Antifungal Susceptibility of Candida albicans Isolated from Tongue and Subgingival Biofilm of Periodontitis Patients
Abstract
1. Introduction
2. Results
2.1. Demographic and Clinical Periodontal Results
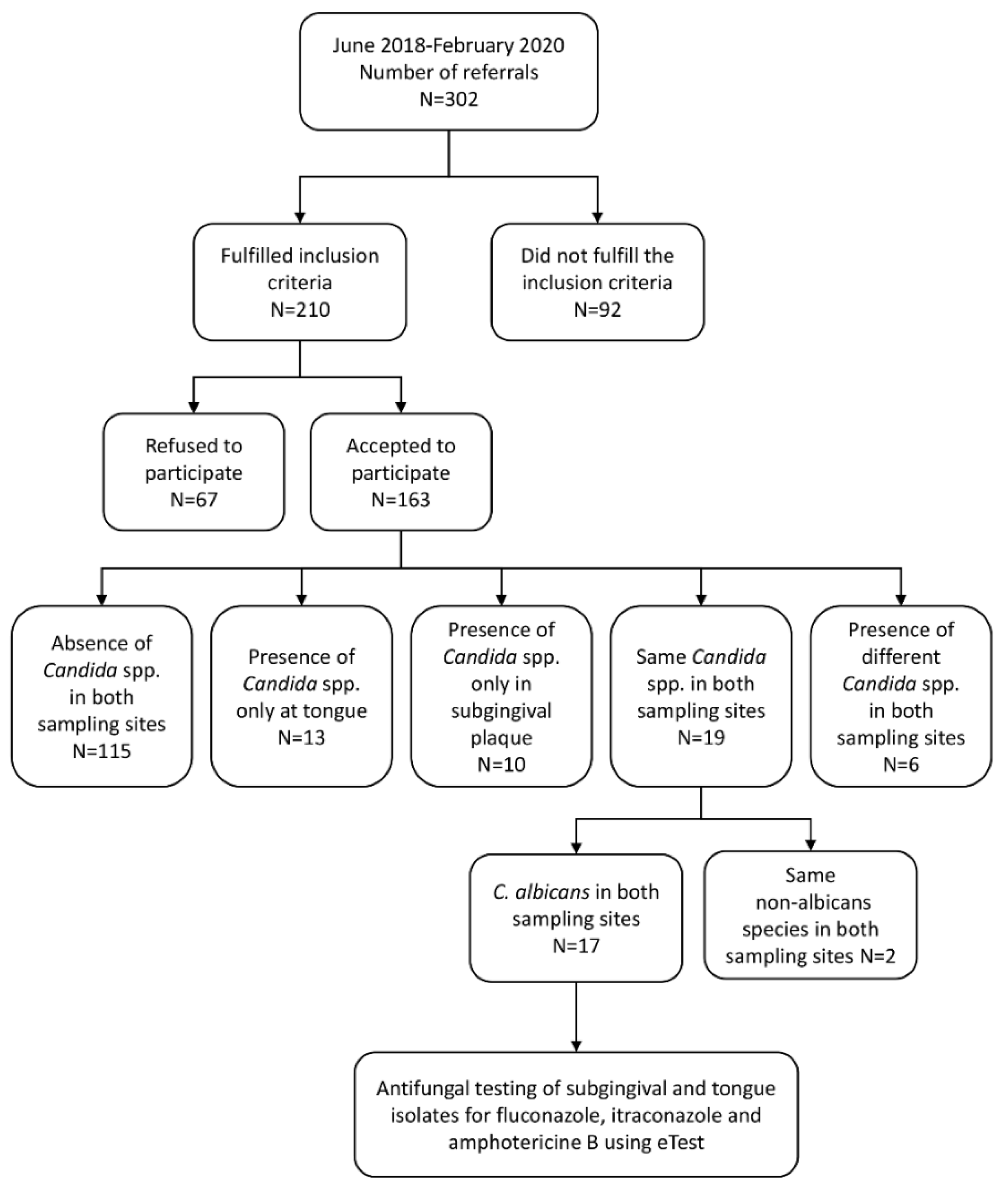
2.2. Distribution of Candida Species in Tongue and Subgingival Samples—Primary Outcome
2.3. Antifungal Susceptibility Results—Secondary Outcome
2.4. Relationship between the Susceptibility of Candida albicans to Antifungals and the Clinical Periodontal Parameters
2.5. Confounding Data Results—Denture Wearers
3. Discussion
4. Materials and Methods
4.1. Study Design, Ethical Approval and Inclusion Criteria
4.2. Periodontal Assessment and Diagnosis of Periodontitis
- (1)
- full-mouth plaque score (FMPS)—expressed as the percentage of sites with soft or mineral debris;
- (2)
- full-mouth bleeding score (FMBS)—expressed as the percentage of bleeding sites 15 s after probing;
- (3)
- mean PPD—mean value for full-mouth probing pocket depth;
- (4)
- mean probing pocket depth at sites with PPD ≥ 6 mm;
- (5)
- mean CAL—mean value for full-mouth clinical attachment loss;
- (6)
- mean CAL at sites ≥ 5 mm;
- (7)
- % of sites 4 mm ≤ PPD < 6 mm—calculated as the percentage of sites with a probing depth of 4 or 5 mm relative to all measured sites;
- (8)
- % of sites PPD ≥6 mm—calculated as the percentage of sites with a probing depth of 6 mm or deeper relative to all measured sites;
- (9)
- No. of sites PPD ≥6 mm—the number of sites with a probing pocket depth of 6 mm or more;
- (10)
- % of sites 3 mm ≤ CAL < 5 mm—calculated as the percentage of sites with clinical attachment loss of 3 or 4 mm relative to all measured sites;
- (11)
- % of sites CAL ≥ 5 mm—calculated as the percentage of sites with clinical attachment loss of 5 mm or higher relative to all measured sites.
4.3. Microbiological Sampling Procedures and Analysis
4.4. Antifungal Susceptibility Testing
4.5. Study Outcomes
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arweiler, N.B.; Netuschil, L. The oral microbiota. Microbiota Hum. Body 2016, 902, 45–60. [Google Scholar]
- Colombo, A.P.V.; Magalhães, C.B.; Hartenbach, F.A.R.R.; do Souto, R.M.; da Silva-Boghossian, C.M. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Meto, A.; Colombari, B.; Sala, A.; Pericolini, E.; Meto, A.; Peppoloni, S.; Blasi, E. Antimicrobial and antibiofilm efficacy of a copper/calcium hydroxide-based endodontic paste against Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. Dent. Mater. J. 2019, 38, 591–603. [Google Scholar] [CrossRef] [PubMed]
