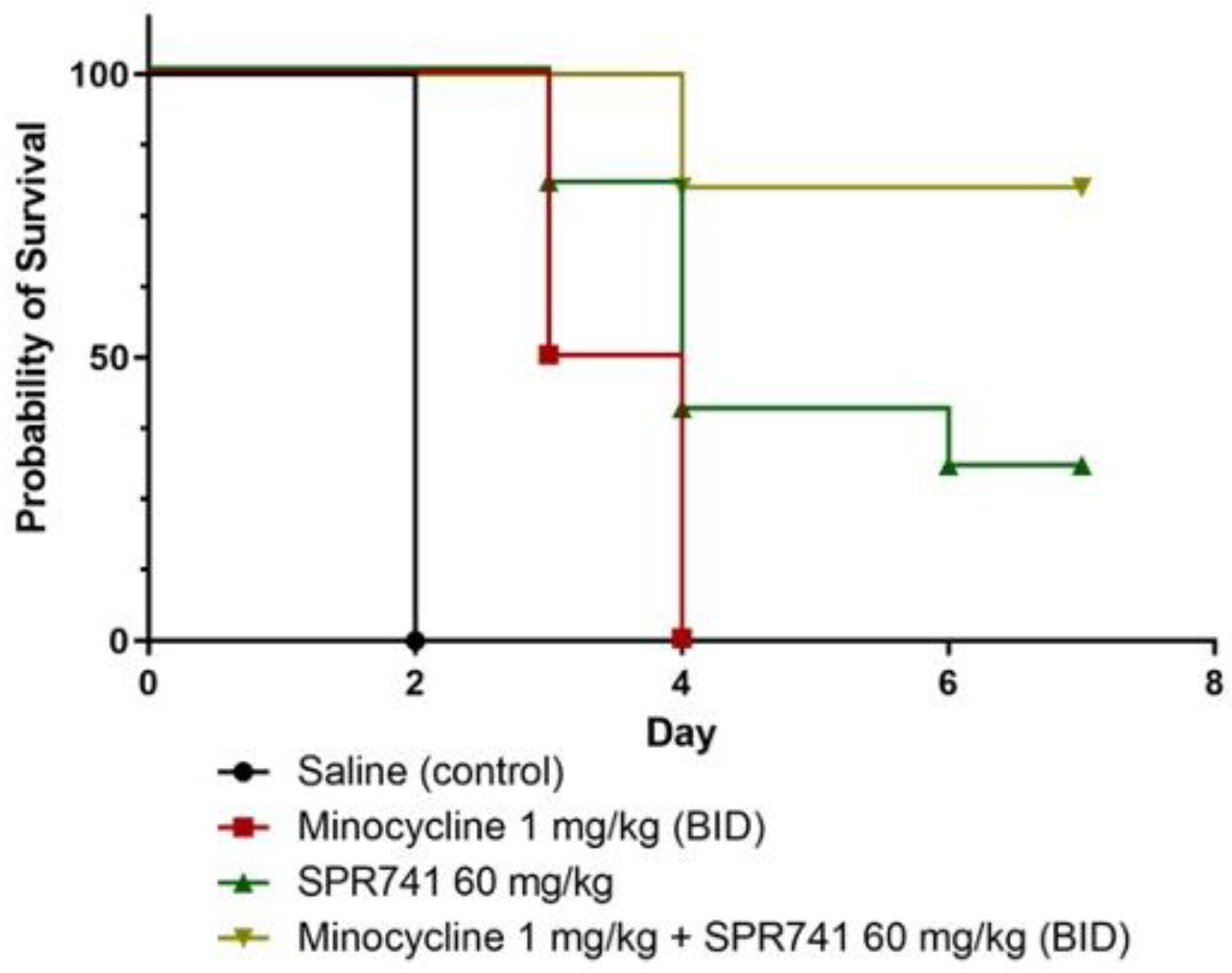
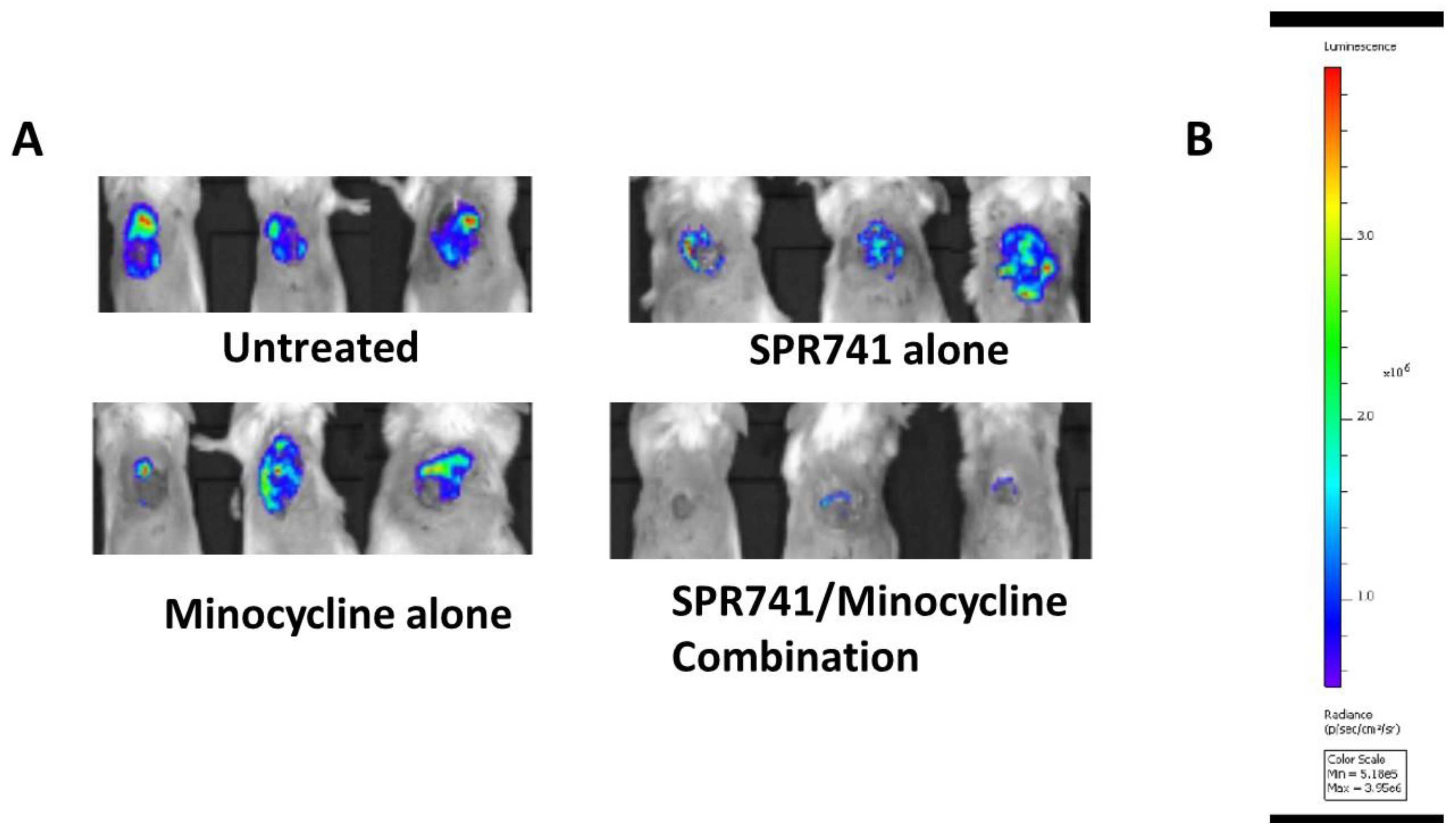
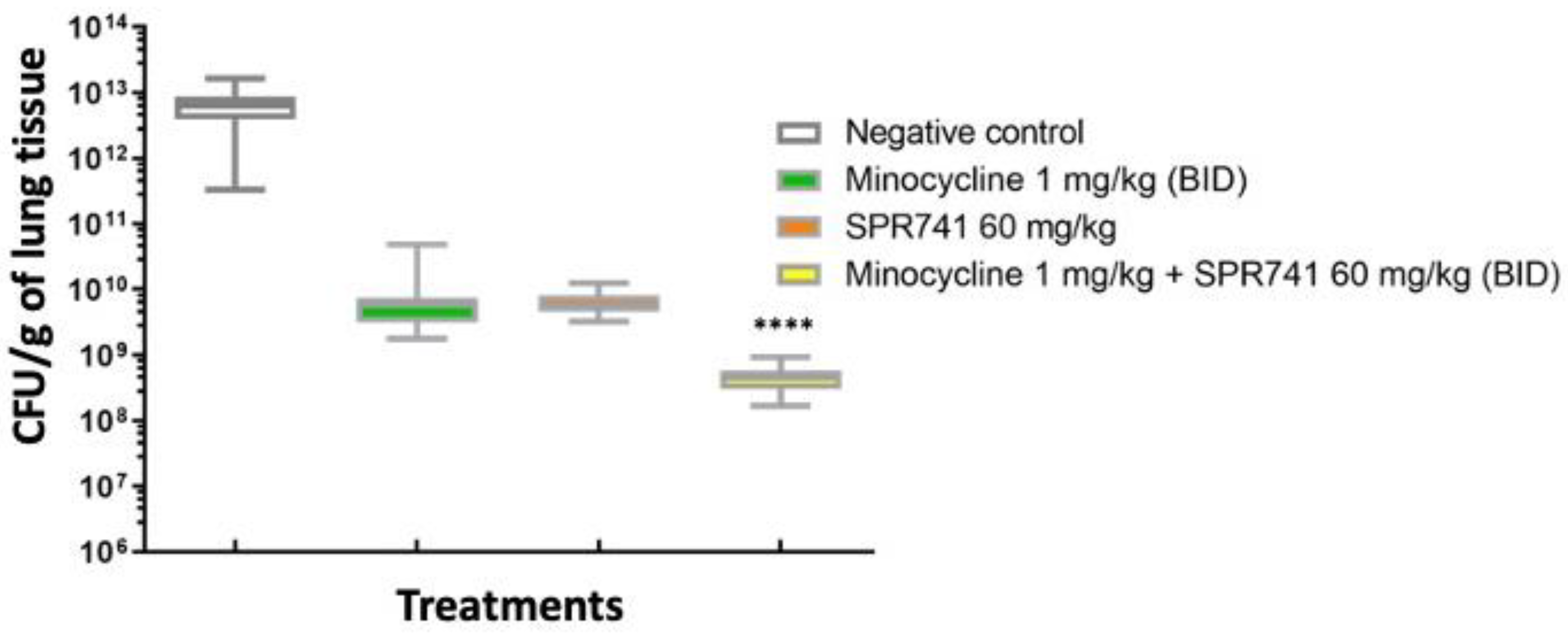
2.2. Pulmonary Infection Analysis
2.3. Wound Infection Model
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Media
4.2. In Vitro Assays of Efficacy
4.3. Murine Pulmonary Model of Infection
4.4. Murine Wound Model of Infection
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Spellberg, B.; Rex, J.H. The value of single-pathogen antibacterial agents. Nat. Rev. Drug Discov. 2013, 12, 963. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Botha, J.; Tiruvoipati, R. Fatal skin and soft tissue infection of multidrug resistant Acinetobacter baumannii: A case report. Int. J. Surg. Case Rep. 2014, 5, 532–536. [Google Scholar] [CrossRef]
- Sinha, N.; Niazi, M.; Lvovsky, D. A fatal case of multidrug resistant acinetobacter necrotizing fasciitis: The changing scary face of nosocomial infection. Case Rep. Infect. Dis. 2014, 2014, 705279. [Google Scholar] [PubMed]
- Freire, M.P.; de Oliveira Garcia, D.; Garcia, C.P.; Campagnari Bueno, M.F.; Camargo, C.H.; Kono Magri, A.S.G.; Francisco, G.R.; Reghini, R.; Vieira, M.F.; Ibrahim, K.Y.; et al. Bloodstream infection caused by extensively drug-resistant Acinetobacter baumannii in cancer patients: High mortality associated with delayed treatment rather than with the degree of neutropenia. Clin. Microbiol. Infect. 2016, 22, 352–358. [Google Scholar] [CrossRef]
- Teng, S.O.; Yen, M.Y.; Ou, T.Y.; Chen, F.L.; Yu, F.L.; Lee, W.S. Comparison of pneumonia- and non-pneumonia-related Acinetobacter baumannii bacteremia: Impact on empiric therapy and antibiotic resistance. J. Microbiol. Immunol. Infect. 2015, 48, 525–530. [Google Scholar] [CrossRef]
- Fu, Q.; Ye, H.; Liu, S. Risk factors for extensive drug-resistance and mortality in geriatric inpatients with bacteremia caused by Acinetobacter baumannii. Am. J. Infect. Control 2015, 43, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, W.; Du, X.; Li, W.; Zhong, T.; Tang, Y.; Feng, Y.; Tao, C.; Xie, Y. Risk and Prognostic Factors for Multidrug-Resistant Acinetobacter Baumannii Complex Bacteremia: A Retrospective Study in a Tertiary Hospital of West China. PLoS ONE 2015, 10, e0130701. [Google Scholar] [CrossRef]
