Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review
Abstract
:1. Introduction
- Changes in the global scale and patterns of antibiotic use in COVID-19 patients since the early stages of the pandemic.
- The proportion of antibiotic use based on the suspicion or confirmation of bacterial infection in COVID-19 patients and the proportion of antibiotic use as an empirical treatment.
- The prevalence of bacterial infection in COVID-19 patients and rates of secondary bacterial infection and bacterial co-infection. These were defined as follows, with the duration of time since hospital admission acting as a proxy for defining hospital-acquired vs. community-acquired infection: Secondary infection—bacterial infection that developed during a hospital stay of >3 days; co-infection—bacterial infection that was already present at admission or detected within 3 days of hospital admission).
- Frequently prescribed antibiotics and the most commonly resistant pathogens in COVID-19 patients.
- The impact of antibiotic use on the clinical outcomes of COVID-19 patients.
- The impacts of bacterial infection on the clinical outcomes of COVID-19 patients.
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria and Study Selection Process
2.2.1. Inclusion Criteria
- All types of clinical studies (cohort, cross-sectional, case–control, randomized control trials (RCT), case reports including case series, other observational studies and some qualitative studies including surveys) reporting the use of antibiotics to treat patients with COVID-19.
- Studies reporting patients diagnosed with COVID-19 and receiving antibiotic treatment, without restrictions on age, race, gender, geographical location or setting.
- Studies which reported both antibiotic treatment and clinical outcomes for COVID-19 patients.
2.2.2. Exclusion Criteria
- Animal studies, in vitro experiments, in silico screening/drug modeling, molecular mechanisms and other aspects of COVID-19 research that were not related to or did not mention antibiotic use (ABU).
- Conference abstracts.
- Commentaries and editorial notes and letters.
- Perspectives.
- Literature reviews.
- Trial protocols.
- Clinical updates or guidelines.
- Case reports and case series with a documented sample size of less than 10 COVID-19 patients.
- Full-text articles not available in English or Chinese.
2.3. Data Extraction
2.4. Data Synthesis and Analysis
3. Results
3.1. Study Selection
3.2. Description of Included Studies
3.3. Antibiotic Prescribing for COVID-19 Patients
3.3.1. Antibiotic Prescribing and Healthcare Settings