- Alberti, A.; Corbella, S.; Taschieri, S.; Francetti, L.; Fakhruddin, K.S.; Samaranayake, L.P. Fungal species in endodontic infections: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0255003. [Google Scholar] [CrossRef] [PubMed]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Bertolini, M.; Dongari-Bagtzoglou, A. The relationship of Candida albicans with the oral bacterial microbiome in health and disease. In Oral Mucosal Immunity and Microbiome; Springer: Berlin/Heidelberg, Germany, 2019; pp. 69–78. [Google Scholar]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-bacterial interactions in health and disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef]
- Boyer, E.; Martin, B.; Le Gall-David, S.; Fong, S.B.; Deugnier, Y.; Bonnaure-Mallet, M.; Meuric, V. Periodontal pathogens and clinical parameters in chronic periodontitis. Mol. Oral Microbiol. 2020, 35, 19–28. [Google Scholar] [CrossRef]
- Klimesova, K.; Jiraskova Zakostelska, Z.; Tlaskalova-Hogenova, H. Oral bacterial and fungal microbiome impacts colorectal carcinogenesis. Front. Microbiol. 2018, 9, 774. [Google Scholar] [CrossRef]
- Matic Petrovic, S.; Radunovic, M.; Barac, M.; Kuzmanovic Pficer, J.; Pavlica, D.; Arsic Arsenijevic, V.; Pucar, A. Subgingival areas as potential reservoirs of different Candida spp in type 2 diabetes patients and healthy subjects. PLoS ONE 2019, 14, e0210527. [Google Scholar] [CrossRef]
- Han, Y.W.; Wang, X. Mobile microbiome: Oral bacteria in extra-oral infections and inflammation. J. Dent. Res. 2013, 92, 485–491. [Google Scholar] [CrossRef]
- McCarty, T.P.; Pappas, P.G. Invasive candidiasis. Infect. Dis. Clin. 2016, 30, 103–124. [Google Scholar] [CrossRef] [PubMed]
- Terzic, M.; Aimagambetova, G.; Terzic, S.; Radunovic, M.; Bapayeva, G.; Laganà, A.S. Periodontal pathogens and preterm birth: Current knowledge and further interventions. Pathogens 2021, 10, 730. [Google Scholar] [CrossRef]
- Sanz, M.; Marco del Castillo, A.; Jepsen, S.; Gonzalez-Juanatey, J.R.; D’Aiuto, F.; Bouchard, P.; Chapple, I.; Dietrich, T.; Gotsman, I.; Graziani, F.; et al. Periodontitis and cardiovascular diseases: Consensus report. J. Clin. Periodontol. 2020, 47, 268–288. [Google Scholar] [CrossRef]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Jeronimo, L.S.; Abreu, L.G.; Cunha, F.A.; Lima, R.E. Association between periodontitis and nosocomial pneumonia: A systematic review and meta-analysis of observational studies. Oral Health Prev. Dent. 2020, 18, 11–17. [Google Scholar] [PubMed]
- Gülses, A.; Açil, Y.; Wiltfang, J. Oral surgery related fungal endocarditis: The need for a novel concept in endocarditis prophylaxy. Med. Hypotheses 2020, 135, 109482. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, M.; Walker, C. Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol. Immunol. 2007, 22, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Al-Fattani, M.A.; Douglas, L.J. Penetration of Candida biofilms by antifungal agents. Antimicrob. Agents Chemother. 2004, 48, 3291–3297. [Google Scholar] [CrossRef]