- Li, W.; Ding, C.; Yin, S. Severe pneumonia in the elderly: A multivariate analysis of risk factors. Int. J. Clin. Exp. Med. 2015, 8, 12463–12475. [Google Scholar]
- Hu, J.; Robinson, J.L. Systematic review of invasive Acinetobacter infections in children. Can. J. Infect. Dis. Med. Microbiol. 2010, 21, 83–88. [Google Scholar] [CrossRef]
- Roy, S.; Basu, S.; Dasgupta, S.; Singh, A.K.; Viswanathan, R. Carbapenem resistance in Acinetobacter baumannii isolated from blood of neonates with sepsis. Indian J. Med. Microbiol. 2010, 28, 416–417. [Google Scholar] [CrossRef]
- Das, P.; Singh, A.K.; Pal, T.; Dasgupta, S.; Ramamurthy, T.; Basu, S. Colonization of the gut with Gram-negative bacilli, its association with neonatal sepsis and its clinical relevance in a developing country. J. Med. Microbiol. 2011, 60, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Keen, E.F., 3rd; Murray, C.K.; Robinson, B.J.; Hospenthal, D.R.; Co, E.M.; Aldous, W.K. Changes in the incidences of multidrug-resistant and extensively drug-resistant organisms isolated in a military medical center. Infect. Control Hosp. Epidemiol. 2010, 31, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.C.; Murray, C.K.; Roop, S.A.; Hospenthal, D.R.; Gourdine, E.; Dooley, D.P. Bacteria recovered from patients admitted to a deployed U.S. military hospital in Baghdad, Iraq. Mil. Med. 2006, 171, 821–825. [Google Scholar] [CrossRef]
- Yun, H.C.; Branstetter, J.G.; Murray, C.K. Osteomyelitis in military personnel wounded in Iraq and Afghanistan. J. Trauma 2008, 64, S163–S168. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.W.; Schuh, J.C.; Zurawski, D.V.; Wang, J.; Arbabi, S.; McVean, M.; Funk, K.A. The First Cut Is the Deepest: The History and Development of Safe Treatments for Wound Healing and Tissue Repair. Int. J. Toxicol. 2016, 35, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Bonomo, R.A. The deadly impact of extreme drug resistance in Acinetobacter baumannii. Crit. Care Med. 2014, 42, 1289–1291. [Google Scholar] [CrossRef]
- Spellberg, B.; Bonomo, R.A. Combination Therapy for Extreme Drug-Resistant Acinetobacter baumannii: Ready for Prime Time? Crit. Care Med. 2015, 43, 1332–1334. [Google Scholar] [CrossRef]
- Potron, A.; Poirel, L.; Nordmann, P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int. J. Antimicrob. Agents 2015, 45, 568–585. [Google Scholar] [CrossRef]