3.3.2. Antibiotic Prescribing and Illness Severity
3.3.3. Antibiotic Prescribing for COVID-19 Patients over the Course of the Pandemic
3.3.4. Antibiotic Prescribing and Health Outcomes
3.3.5. Severity Categories and Outcomes
3.3.6. Antibiotic Prescribing with Health Outcomes by Country Economic Status, Geographical Region, and Study Type
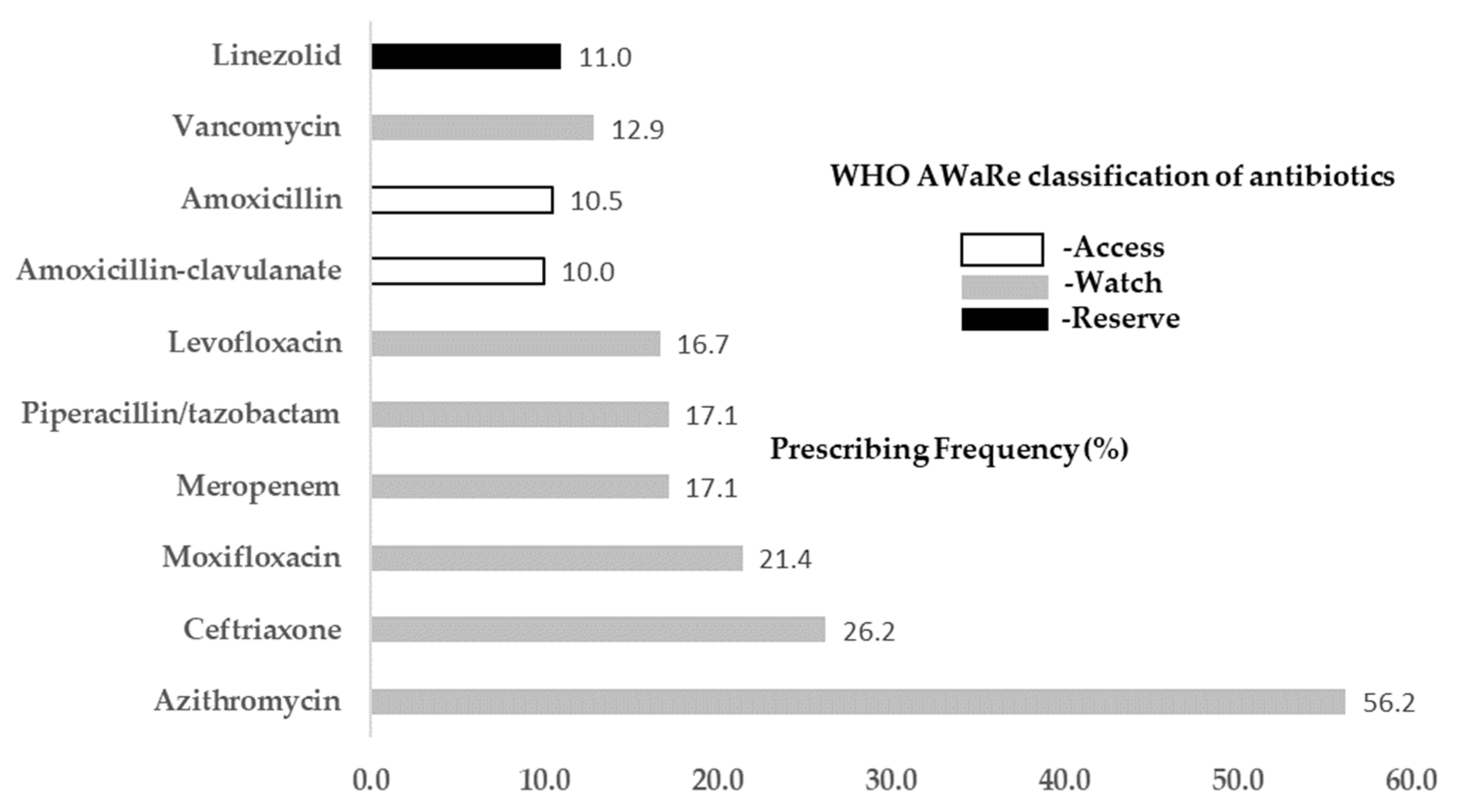
3.4. Frequently Prescribed Antibiotics for COVID-19 Patients
3.5. Frequent Antibiotic Prescribing Scenarios for COVID-19 Patients
3.6. Bacterial Infection in COVID-19 Patients
3.7. Frequently Reported Resistant Pathogens in COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jim, O. Antimicrobial Resistance Antimicrobial Resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 20, 1–16. [Google Scholar]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- COVID-19 and Antibiotic Resistance: What Is the Link? Available online: https://www.medicalnewstoday.com/articles/covid-19-may-have-led-to-a-spike-in-antibiotic-resistance (accessed on 2 May 2022).
- Cong, W.; Poudel, A.N.; Alhusein, N.; Wang, H.; Yao, G.; Lambert, H. Antimicrobial Use in COVID-19 Patients in the First Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2021, 10, 745. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A.H. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Baghdadi, J.D.; Coffey, K.C.; Adediran, T.; Goodman, K.E.; Pineles, L.; Magder, L.S.; O’Hara, L.M.; Pineles, B.L.; Nadimpalli, G.; Morgan, D.J.; et al. Antibiotic Use and Bacterial Infection among Inpatients in the First Wave of COVID-19: A Retrospective Cohort Study of 64,691 Patients. Antimicrob. Agents Chemother. 2021, 65, e0134121. [Google Scholar] [CrossRef] [PubMed]
- Goncalves Mendes Neto, A.; Lo, K.B.; Wattoo, A.; Salacup, G.; Pelayo, J.; DeJoy III, R.; Bhargav, R.; Gul, F.; Peterson, E.; Albano, J.; et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 2021, 93, 1489–1495. [Google Scholar] [CrossRef]
- Pettit, N.N.; Nguyen, C.T.; Lew, A.K.; Bhagat, P.H.; Nelson, A.; Olson, G.; Ridgway, J.P.; Pho, M.T.; Pagkas-Bather, J. Reducing the use of empiric antibiotic therapy in COVID-19 on hospital admission. BMC Infect. Dis. 2021, 1, 516. [Google Scholar] [CrossRef]
- Antibiotic Use at Veterans Affairs’ Hospitals Increases During COVID-19 Pandemic, Reversing a Four-Year Downward Trend. Available online: https://www.idsociety.org/news--publications-new/articles/2020/antibiotic-use-at-veterans-affairs-hospitals-increases-during-covid-19-pandemic-reversing-a-four-year-downward-trend/ (accessed on 4 December 2020).