- Kim, S.-M.; Kim, H.C.; Lee, S.-W.S. Characterization of antibiotic resistance determinants in oral biofilms. J. Microbiol. 2011, 49, 595–602. [Google Scholar] [CrossRef]
- Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 10.0, 2020. CLinical Breakpoint for Fungi. Available online: https://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals (accessed on 24 April 2021).
- Monroy-Pérez, E.; Rodríguez-Bedolla, R.M.; Garzón, J.; Vaca-Paniagua, F.; Jiménez, E.A.-R.; Paniagua-Contreras, G.L. Marked virulence and azole resistance in Candida albicans isolated from patients with periodontal disease. Microb. Pathog. 2020, 148, 104436. [Google Scholar] [CrossRef]
- Krishnan, G.S.; Naik, D.; Uppoor, A.; Nayak, S.; Baliga, S.; Maddi, A. Candidal carriage in saliva and subgingival plaque among smokers and non-smokers with chronic periodontitis—A cross-sectional study. PeerJ 2020, 8, e8441. [Google Scholar] [CrossRef] [PubMed]
- Urzúa, B.; Hermosilla, G.; Gamonal, J.; Morales-Bozo, I.; Canals, M.; Barahona, S.; Cóccola, C.; Cifuentes, V. Yeast diversity in the oral microbiota of subjects with periodontitis: Candida albicans and Candida dubliniensis colonize the periodontal pockets. Sabouraudia 2008, 46, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Cimbaljević, M.; Radunović, M.; Jotić, A.; Pucar, A. Detection and sampling methods for isolation of Candida spp. from oral cavities in diabetics and non-diabetics. Braz. Oral Res. 2015, 29, 77. [Google Scholar]
- Marsh, P.D. Role of the Oral Microflora in Health. Microb. Ecol. Health Dis. 2000, 12, 130–137. [Google Scholar] [CrossRef]
- Thomas, J.G.; Nakaishi, L.A. Managing the complexity of a dynamic biofilm. J. Am. Dent. Assoc. 2006, 137, S10–S15. [Google Scholar] [CrossRef]
- Rosa, E.A.R.; Rached, R.N.; Ignácio, S.A.; Rosa, R.T.; da Silva, W.J.; Yau, J.Y.Y.; Samaranayake, L.P. Phenotypic evaluation of the effect of anaerobiosis on some virulence attributes of Candida albicans. J. Med. Microbiol. 2008, 57, 1277–1281. [Google Scholar] [CrossRef]
- Shapiro, R.S.; Ryan, O.; Boone, C.; Cowen, L.E. Regulatory circuitry governing morphogenesis in Saccharomyces cerevisiae and Candida albicans. Cell Cycle 2012, 11, 4294–4295. [Google Scholar] [CrossRef]
- Dumitru, R.; Hornby, J.M.; Nickerson, K.W. Defined anaerobic growth medium for studying Candida albicans basic biology and resistance to eight antifungal drugs. Antimicrob. Agents Chemother. 2004, 48, 2350–2354. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chaffin, W.L. Anaerobic growth of Candida albicans does not support biofilm formation under similar conditions used for aerobic biofilm. Curr. Microbiol. 2005, 51, 100–104. [Google Scholar] [CrossRef]
