Dual Infection of an Open Fracture Caused by Mycobacterium setense and Clostridium celerecrescens
Abstract
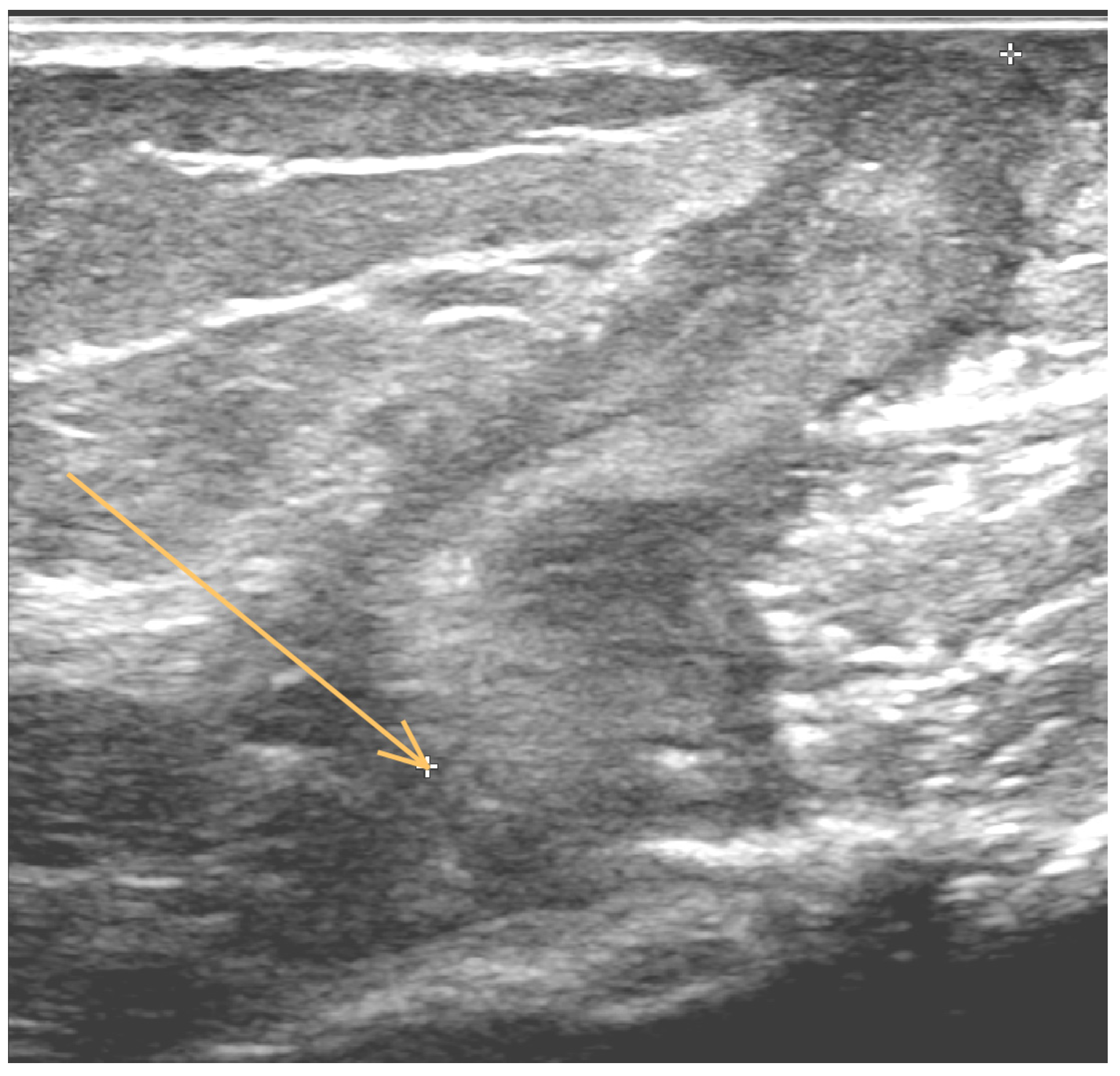
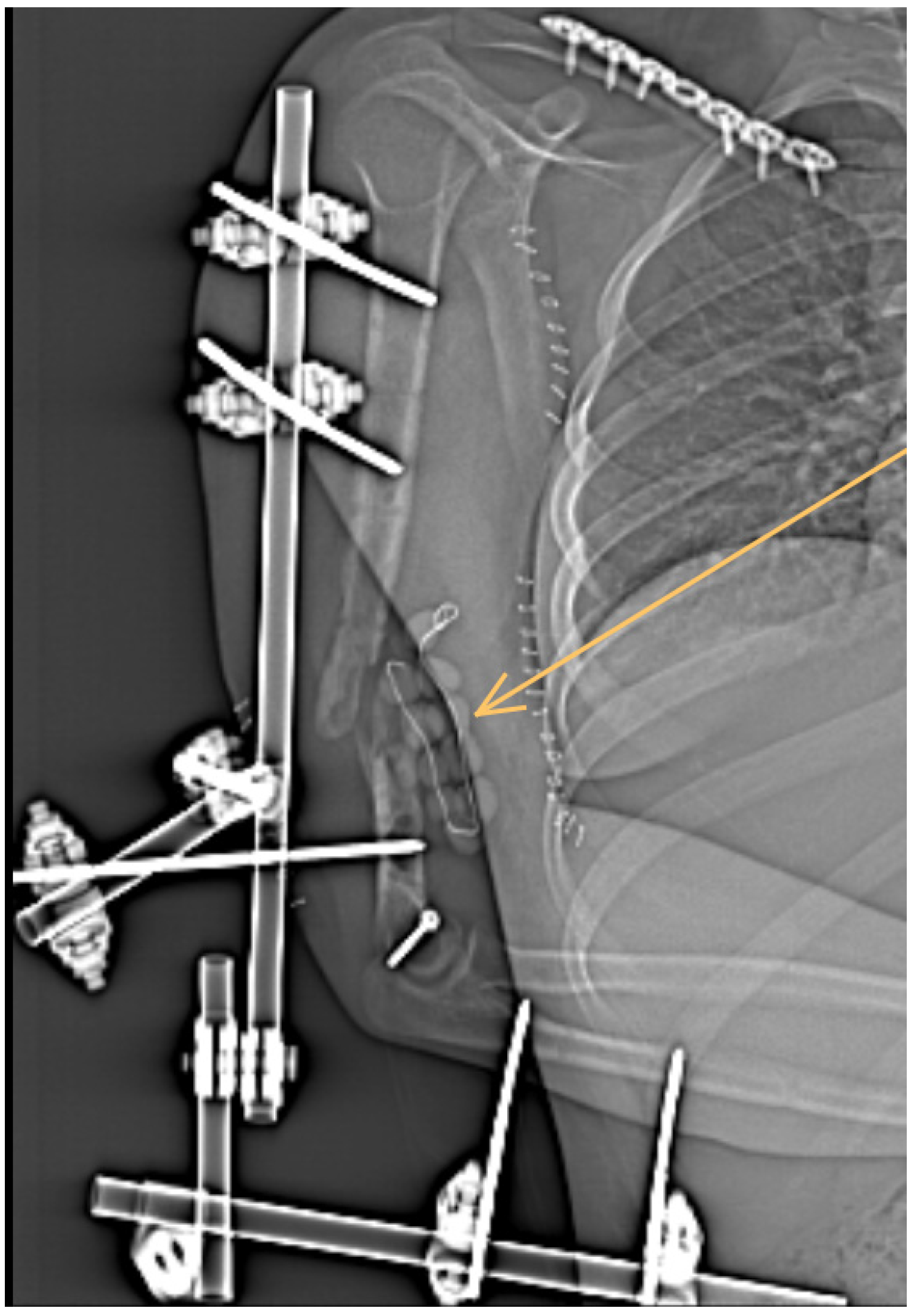
1. Case Report
2. Literature Review and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNally, M.; Corrigan, R.; Sliepen, J.; Dudareva, M.; Rentenaar, R.; IJpma, F.; Atkins, B.L.; Wouthuyzen-Bakker, M.; Govaert, G. What Factors Affect Outcome in the Treatment of Fracture-Related Infection? Antibiotics 2022, 11, 946. [Google Scholar] [CrossRef]
- Depypere, M.; Morgenstern, M.; Kuehl, R.; Senneville, E.; Moriarty, T.F.; Obremskey, W.T.; Zimmerli, W.; Trampuz, A.; Lagrou, K.; Metsemakers, W.J. Pathogenesis and management of fracture-related infection. Clin. Microbiol. Infect. 2020, 26, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, R.; Tschudin-Sutter, S.; Morgenstern, M.; Dangel, M.; Egli, A.; Nowakowski, A.; Suhm, N.; Theilacker, C.; Widmer, A.F. Time-dependent differences in management and microbiology of orthopaedic internal fixation-associated infections: An observational prospective study with 229 patients. Clin. Microbiol. Infect. 2019, 25, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Depypere, M.; Sliepen, J.; Onsea, J.; Debaveye, Y.; Govaert, G.; IJpma, F.; Zimmerli, W.; Metsemakers, W.J. The Microbiological Etiology of Fracture-Related Infection. Front. Cell. Infect. Microbiol. 2022, 12, 934485. [Google Scholar] [CrossRef] [PubMed]
- Lamy, B.; Marchandin, H.; Hamitouche, K.; Laurent, F. Mycobacterium setense sp. nov., a Mycobacterium fortuitum-group organism isolated from a patient with soft tissue infection and osteitis. Int. J. Syst. Evol. Microbiol. 2008, 58, 486–490. [Google Scholar] [CrossRef]
- Mormeneo Bayo, S.; Ferrer Cerón, I.; Martín Juste, P.; Lallana Dupla, J.; Millán Lou, M.I.; García-Lechuz Moya, J.M. A review of difficult-to-treat post-traumatic osteomyelitis: Role of Clostridium celerecrescens. Rev. Esp. Cir. Ortop. Traumatol. (Engl. Ed.) 2020, 64, 281–285, (In English, Spanish). [Google Scholar] [CrossRef]
- Pavlik, I.; Ulmann, V.; Weston, R.T. Clinical relevance and environmental prevalence of Mycobacterium fortuitum group members. Comment on Mugetti et al. gene sequencing and phylogenetic analysis: Powerful tools for an improved diagnosis of fish mycobacteriosis caused by Mycobacterium fortuitum group members. Microorganisms 2021, 9, 2345. [Google Scholar] [CrossRef]