- Lin, L.; Tan, B.; Pantapalangkoor, P.; Ho, T.; Baquir, B.; Tomaras, A.; Montgomery, J.I.; Reilly, U.; Barbacci, E.G.; Hujer, K.; et al. Inhibition of LpxC protects mice from resistant Acinetobacter baumannii by modulating inflammation and enhancing phagocytosis. mBio 2012, 3, e00312-12. [Google Scholar] [CrossRef]
- de Leeuw, E.P. Efficacy of the small molecule inhibitor of Lipid II BAS00127538 against Acinetobacter baumannii. Drug Des. Devel. Ther. 2014, 8, 1061–1064. [Google Scholar] [CrossRef]
- Corey, B.W.; Thompson, M.G.; Hittle, L.E.; Jacobs, A.C.; Asafo-Adjei, E.A.; Huggins, W.M.; Melander, R.J.; Melander, C.; Ernst, R.K.; Zurawski, D.V. 1,2,4-Triazolidine-3-thiones Have Specific Activity against Acinetobacter baumannii among Common Nosocomial Pathogens. ACS Infect. Dis. 2017, 3, 62–71. [Google Scholar]
- Huggins, W.M.; Minrovic, B.M.; Corey, B.W.; Jacobs, A.C.; Melander, R.J.; Sommer, R.D.; Zurawski, D.V.; Melander, C. 1,2,4-Triazolidine-3-thiones as Narrow Spectrum Antibiotics against Multidrug-Resistant Acinetobacter baumannii. ACS Med. Chem. Lett. 2017, 8, 27–31. [Google Scholar]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2018, 63, e01110-18. [Google Scholar] [CrossRef] [PubMed]
- Mood, E.H.; Goltermann, L.; Brolin, C.; Cavaco, L.M.; Nejad, A.J.; Yavari, N.; Frederiksen, N.; Franzyk, H.; Nielsen, P.E. Antibiotic Potentiation in Multidrug-Resistant Gram-Negative Pathogenic Bacteria by a Synthetic Peptidomimetic. ACS Infect. Dis. 2021, 7, 2152–2163. [Google Scholar] [CrossRef]
- Liu, Y.; Li, R.; Xiao, X.; Wang, Z. Antibiotic adjuvants: An alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit. Rev. Microbiol. 2019, 45, 301–314. [Google Scholar] [CrossRef]
- Thompson, R.J.; Bobay, B.G.; Stowe, S.D.; Olson, A.L.; Peng, L.; Su, Z.; Actis, L.A.; Melander, C.; Cavanagh, J. Identification of BfmR, a response regulator involved in biofilm development, as a target for a 2-Aminoimidazole-based antibiofilm agent. Biochemistry 2012, 51, 9776–9778. [Google Scholar] [CrossRef]
- Cox, G.; Koteva, K.; Wright, G.D. An unusual class of anthracyclines potentiate Gram-positive antibiotics in intrinsically resistant Gram-negative bacteria. J. Antimicrob. Chemother. 2014, 69, 1844–1855. [Google Scholar] [CrossRef]
