Anticandidal and Antibiofilm Effect of Synbiotics including Probiotics and Inulin-Type Fructans
Abstract
:1. Introduction
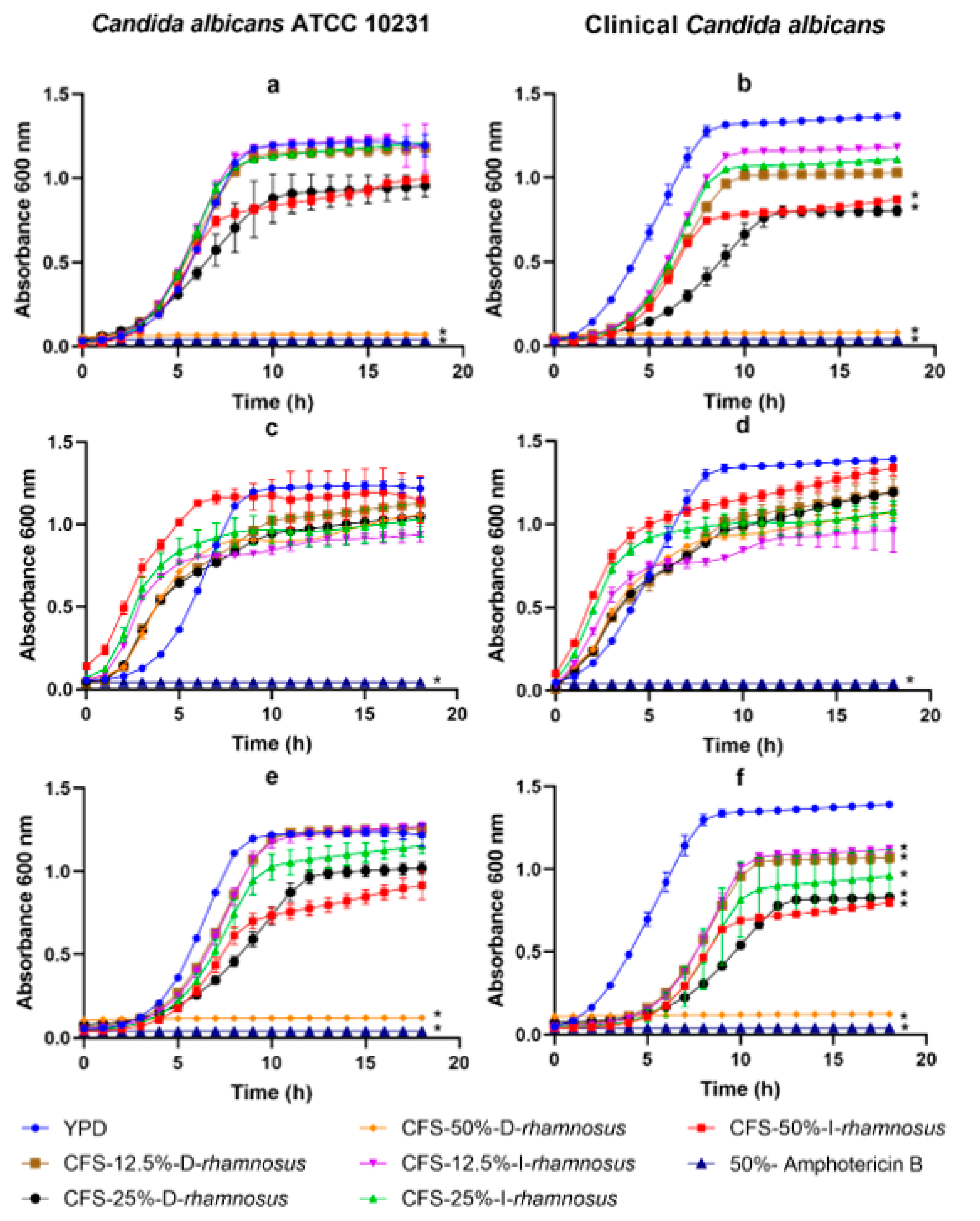
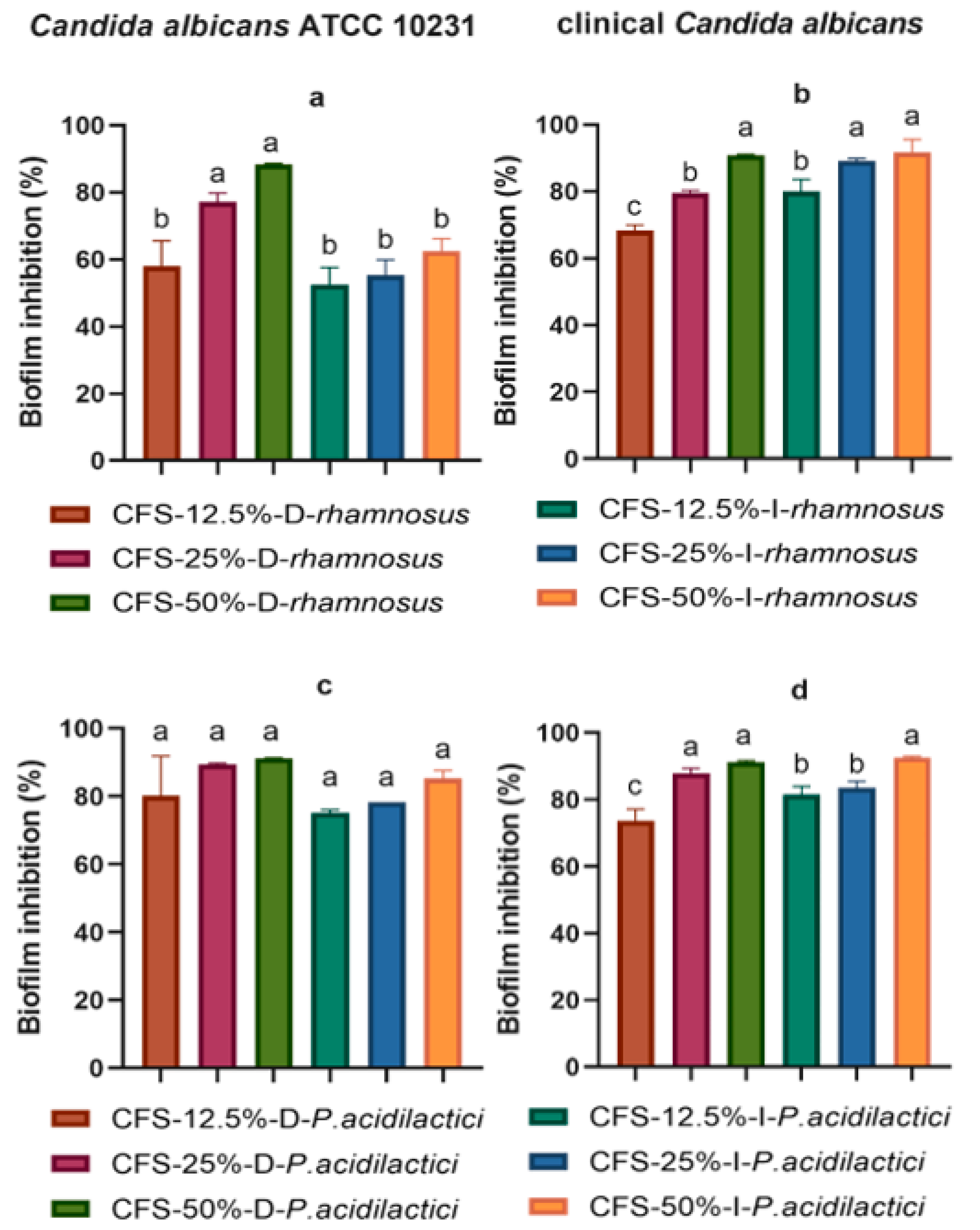
2. Results
3. Discussion
4. Materials and Methods
4.1. Candida albicans Culture Conditions
4.2. Probiotics Cultivations and Cell-Free Supernatants Obtention
4.3. Growth Inhibition of Candida albicans
4.4. Antibiofilm Activity against Candida albicans
- O.D.1 = absorbance of wells containing cell-free supernatant and Candida spp.
- O.D.2 = absorbance of wells containing only Candida spp. (control).
4.5. Determination of SCFAs in Cell-Free Supernatants
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a New Approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Monteagudo-Mera, A.; Rastall, R.A.; Gibson, G.R.; Charalampopoulos, D.; Chatzifragkou, A. Adhesion Mechanisms Mediated by Probiotics and Prebiotics and Their Potential Impact on Human Health. Appl. Microbiol. Biotechnol. 2019, 103, 6463–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, N.; Li, P.; Gu, Q. Probiotic Therapy in Helicobacter pylori Infection: A Potential Strategy against a Serious Pathogen? Appl. Microbiol. Biotechnol. 2019, 103, 1573–1588. [Google Scholar] [CrossRef] [PubMed]
- Sales-Campos, H.; Soares, S.C.; Oliveira, C.J.F. An Introduction of the Role of Probiotics in Human Infections and Autoimmune Diseases. Crit. Rev. Microbiol. 2019, 45, 413–432. [Google Scholar] [CrossRef]
- Yazbeck, R.; Lindsay, R.J.; Geier, M.S.; Butler, R.N.; Howarth, G.S. Prebiotics Fructo-, Galacto-, and Mannan-Oligosaccharide Do Not Protect against 5-Fluorouracil–Induced Intestinal Mucositis in Rats. J. Nutr. 2019, 149, 2164–2173. [Google Scholar] [CrossRef]
- Senés-Guerrero, C.; Gradilla-Hernández, M.S.; García-Gamboa, R.; García-Cayuela, T. Dietary Fiber and Gut Microbiota. In Science and Technology of Fibers in Food Systems; Springer International Publishing: Cham, Switzerland, 2020; pp. 277–298. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In Vitro Evaluation of the Antimicrobial Activity of a Range of Probiotics against Pathogens: Evidence for the Effects of Organic Acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef]