- Toro, A.; Adekambi, T.; Cheynet, F.; Fournier, P.E.; Drancourt, M. Mycobacterium setense infection in humans. Emerg. Infect. Dis. 2008, 14, 1330–1332. [Google Scholar] [CrossRef]
- Kušar, D.; Zajc, U.; Jenčič, V.; Ocepek, M.; Higgins, J.; Žolnir-Dovč, M.; Pate, M. Mycobacteria in aquarium fish: Results of a 3-year survey indicate caution required in handling pet-shop fish. J. Fish Dis. 2017, 40, 773–784. [Google Scholar] [CrossRef]
- Mugetti, D.; Tomasoni, M.; Pastorino, P.; Esposito, G.; Menconi, V.; Dondo, A.; Prearo, M. Gene Sequencing and Phylogenetic Analysis: Powerful Tools for an Improved Diagnosis of Fish Mycobacteriosis Caused by Mycobacterium fortuitum Group Members. Microorganisms 2021, 10, 797. [Google Scholar] [CrossRef]
- Apostolopoulos, N.; Prenger-Berninghoff, E.; Wildermuth, B.; Moser, I.; Hillemann, D.; Nobach, D.; Herden, C.; Ewers, C.; Thom, N. Mycobacterium setense isolated from a cat with atypical mycobacterial panniculitis. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 2021, 49, 390–396. (In English) [Google Scholar] [CrossRef]
- Shojaei, H.; Heidarieh, P.; Hashemi, A.; Feizabadi, M.M.; Daei Naser, A. Species identification of neglected nontuberculous mycobacteria in a developing country. Jpn. J. Infect. Dis. 2011, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, S.; Ohama, Y.; Okazaki, M.; Nukui, Y.; Moriya, K. Antimicrobial susceptibility testing of rapidly growing mycobacteria isolated in Japan. BMC Infect. Dis. 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Bouam, A.; Levasseur, A.; Drancourt, M. Draft Genome Sequence of Mycobacterium setense CSUR47. Genome Announc. 2018, 18, e01415–e01417. [Google Scholar] [CrossRef] [PubMed]
- Azadi, D.; Shojaei, H.; Pourchangiz, M.; Dibaj, R.; Davarpanah, M.; Naser, A.D. Species diversity and molecular characterization of nontuberculous mycobacteria in hospital water system of a developing country, Iran. Microb. Pathog. 2016, 100, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M. Case report of isolation of Mycobacterium setense from a hospital water supply. Environ. Dis. 2018, 3, 52–54. [Google Scholar] [CrossRef]
- Davarpanah, M.; Azadi, D.; Shojaei, H. Prevalence and molecular characterization of non-tuberculous mycobacteria in hospital soil and dust of a developing country, Iran. Microbiology 2019, 165, 1306–1314. [Google Scholar] [CrossRef]