- Zabawa, T.P.; Pucci, M.J.; Parr, T.R.; Lister, T., Jr. Treatment of Gram-negative bacterial infections by potentiation of antibiotics. Curr. Opin. Microbiol. 2016, 33, 7–12. [Google Scholar] [CrossRef]
- Vaara, M.; Siikanen, O.; Apajalahti, J.; Fox, J.; Frimodt-Møller, N.; He, H.; Poudyal, A.; Li, J.; Nation, R.L.; Vaara, T. A novel polymyxin derivative that lacks the fatty acid tail and carries only three positive charges has strong synergism with agents excluded by the intact outer membrane. Antimicrob. Agents Chemother. 2010, 54, 3341–3346. [Google Scholar] [CrossRef]
- French, S.; Farha, M.; Ellis, M.J.; Sameer, Z.; Côté, J.P.; Cotroneo, N.; Lister, T.; Rubio, A.; Brown, E.D. Potentiation of Antibiotics against Gram-Negative Bacteria by Polymyxin B Analogue SPR741 from Unique Perturbation of the Outer Membrane. ACS Infect. Dis. 2020, 6, 1405–1412. [Google Scholar] [CrossRef]
- Zurawski, D.V.; Reinhart, A.A.; Alamneh, Y.A.; Pucci, M.J.; Si, Y.; Abu-Taleb, R.; Shearer, J.P.; Demons, S.T.; Tyner, S.D.; Lister, T. SPR741, an Antibiotic Adjuvant, Potentiates the In Vitro and In Vivo Activity of Rifampin against Clinically Relevant Extensively Drug-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2017, 61, e01239-17. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Rhomberg, P.R.; Lister, T.; Cotroneo, N.; Rubio, A.; Flamm, R.K. Evaluation of Antimicrobial Effects of a New Polymyxin Molecule (SPR741) When Tested in Combination with a Series of beta-Lactam Agents against a Challenge Set of Gram-Negative Pathogens. Microb. Drug Resist. 2020, 26, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.E.; Rhomberg, P.R.; Lister, T.; Cotroneo, N.; Parr, T.R.; Castanheira, M. In Vitro Activity Analysis of a New Polymyxin, SPR741, Tested in Combination with Antimicrobial Agents against a Challenge Set of Enterobacteriaceae, Including Molecularly Characterized Strains. Antimicrob. Agents Chemother. 2020, 65, e00742-20. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.C.; Thompson, M.G.; Black, C.C.; Kessler, J.L.; Clark, L.P.; McQueary, C.N.; Gancz, H.Y.; Corey, B.W.; Moon, J.K.; Si, Y.; et al. AB5075, a Highly Virulent Isolate of Acinetobacter baumannii, as a Model Strain for the Evaluation of Pathogenesis and Antimicrobial Treatments. mBio 2014, 5, e01076-14. [Google Scholar] [CrossRef]