- Karkowska-Kuleta, J.; Bartnicka, D.; Zawrotniak, M.; Zielinska, G.; Kierońska, A.; Bochenska, O.; Ciaston, I.; Koziel, J.; Potempa, J.; Baster, Z. The activity of bacterial peptidylarginine deiminase is important during formation of dual-species biofilm by periodontal pathogen Porphyromonas gingivalis and opportunistic fungus Candida albicans. Pathog. Dis. 2018, 76, fty033. [Google Scholar] [CrossRef]
- Bachtiar, E.W.; Bachtiar, B.M.; Jarosz, L.M.; Amir, L.R.; Sunarto, H.; Ganin, H.; Meijler, M.M.; Krom, B.P. AI-2 of Aggregatibacter actinomycetemcomitans inhibits Candida albicans biofilm formation. Front. Cell. Infect. Microbiol. 2014, 4, 94. [Google Scholar] [CrossRef] [PubMed]
- Bor, B.; Cen, L.; Agnello, M.; Shi, W.; He, X. Morphological and physiological changes induced by contact-dependent interaction between Candida albicans and Fusobacterium nucleatum. Sci. Rep. 2016, 6, 27956. [Google Scholar] [CrossRef] [PubMed]
- Bachtiar, E.W.; Bachtiar, B.M. Effect of cell-free spent media prepared from Aggregatibacter actinomycetemcomitans on the growth of Candida albicans and Streptococcus mutans in co-species biofilms. Eur. J. Oral Sci. 2020, 128, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.; Lundgren, B.; Jensen, I.M.; Hansen, B.S.; Frimodt-Møller, N. Comparison of Etest and a tablet diffusion test with the NCCLS broth microdilution method for fluconazole and amphotericin B susceptibility testing of Candida isolates. J. Antimicrob. Chemother. 2001, 47, 521–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elder, J.V. Fluconazole and amphotericine B antifungal susceptibility testing by National Committee for Clinical Laboratory Standards broth macrodilution method compared with E test and semiautomated broth microdilution test. J. Clin. Microbiol. 1996, 32, 2099–2102. [Google Scholar]
- Sharma, J.; Rosiana, S.; Razzaq, I.; Shapiro, R.S. Linking Cellular Morphogenesis with Antifungal Treatment and Susceptibility in Candida Pathogens. J. Fungi 2019, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Jensen, R.H.; Astvad, K.M.T.; Silva, L.V.; Sanglard, D.; Jørgensen, R.; Nielsen, K.F.; Mathiasen, E.G.; Doroudian, G.; Perlin, D.S.; Arendrup, M.C. Stepwise emergence of azole, echinocandin and amphotericin B multidrug resistance in vivo in Candida albicans orchestrated by multiple genetic alterations. J. Antimicrob. Chemother. 2015, 70, 2551–2555. [Google Scholar] [CrossRef]
- Furlletti, V.F.; de Cássia Mardegan, R.; Obando-Pereda, G.A.; Aníbal, P.C.; Duarte, M.C.T.; Gonçalves, R.B.; Höfling, J.F. Susceptibility of Candida spp. Oral isolates for azolic antifungals and amphotericin B. Braz. J. Oral Sci. 2008, 7, os08015. [Google Scholar]
- Ellis, D. Amphotericin B: Spectrum and resistance. J. Antimicrob. Chemother. 2002, 49, 7–10. [Google Scholar] [CrossRef]