- Buetti, N.; Mazzuchelli, T.; Priore, E.L.; Balmelli, C.; Llamas, M.; Pallanza, M.; Elzi, L.; Consonni, V.; Trimboli, P.; Forni-Ogna, V.; et al. Early administered antibiotics do not impact mortality in critically ill patients with COVID-19. J. Infect. 2020, 81, e148–e149. [Google Scholar] [CrossRef]
- Ng, T.M.; Ong, S.W.X.; Loo, A.Y.X.; Tan, S.H.; Tay, H.L.; Yap, M.Y.; Lye, D.C.; Lee, T.H.; Young, B.E. Antibiotic Therapy in the Treatment of COVID-19 Pneumonia: Who and When? Antibiotics 2022, 11, 184. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Management of COVID-19 Interim Guidance; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 2 May 2022).
- National Institute for Health and Care Excellence. COVID-19 Rapid Guideline: Managing COVID-19. 2022. Available online: https://www.nice.org.uk/guidance/ng191/resources/covid19-rapid-guideline-managing-covid19-pdf-51035553326 (accessed on 2 May 2022).
- National Institutes of Health. COVID-19 Treatment Guidelines. 2021. Available online: https://www.covid19treatmentguidelines.nih.gov/management/critical-care-for-adults/pharmacologic-interventions-for-critically-ill-patients/ (accessed on 2 May 2022).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Bank Data Help Desk. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 4 December 2021).
- World Health Organization: 2021 AWaRe Classification. 2021. Available online: https://www.who.int/publications/i/item/2021-aware-classification (accessed on 10 March 2022).
- Spotlight on Antimicrobial Resistance: The Slow Pandemic. Royal Society of Medicine website. Available online: https://www.rsm.ac.uk/events/rsm-studios/2021-22/ceq03/ (accessed on 24 May 2022).
- Staub, M.B.; Beaulieu, R.M.; Graves, J.; Nelson, G.E. Changes in antimicrobial utilization during the coronavirus disease 2019 (COVID-19) pandemic after implementation of a multispecialty clinical guidance team. Infect. Control Hosp. Epidemiol. 2020, 42, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Aylin, P.; Rawson, T.; Gilchrist, M.; Majeed, A.; Holmes, A. Investigating the impact of COVID-19 on primary care antibiotic prescribing in North West London across two epidemic waves. Clin. Microbiol. Infect. 2021, 27, 762–768. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.; Ong’Uti, S.; Chang, A.; Mui, E.; Nelligan, I.; Betts, B.; Lentz, C.; Alegria, W.; Fox, E.; Meng, L.; et al. Sustained Reduction in Urgent Care Antibiotic Prescribing During the Coronavirus Disease 2019 Pandemic: An Academic Medical Center’s Experience. Open Forum Infect. Dis. 2022, 9, ofab662. [Google Scholar] [CrossRef]
- Hayat, K.; Mustafa, Z.U.; Ikram, M.N.; Ijaz-Ul-Haq, M.; Noor, I.; Rasool, M.F.; Ishaq, H.M.; Rehman, A.U.; Hasan, S.S.; Fang, Y. Perception, Attitude, and Confidence of Physicians About Antimicrobial Resistance and Antimicrobial Prescribing Among COVID-19 Patients: A Cross-Sectional Study from Punjab, Pakistan. Front. Pharmacol. 2022, 12, 794453. [Google Scholar] [CrossRef]