- Regeimbal, J.M.; Jacobs, A.C.; Corey, B.W.; Henry, M.S.; Thompson, M.G.; Pavlicek, R.L.; Quinones, J.; Hannah, R.M.; Ghebremedhin, M.; Crane, N.J.; et al. Personalized Therapeutic Cocktail of Wild Environmental Phages Rescues Mice from Acinetobacter baumannii Wound Infections. Antimicrob. Agents Chemother. 2016, 60, 5806–5816. [Google Scholar] [CrossRef]
- Krašovec, R.; Belavkin, R.V.; Aston, J.A.; Channon, A.; Aston, E.; Rash, B.M.; Kadirvel, M.; Forbes, S.; Knight, C.G. Mutation rate plasticity in rifampicin resistance depends on Escherichia coli cell-cell interactions. Nat. Commun. 2014, 5, 3742. [Google Scholar] [CrossRef]
- Jaidane, N.; Naas, T.; Mansour, W.; Radhia, B.B.; Jerbi, S.; Boujaafar, N.; Bouallegue, O.; Bonnin, R.A. Genomic analysis of in vivo acquired resistance to colistin and rifampicin in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2018, 51, 266–269. [Google Scholar] [CrossRef]
- Sader, H.S.; Mendes, R.E.; Streit, J.M.; Carvalhaes, C.G.; Castanheira, M. Antimicrobial susceptibility of Gram-negative bacteria from intensive care unit and non-intensive care unit patients from United States hospitals (2018–2020). Diagn. Microbiol. Infect. Dis. 2022, 102, 115557. [Google Scholar] [CrossRef]
- Lodise, T.P.; Van Wart, S.; Sund, Z.M.; Bressler, A.M.; Khan, A.; Makley, A.T.; Hamad, Y.; Salata, R.A.; Silveira, F.P.; Sims, M.D.; et al. Pharmacokinetic and Pharmacodynamic Profiling of Minocycline for Injection following a Single Infusion in Critically Ill Adults in a Phase IV Open-Label Multicenter Study (ACUMIN). Antimicrob. Agents Chemother. 2021, 65, e01809-20. [Google Scholar] [CrossRef]
- Fragkou, P.C.; Poulakou, G.; Blizou, A.; Blizou, M.; Rapti, V.; Karageorgopoulos, D.E.; Koulenti, D.; Papadopoulos, A.; Matthaiou, D.K.; Tsiodras, S. The Role of Minocycline in the Treatment of Nosocomial Infections Caused by Multidrug, Extensively Drug and Pandrug Resistant Acinetobacter baumannii: A Systematic Review of Clinical Evidence. Microorganisms 2019, 7, 159. [Google Scholar] [CrossRef]
- Lashinsky, J.N.; Henig, O.; Pogue, J.M.; Kaye, K.S. Minocycline for the Treatment of Multidrug and Extensively Drug-Resistant A. baumannii: A Review. Infect. Dis. Ther. 2017, 6, 199–211. [Google Scholar] [CrossRef]