- Kamińska, A.; Malm, A.; Szymańska, J. Antifungal drugs resistance profiles of C. albicans strains isolated from the oral cavity of children and adolescents. Acta Pol. Pharm. Drug Res. 2019, 76, 873–876. [Google Scholar] [CrossRef]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Douglas, L.J. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob. Agents Chemother. 1998, 42, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- Jelesić, Z.Z.; Medić, D.D.; Mihajlović-Ukropina, M.M.; Jevtić, M.; Gusman, V.P.; Radosavljević, B.J.; Milosavljević, B.T. Susceptibility to antifungal agents of Candida spp. from blood and feces collected in Novi Sad in 3-year period (2008–2010). Zb. Matice Srp. Za Prir. Nauk. 2011, 2011, 19–26. [Google Scholar] [CrossRef]
- Matić-Petrović, S.; Đorđević, M.; Radunović, M.; Živanović, T.; Pavlica, D.; Pucar, A. Geographic tongue: Does Candida play a role in its pathogenesis. Balk. J. Dent. Med. 2019, 23, 152–156. [Google Scholar] [CrossRef]
- Arendrup, M.C. Candida and candidaemia. Susceptibility and epidemiology. Dan. Med. J. 2013, 60, B4698. [Google Scholar] [PubMed]
- Volpato Sanitá, P.; Lúcia Machado, A.; Cláudia Pavarina, A.; Maria Sgavioli Massucato, E.; Lopes Colombo, A.; Eduardo Vergani, C. Microwave denture disinfection versus nystatin in treating patients with well-controlled type 2 diabetes and denture stomatitis: A randomized clinical trial. Int. J. Prosthodont. 2012, 25, 232–244. [Google Scholar]
- Bachtiar, B.M.; Fath, T.; Widowati, R.; Bachtiar, E.W. Quantification and pathogenicity of Candida albicans in denture-wearing and nondenture-wearing elderly. Eur. J. Dent. 2020, 14, 423–428. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.; Leidich, S.; Faddoul, F.; Hoyer, L.; Douglas, L.; Ghannoum, M. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 2001, 80, 903–908. [Google Scholar] [CrossRef]
- Lyon, J.; Moreira, L.; Cardoso, M.; Saade, J.; Resende, M. Antifungal suscepitibility profile of Candida spp. oral isolates obtained from denture wearers. Braz. J. Microbiol. 2008, 39, 668–672. [Google Scholar] [CrossRef][Green Version]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions–Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Villafuerte, K.R.; Dantas, F.T.; Messora, M.R.; Novaes, A.B., Jr.; Grisi, M.F.; Taba Jr, M.; Souza, S.L.; Candido dos Reis, F.J.; Carrara, H.H.; Palioto, D.B. Preliminary results of non-surgical periodontal treatment in patients with breast cancer undergoing chemotherapy. J. Periodontol. 2016, 87, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- O'leary, T.J. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Marinho, S.A.; Teixeira, A.B.; Santos, O.S.; Cazanova, R.F.; Ferreira, C.A.S.; Cherubini, K.; Oliveira, S.D.d. Identification of Candida spp. by phenotypic tests and PCR. Braz. J. Microbiol. 2010, 41, 286–294. [Google Scholar] [CrossRef] [PubMed]