- Gradilla-Hernández, M.S.; García-González, A.; Gschaedler, A.; Herrera-López, E.J.; González-Avila, M.; García-Gamboa, R.; Yebra Montes, C.; Fuentes-Aguilar, R.Q. Applying Differential Neural Networks to Characterize Microbial Interactions in an Ex Vivo Gastrointestinal Gut Simulator. Processes 2020, 8, 593. [Google Scholar] [CrossRef]
- Xu, C.; Fu, Y.; Liu, F.; Liu, Z.; Ma, J.; Jiang, R.; Song, C.; Jiang, Z.; Hou, J. Purification and Antimicrobial Mechanism of a Novel Bacteriocin Produced by Lactobacillus rhamnosus 1.0320. LWT 2021, 137, 110338. [Google Scholar] [CrossRef]
- Yan, X.; Gu, S.; Cui, X.; Shi, Y.; Wen, S.; Chen, H.; Ge, J. Antimicrobial, Anti-Adhesive and Anti-Biofilm Potential of Biosurfactants Isolated from Pediococcus acidilactici and Lactobacillus plantarum against Staphylococcus aureus CMCC26003. Microb. Pathog. 2019, 127, 12–20. [Google Scholar] [CrossRef]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. Invasive Candidiasis: Update and Current Challenges in the Management of This Mycosis in South America. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef]
- Oliveira, V.M.C.; Santos, S.S.F.; Silva, C.R.G.; Jorge, A.O.C.; Leão, M.V.P. Lactobacillus Is Able to Alter the Virulence and the Sensitivity Profile of Candida albicans. J. Appl. Microbiol. 2016, 121, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Ropars, J.; Maufrais, C.; Diogo, D.; Marcet-Houben, M.; Perin, A.; Sertour, N.; Mosca, K.; Permal, E.; Laval, G.; Bouchier, C. Gene Flow Contributes to Diversification of the Major Fungal Pathogen Candida albicans. Nat. Commun. 2018, 9, 2253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Gamboa, R.; Kirchmayr, M.R.; Gradilla-Hernández, M.S.; Pérez-Brocal, V.; Moya, A.; González-Avila, M. The Intestinal Mycobiota and Its Relationship with Overweight, Obesity and Nutritional Aspects. J. Hum. Nutr. Diet. 2021, 34, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Lv, X.; Fu, J.; He, C.; Hua, H.; Yan, Z. In Vitro Inhibitory Activity of Probiotic Products against Oral Candida Species. J. Appl. Microbiol. 2016, 121, 254–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Wei, C.; Liu, C.; Li, D.; Sun, J.; Huang, H.; Zhou, H. Inhibitory Effects of Oral Actinomyces on the Proliferation, Virulence and Biofilm Formation of Candida albicans. Arch. Oral Biol. 2015, 60, 1368–1374. [Google Scholar] [CrossRef]