- Greig, S.L.; Scott, L.J. Intravenous Minocycline: A Review in Acinetobacter Infections. Drugs 2016, 76, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Mendes, R.E.; Jones, R.N. Update on Acinetobacter species: Mechanisms of antimicrobial resistance and contemporary in vitro activity of minocycline and other treatment options. Clin. Infect. Dis. 2014, 59, S36–S373. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Liu, X.F.; Huang, J.; Zhu, D.M.; Li, J.; Zhang, J. Activities of colistin- and minocycline-based combinations against extensive drug resistant Acinetobacter baumannii isolates from intensive care unit patients. BMC Infect. Dis. 2011, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.S.; Lee, Y.; Tseng, K.C.; Huang, W.C.; Chuang, M.F.; Kuo, S.C.; Lauderdale, T.L.; Chen, T.L. In Vivo and In Vitro Efficacy of Minocycline-Based Combination Therapy for Minocycline-Resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 4047–4054. [Google Scholar] [CrossRef]
- Lodise, T.P.; Fan, W.; Griffith, D.C.; Dudley, M.N.; Sulham, K.A. A Retrospective Cohort Analysis Shows that Coadministration of Minocycline with Colistin in Critically Ill Patients Is Associated with Reduced Frequency of Acute Renal Failure. Antimicrob. Agents Chemother. 2017, 6, e01165-17. [Google Scholar] [CrossRef]
- Arroyo, L.A.; Mateos, I.; González, V.; Aznar, J. In vitro activities of tigecycline, minocycline, and colistin-tigecycline combination against multi- and pandrug-resistant clinical isolates of Acinetobacter baumannii group. Antimicrob. Agents Chemother. 2009, 53, 1295–1296. [Google Scholar] [CrossRef]
- Qu, X.; Bian, X.; Chen, Y.; Hu, J.; Huang, X.; Wang, Y.; Fan, Y.; Wu, H.; Li, X.; Li, Y.; et al. Polymyxin B Combined with Minocycline: A Potentially Effective Combination against bla(OXA-23)-harboring CRAB in In Vitro PK/PD Model. Molecules 2022, 27, 1085. [Google Scholar] [CrossRef]
- Liu, P.Y.; Ko, W.C.; Lee, W.S.; Lu, P.L.; Chen, Y.H.; Cheng, S.H.; Lu, M.C.; Lin, C.Y.; Wu, T.S.; Yen, M.Y.; et al. In vitro activity of cefiderocol, cefepime/enmetazobactam, cefepime/zidebactam, eravacycline, omadacycline, and other comparative agents against carbapenem-non-susceptible Pseudomonas aeruginosa and Acinetobacter baumannii isolates associated from bloodstream infection in Taiwan between 2018–2020. J. Microbiol. Immunol. Infect. 2021, S1684–1182, 00186-9. [Google Scholar]