- Kamara, I.F.; Kumar, A.M.V.; Maruta, A.; Fofanah, B.D.; Njuguna, C.K.; Shongwe, S.; Moses, F.; Tengbe, S.M.; Kanu, J.S.; Lakoh, S.; et al. Antibiotic Use in Suspected and Confirmed COVID-19 Patients Admitted to Health Facilities in Sierra Leone in 2020–2021: Practice Does Not Follow Policy. Int. J. Environ. Res. Public Health 2022, 19, 4005. [Google Scholar] [CrossRef]
- Mustafa, Z.U.; Saleem, M.S.; Ikram, M.N.; Salman, M.; Butt, S.A.; Khan, S.; Godman, B.; Seaton, R.A. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: Findings from a multicenter, point prevalence survey. Pathog. Glob. Health 2021, 16, 1–7. [Google Scholar] [CrossRef]
- Butler, C.C.; Dorward, J.; Yu, L.-M.; Gbinigie, O.; Hayward, G.; Saville, B.R.; Van Hecke, O.; Berry, N.; Detry, M.; Saunders, C.; et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet 2021, 397, 1063–1074. [Google Scholar] [CrossRef]
- Hinks, T.S.C.; Cureton, L.; Knight, R.; Wang, A.; Cane, J.L.; Barber, V.S.; Black, J.; Dutton, S.J.; Melhorn, J.; Jabeen, M.; et al. Azithromycin versus standard care in patients with mild-to-moderate COVID-19 (ATOMIC2): An open-label, randomised trial. Lancet Respir. Med. 2021, 9, 1130–1140. [Google Scholar] [CrossRef]
- Lu, Z.K.; Yuan, J.; Li, M.; Sutton, S.S.; Rao, G.A.; Jacob, S.; Bennett, C.L. Cardiac risks associated with antibiotics: Azithromycin and levofloxacin. Expert Opin. Drug Saf. 2014, 14, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, H. Bacterial co-infections and antibiotic resistance in patients with COVID-19. GMS. Hyg. Infect. Control 2020, 15, Doc35. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Torres, V.; de Mendoza, C.; de la Fuente, S.; Sánchez, E.; Martínez-Urbistondo, M.; Herráiz, J.; Gutiérrez, A.; Gutiérrez, Á.; Hernández, C.; Callejas, A.; et al. Bacterial infections in patients hospitalized with COVID-19. Intern. Emerg. Med. 2022, 17, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Choi, J.J.; Pinheiro, L.C.; Schenck, E.J.; Chen, R.; Jabri, A.; Satlin, M.J.; Campion, T.R., Jr.; Nahid, M.; Ringel, J.B.; et al. Clinical Characteristics of Covid-19 in New York City. N. Engl. J. Med. 2020, 382, 2372–2374. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1578. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Farrell, J.M.; Zhao, C.Y.; Tarquinio, K.M.; Brown, S.P. Causes and Consequences of COVID-19-Associated Bacterial Infections. Front. Microbiol. 2021, 12, 682571. [Google Scholar] [CrossRef]
- Ahmed, N.; Khan, M.; Saleem, W.; Karobari, M.I.; Mohamed, R.N.; Heboyan, A.; Rabaan, A.A.; Al Mutair, A.; Alhumaid, S.; Alsadiq, S.A.; et al. Evaluation of Bi-Lateral Co-Infections and Antibiotic Resistance Rates among COVID-19 Patients. Antibiotics 2022, 11, 276. [Google Scholar] [CrossRef]
- Hirabayashi, A.; Kajihara, T.; Yahara, K.; Shibayama, K.; Sugai, M. Impact of the COVID-19 pandemic on the surveillance of antimicrobial resistance. J. Hosp. Infect. 2021, 117, 147–156. [Google Scholar] [CrossRef]
- Chatha, N.; Fortin, D.; Bosma, K.J. Management of necrotizing pneumonia and pulmonary gangrene: A case series and review of the literature. Can. Respir. J. 2014, 21, 239–245. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 20 May 2022).