| Variable Mean ± SD (Min–Max) | |
|---|---|
| No. of teeth | 20.88 ± 4.90 |
| FMPS (%) | 72.75 ± 26.74 |
| FMBS (%) | 62.07 ± 27.71 |
| Mean PPD (mm) | 3.81 ± 1.06 |
| Mean CAL (mm) | 4.04 ± 1.59 |
| Mean PPD at sites ≥6 mm (mm) | 6.69 ± 0.88 |
| Mean CAL at sites ≥5 mm (mm) | 6.14 ± 1.06 |
| % of sites 4 mm ≤ PPD < 6 mm | 27.65 ± 15.79 |
| % of sites PPD ≥ 6 mm | 11.45 ± 18.06 |
| No. of sites PPD ≥ 6 mm | 14.84 ± 23.67 |
| % of sites 3 mm ≤ CAL < 5 mm | 32.36 ± 16.04 |
| % of sites CAL ≥ 5 mm | 29.35 ± 26.98 |
| Tongue Sample | Subgingival Sample | p Value | ||
|---|---|---|---|---|
| Mean ± SD Median (Min–Max) | Mean ± SD Median (Min–Max) | |||
| MIC (μL/mL) | Fluconazole | 4.79 ± 4.61 2.00 (0.38–16.00) | 5.88 ± 8.13 2.00 (1.00–32.00) | 0.709 |
| Amphotericin B | 0.42 ± 0.27 0.25 (0.19–1.00) | 0.38 ± 0.18 0.25 (0.19–0.75) | 0.919 | |
| Itraconazole | 2.22 ± 7.69 0.12 (0.019–32) | 2.80 ± 7.78 0.12 (0.019–32) | 0.708 | |
| Clinical Parameter | Antimycotic (MIC) | Sampling Site | R | p Value |
|---|---|---|---|---|
| FMPS | Amphotericin B | Tongue | 0.566 | 0.018 |
| Fluconazole | 0.681 | 0.003 | ||
| Itraconazole | 0.529 | 0.029 | ||
| FMBS | Itraconazole | Tongue | 0.524 | 0.031 |
| Mean PPD at sites ≥ 6mm | Itraconazole | Tongue | −0.723 | 0.012 |
| % site 3 mm ≤ CAL < 5 mm | Amphotericin B | Tongue | 0.596 | 0.012 |
| Amphotericin | Periodontal pocket | 0.557 | 0.020 | |
| Fluconazole | Tongue | 0.639 | 0.006 | |
| Fluconazole | Periodontal pocket | 0.592 | 0.012 | |
| Itraconazole | Tongue | 0.547 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radunovic, M.; Barac, M.; Kuzmanovic Pficer, J.; Pavlica, D.; Jovanovic, A.; Pucar, A.; Petrovic, S. Antifungal Susceptibility of Candida albicans Isolated from Tongue and Subgingival Biofilm of Periodontitis Patients. Antibiotics 2022, 11, 802. https://doi.org/10.3390/antibiotics11060802
Radunovic M, Barac M, Kuzmanovic Pficer J, Pavlica D, Jovanovic A, Pucar A, Petrovic S. Antifungal Susceptibility of Candida albicans Isolated from Tongue and Subgingival Biofilm of Periodontitis Patients. Antibiotics. 2022; 11(6):802. https://doi.org/10.3390/antibiotics11060802
Chicago/Turabian StyleRadunovic, Milena, Milena Barac, Jovana Kuzmanovic Pficer, Dusan Pavlica, Aleksandar Jovanovic, Ana Pucar, and Sanja Petrovic. 2022. "Antifungal Susceptibility of Candida albicans Isolated from Tongue and Subgingival Biofilm of Periodontitis Patients" Antibiotics 11, no. 6: 802. https://doi.org/10.3390/antibiotics11060802
APA StyleRadunovic, M., Barac, M., Kuzmanovic Pficer, J., Pavlica, D., Jovanovic, A., Pucar, A., & Petrovic, S. (2022). Antifungal Susceptibility of Candida albicans Isolated from Tongue and Subgingival Biofilm of Periodontitis Patients. Antibiotics, 11(6), 802. https://doi.org/10.3390/antibiotics11060802