- Pfaller, M.A.; Huband, M.D.; Shortridge, D.; Flamm, R.K. Surveillance of omadacycline activity tested against clinical isolates from the USA: Report from the SENTRY Antimicrobial Surveillance Program, 2019. J. Glob. Antimicrob. Resist. 2021, 27, 337–351. [Google Scholar] [CrossRef]
- Chukwudi, C.U. rRNA Binding Sites and the Molecular Mechanism of Action of the Tetracyclines. Antimicrob. Agents Chemother. 2016, 60, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Di Noto, G.P.; Molina, M.C.; Quiroga, C. Insights Into Non-coding RNAs as Novel Antimicrobial Drugs. Front. Genet. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Galac, M.R.; Snesrud, E.; Lebreton, F.; Stam, J.; Julius, M.; Ong, A.C.; Maybank, R.; Jones, A.R.; Kwak, Y.I.; Hinkle, K.; et al. A Diverse Panel of Clinical Acinetobacter baumannii for Research and Development. Antimicrob Agents Chemother. 2020, 64, e00840-20. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yu, B.; Diep, J.K.; Sharma, R.; Dudley, M.; Monteiro, J.; Kaye, K.S.; Pogue, J.M.; Abboud, C.S.; Rao, G.G. In Vitro Assessment of Combined Polymyxin B and Minocycline Therapy against Klebsiella pneumoniae Carbapenemase (KPC)-Producing K. pneumoniae. Antimicrob. Agents Chemother. 2017, 61, e00073-17. [Google Scholar] [CrossRef]
- Seaton, M.; Hocking, A.; Gibran, N.S. Porcine models of cutaneous wound healing. ILAR J. 2015, 56, 127–138. [Google Scholar] [CrossRef]
- Swindle, M.; Makin, A.; Herron, A.; Clubb, F., Jr.; Frazier, K. Swine as models in biomedical research and toxicology testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef]
- Thompson, M.G.; Black, C.C.; Pavlicek, R.L.; Honnold, C.L.; Wise, M.C.; Alamneh, Y.A.; Moon, J.K.; Kessler, J.L.; Si, Y.; Williams, R.; et al. Validation of a novel murine wound model of Acinetobacter baumannii infection. Antimicrob Agents Chemother. 2014, 58, 1332–1342. [Google Scholar] [CrossRef]
- Stainton, S.M.; Abdelraouf, K.; Utley, L.; Pucci, M.J.; Lister, T.; Nicolau, D.P. Assessment of the In Vivo Activity of SPR741 in Combination with Azithromycin against Multidrug-Resistant Enterobacteriaceae Isolates in the Neutropenic Murine Thigh Infection Model. Antimicrob. Agents Chemother. 2018, 62, e00239-18. [Google Scholar] [CrossRef]