- National Institute for Health and Care Excellence. Pneumonia (Hospital-Acquired): Antimicrobial Prescribing. 2019. Available online: https://www.nice.org.uk/guidance/ng139/chapter/Recommendations (accessed on 18 July 2022).
- Loomba, P.P.; Taneja, J.; Mishra, B. Methicillin- and Vancomycin-resistant Staphylococcus aureus in hospitalized patients. J. Global. Infect. Dis. 2010, 2, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Alvarez-Uria, G.; Turner, P.; Joshi, J.; Limmathurotsakul, D.; van Doorn, H.R. Antimicrobial Resistance Surveillance in Low- and Middle-Income Countries: Progress and Challenges in Eight South Asian and Southeast Asian Countries. Clin. Microbiol. Rev. 2020, 33, e00048-19. [Google Scholar] [CrossRef] [PubMed]
- Hoque, R.; Ahmed, S.M.; Naher, N.; Islam, M.A.; Rousham, E.K.; Islam, B.Z.; Hassan, S. Tackling antimicrobial resistance in Bangladesh: A scoping review of policy and practice in human, animal and environment sectors. PLoS ONE 2020, 15, e0227947. [Google Scholar] [CrossRef] [Green Version]
- Ranjalkar, J.; Chandy, S.J. India’s National Action Plan for antimicrobial resistance—An overview of the context, status, and way ahead. J. Family Med. Prim. Care 2019, 8, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Hamers, R.L.; van Doorn, H.R. Antibiotic consumption in low-income and middle-income countries. Lancet Glob. Health 2018, 6, e732. [Google Scholar] [CrossRef] [Green Version]


| Patient Category | Number of Studies | Number of Prescribed Antibiotics | Total Number of Patients | Proportion of Prescribed Antibiotics |
|---|---|---|---|---|
| Inpatients | 375 | 82,779 | 156,716 | 52.8% |
| Outpatients | 14 | 8493 | 37,326 | 22.7% |
| Mixed (inpatients and outpatients) | 44 | 72,630 | 218,434 | 33.3% |
| Illness Severity of COVID-19 Patients | Number of Studies | Number of Prescribed Antibiotics | Total Number of Patients | Proportion of Prescribed Antibiotics |
|---|---|---|---|---|
| Severe and critical | 176 | 14,444 | 29,912 | 48.3% |
| Mild and moderate | 144 | 23,761 | 152,975 | 15.5% |
| Illness Severity of COVID-19 Patients | Average Antibiotic Prescribing Rate (%) | |
|---|---|---|
| The First Phase of the Pandemic (from December 2019 to June 2020) | The Second Phase of the Pandemic (from June 2020 to March 2021) | |
| Severe and critical | 75.4% | 48.3% |
| Mild and moderate | 75.1% | 15.5% |
| Overall | 82.3% | 39.7% |
| Category of Antibiotic Prescribing | LOS (Mean Days) | Discharge (Mean %) | Mortality (Mean %) |
|---|---|---|---|
| All given abs | 16.4 (25 studies) | 71.0% (40 studies) | 15.9% (55 studies) |
| Majority are given abs | 14.2 (102 studies) | 75.3% (143 studies) | 16.9% (208 studies) |
| Majority not given abs | 14.5 (43 studies) | 75.7% (68 studies) | 9.0% (101 studies) |
| Illness Severity of COVID-19 Patients | LOS (Mean Days) | Discharge (Mean %) | Mortality (Mean %) |
|---|---|---|---|
| Severe and critical (86 studies) | 15.6 (10 studies) | 59.8% (40 studies) | 27.1% (68 studies) |
| Mild and moderate (47 studies) | 12.0 (30 studies) | 79.2% (25 studies) | 2.0% (35 studies) |
| World Bank Classification | Antibiotic Prescribing Rate (%) | LOS (Mean Days) | Discharge (Mean %) | Mortality (Mean %) |
|---|---|---|---|---|
| High Income | 50.0% (192 studies) | 13.7 (86 studies) | 72.2% (97 studies) | 14.3% (177 studies) |
| Low and Middle income | 28.5% (240 studies) | 15.3 (91 studies) | 79.1% (175 studies) | 9.1% (219 studies) |