- Siavashifar, M.; Rezaei, F.; Motallebirad, T.; Azadi, D.; Absalan, A.; Naserramezani, Z.; Golshani, M.; Jafarinia, M.; Ghaffari, K. Species diversity and molecular analysis of opportunistic Mycobacterium, Nocardia and Rhodococcus isolated from the hospital environment in a developing country, a potential resources for nosocomial infection. Genes Environ. 2021, 28, 43. [Google Scholar] [CrossRef]
- Pang, H.; Li, G.; Wan, L.; Jiang, Y.; Liu, H.; Zhao, X.; Zhao, Z.; Wan, K. In vitro drug susceptibility of 40 international reference rapidly growing mycobacteria to 20 antimicrobial agents. Int. J. Clin. Exp. Med. 2015, 15, 15423–15431. [Google Scholar]
- Varghese, B.; Al-Hajoj, S. A global update on rare non-tuberculous mycobacteria in humans: Epidemiology and emergence. Int. J. Tuberc. Lung Dis. 2020, 24, 214–223. [Google Scholar] [CrossRef]
- Palop, M.L.; Valles, S.; Pinaga, F.; Flors, A. Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium celerecrescens sp. nov. Int. J. Syst. Evol. Microbiol. 1989, 39, 68–71. [Google Scholar] [CrossRef]
- Glazunova, O.O.; Raoult, D.; Roux, V. First identification of Clostridium celerecrescens in liquid drained from an abscess. J. Clin. Microbiol. 2005, 43, 3007–3008. [Google Scholar] [CrossRef] [PubMed]
- Mischnik, A.; Zimmermann, S.; Bekeredjian-Ding, I.; Egermann, M. Relapse of posttraumatic osteomyelitis due to Clostridium celerecrescens. Infection 2011, 39, 491–494. [Google Scholar] [CrossRef]
- Griffith, D.E.; Aksamit, T.; Brown-Elliott, B.A.; Catanzaro, A.; Daley, C.; Gordin, F.; Holland, S.M.; Horsburgh, R.; Huitt, G.; Iademarco, M.F.; et al. An official ATS/IDSA statement: Diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007, 175, 367–416. [Google Scholar] [CrossRef] [PubMed]
- Wi, Y.M. Treatment of extrapulmonary nontuberculous mycobacterial diseases. Infect. Chemother. 2019, 51, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kucera, T.; Ryskova, L.; Soukup, T.; Malakova, J.; Cermakova, E.; Mericka, P.; Suchanek, J.; Sponer, P. Elution kinetics of vancomycin and gentamicin from carriers and their effects on mesenchymal stem cell proliferation: An in vitro study. BMC Musculoskelet. Disord. 2017, 2, 381. [Google Scholar] [CrossRef]