- Martins, A.M.; Marto, J.M.; Johnson, J.L.; Graber, E.M. A Review of Systemic Minocycline Side Effects and Topical Minocycline as a Safer Alternative for Treating Acne and Rosacea. Antibiotics 2021, 10, 757. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Lister, T.; Walpole, S.; Keutzer, T.; Utley, L.; Tomayko, J.; Kopp, E.; Farinola, N.; Coleman, S. Safety, Tolerability, Pharmacokinetics, and Drug Interaction Potential of SPR741, an Intravenous Potentiator, after Single and Multiple Ascending Doses and When Combined with beta-Lactam Antibiotics in Healthy Subjects. Antimicrob. Agents Chemother. 2019, 63, e00892-19. [Google Scholar] [CrossRef]
- CLSI Supplement M100; Performance Standards for Antimicrobial Susceptibility Testing; 32nd Informational Supplement. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2022.

| Bacteria | Minocycline (µg/mL) | SPR741 (µg/mL) | SPR741 + Minocycline (µg/mL) | Fold-Change |
|---|---|---|---|---|
| AB5075 | 0.5 | >64 | 0.0125 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamneh, Y.A.; Antonic, V.; Garry, B.; Pucci, M.J.; Abu-Taleb, R.; Shearer, J.P.; Demons, S.T.; Getnet, D.; Swierczewski, B.E.; Lister, T.; et al. Minocycline and the SPR741 Adjuvant Are an Efficacious Antibacterial Combination for Acinetobacter baumannii Infections. Antibiotics 2022, 11, 1251. https://doi.org/10.3390/antibiotics11091251
Alamneh YA, Antonic V, Garry B, Pucci MJ, Abu-Taleb R, Shearer JP, Demons ST, Getnet D, Swierczewski BE, Lister T, et al. Minocycline and the SPR741 Adjuvant Are an Efficacious Antibacterial Combination for Acinetobacter baumannii Infections. Antibiotics. 2022; 11(9):1251. https://doi.org/10.3390/antibiotics11091251
Chicago/Turabian StyleAlamneh, Yonas A., Vlado Antonic, Brittany Garry, Michael J. Pucci, Rania Abu-Taleb, Jonathan P. Shearer, Samandra T. Demons, Derese Getnet, Brett E. Swierczewski, Troy Lister, and et al. 2022. "Minocycline and the SPR741 Adjuvant Are an Efficacious Antibacterial Combination for Acinetobacter baumannii Infections" Antibiotics 11, no. 9: 1251. https://doi.org/10.3390/antibiotics11091251
APA StyleAlamneh, Y. A., Antonic, V., Garry, B., Pucci, M. J., Abu-Taleb, R., Shearer, J. P., Demons, S. T., Getnet, D., Swierczewski, B. E., Lister, T., & Zurawski, D. V. (2022). Minocycline and the SPR741 Adjuvant Are an Efficacious Antibacterial Combination for Acinetobacter baumannii Infections. Antibiotics, 11(9), 1251. https://doi.org/10.3390/antibiotics11091251