| Mixed | 52.9% (7 studies) | 9.3 (2 studies) | 64.9% (3 studies) | 13.0% (5 studies) |
| Geographical Region | Antibiotic Prescribing Percentage | LOS (Mean Days) | Discharge (Mean %) | Mortality (Mean %) |
|---|---|---|---|---|
| East Asia and Pacific | 53.7% (206 studies) | 16.2 (80 studies) | 80.2% (147 studies) | 8.1% (186 studies) |
| Europe and Central Asia | 34.7% (134 studies) | 13.5 (57 studies) | 72.3% (64 studies) | 21.2% (123 studies) |
| Latin America and the Caribbean | 14.8% (15 studies) | 11.1 (10 studies) | 73.8% (12 studies) | 8.9% (17 studies) |
| Middle East and North Africa | 82.2% (25 studies) | 11.7 (16 studies) | 61.4% (20 studies) | 16.0% (22 studies) |
| North America | 73.7% (44 studies) | 13.5 (22 studies) | 75.4% (26 studies) | 11.6% (41 studies) |
| South Asia | 88.7% (7 studies) | 4.2 (1 study) | 95.7% (3 studies) | 10.0% (6 studies) |
| Mixed | 53.5% (8 studies) | 13.5 (3 studies) | 64.9% (3 studies) | 14.4% (6 studies) |
| Antibiotic Prescribing Scenario | Reason for Antibiotic Prescribing for COVID-19 Patients, If Reported | Number of Studies Reported | % of Total Studies |
|---|---|---|---|
| Suspicious or confirmed bacterial infection | Microbiological analysis of samples such as blood, stool, urine or sputum culture was conducted | 77 | 15.8% |
| Empirical antibiotic therapy * | Antibiotics were used as an empirical/adjuvant/concomitant/standard treatment | 143 | 29.4% |
| Antibiotics were prescribed, but unclear whether it was based on suspicion or confirmation of bacterial infections (likely empirical treatment) | 122 | 25.1% |
| Bacterial Infection Category | Number of Studies | Number With Bacterial Infection | Total Number of Patients Accounting for Bacterial Infection | Bacterial Infection Rate (%) |
|---|---|---|---|---|
| Secondary infection | 32 | 1954 | 14,416 | 13.5% |
| Co-infection | 28 | 992 | 14,416 | 7.0% |
| Secondary infection and co-infection | 6 | 198 | 1697 | 11.7% |
| Description | Percentage of Severely/Critically Ill Patients (n) | Percentage of Mildly/Moderately Ill Patients (n) | LOS (Mean Days) | Discharge (Mean %) | Mortality (Mean %) |
|---|---|---|---|---|---|
| Patients with bacterial infection | 22.7% (34 studies) | 3.7% (34 studies) | 21.8 (17 studies) | 59.6% (19 studies) | 30.2% (42 studies) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cong, W.; Stuart, B.; AIhusein, N.; Liu, B.; Tang, Y.; Wang, H.; Wang, Y.; Manchundiya, A.; Lambert, H. Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics 2022, 11, 991. https://doi.org/10.3390/antibiotics11080991
Cong W, Stuart B, AIhusein N, Liu B, Tang Y, Wang H, Wang Y, Manchundiya A, Lambert H. Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics. 2022; 11(8):991. https://doi.org/10.3390/antibiotics11080991
Chicago/Turabian StyleCong, Wenjuan, Beth Stuart, Nour AIhusein, Binjuan Liu, Yunyi Tang, Hexing Wang, Yi Wang, Amit Manchundiya, and Helen Lambert. 2022. "Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review" Antibiotics 11, no. 8: 991. https://doi.org/10.3390/antibiotics11080991
APA StyleCong, W., Stuart, B., AIhusein, N., Liu, B., Tang, Y., Wang, H., Wang, Y., Manchundiya, A., & Lambert, H. (2022). Antibiotic Use and Bacterial Infection in COVID-19 Patients in the Second Phase of the SARS-CoV-2 Pandemic: A Scoping Review. Antibiotics, 11(8), 991. https://doi.org/10.3390/antibiotics11080991