- Adekambi, T.; Colson, P.; Drancourt, M. rpoB-based identification of nonpigmented and late-pigmenting rapidly growing mycobacteria. J. Clin. Microbiol. 2003, 41, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Helb, D.; Burday, M.; Connell, N.; Alland, D. A detailed analysis of 16S ribosomal RNA gene segments for the diagnosis of pathogenic bacteria. J. Microbiol. Methods 2007, 69, 330–339. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Susceptibility Testing of Mycobacteria, Nocardia spp, and Other Aerobic Actinomyces, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018; Volume M24. [Google Scholar]

| Mycobacterium setense | Clostridium celerecrescens | ||||
|---|---|---|---|---|---|
| Antimicrobial | MIC | Interpretation | Antimicrobial | MIC | Interpretation |
| Clarithromycin | 24 | R | Penicillin | 0.19 | S |
| Amikacin | 0.38 | S | Amoxicillin | 0.25 | S |
| Imipenem | 0.5 | S | Clindamycin | 12 | R |
| Linezolid | 12 | R | Metronidazole | 0.047 | S |
| Tigecycline | 0.032 | S | Vancomycin | 0.5 | S |
| Ciprofloxacin | 0.032 | S | Linezolid | 0.16 | S |
| Moxifloxacin | 0.064 | S | Imipenem | 0.25 | S |
| Trimethoprim–sulfamethoxazole | 0.125 | S | |||
| Doxycycline | 24 | R | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryskova, L.; Zahradnicek, J.; Kukla, R.; Bolehovska, R.; Vajda, M.; Pavlik, I.; Bostik, P.; Ryska, P. Dual Infection of an Open Fracture Caused by Mycobacterium setense and Clostridium celerecrescens. Antibiotics 2022, 11, 1254. https://doi.org/10.3390/antibiotics11091254
Ryskova L, Zahradnicek J, Kukla R, Bolehovska R, Vajda M, Pavlik I, Bostik P, Ryska P. Dual Infection of an Open Fracture Caused by Mycobacterium setense and Clostridium celerecrescens. Antibiotics. 2022; 11(9):1254. https://doi.org/10.3390/antibiotics11091254
Chicago/Turabian StyleRyskova, Lenka, Jan Zahradnicek, Rudolf Kukla, Radka Bolehovska, Milan Vajda, Ivo Pavlik, Pavel Bostik, and Pavel Ryska. 2022. "Dual Infection of an Open Fracture Caused by Mycobacterium setense and Clostridium celerecrescens" Antibiotics 11, no. 9: 1254. https://doi.org/10.3390/antibiotics11091254
APA StyleRyskova, L., Zahradnicek, J., Kukla, R., Bolehovska, R., Vajda, M., Pavlik, I., Bostik, P., & Ryska, P. (2022). Dual Infection of an Open Fracture Caused by Mycobacterium setense and Clostridium celerecrescens. Antibiotics, 11(9), 1254. https://doi.org/10.3390/antibiotics11091254