- Popp, C.; Ramírez-Zavala, B.; Schwanfelder, S.; Krüger, I.; Morschhäuser, J. Evolution of Fluconazole-Resistant Candida albicans Strains by Drug-Induced Mating Competence and Parasexual Recombination. mBio 2019, 10, e02740-18. [Google Scholar] [CrossRef] [Green Version]
- Prabhurajeshwar, C.; Chandrakanth, K. Evaluation of Antimicrobial Properties and their Substances against Pathogenic Bacteria In-Vitro by Probiotic Lactobacilli Strains Isolated from Commercial Yoghurt. Clin. Nutr. Exp. 2019, 23, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Hu, P.; Zhao, F.; Wang, J.; Zhu, W. Early-Life Lactoferrin Intervention Modulates the Colonic Microbiota, Colonic Microbial Metabolites and Intestinal Function in Suckling Piglets. Appl. Microbiol. Biotechnol. 2020, 104, 6185–6197. [Google Scholar] [CrossRef]
- Hong, L.; Kim, W.-S.; Lee, S.-M.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Pullulan Nanoparticles as Prebiotics Enhance the Antibacterial Properties of Lactobacillus plantarum through the Induction of Mild Stress in Probiotics. Front. Microbiol. 2019, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Kang, S.-S. Antifungal Activities against Candida albicans, of Cell-Free Supernatants Obtained from Probiotic Pediococcus acidilactici HW01. Arch. Oral Biol. 2019, 99, 113–119. [Google Scholar] [CrossRef]
- Papaianni, M.; Ricciardelli, A.; Casillo, A.; Corsaro, M.M.; Borbone, F.; Della Ventura, B.; Velotta, R.; Fulgione, A.; Woo, S.L.; Tutino, M.L. The Union Is Strength: The Synergic Action of Long Fatty Acids and a Bacteriophage against Xanthomonas campestris Biofilm. Microorganisms 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Roller, M.; Rechkemmer, G.; Watzl, B. Prebiotic Inulin Enriched with Oligofructose in Combination with the Probiotics Lactobacillus rhamnosus and Bifidobacterium lactis Modulates Intestinal Immune Functions in Rats. J. Nutr. 2004, 134, 153–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and Characterization of Inulin-Type Fructans from Artichoke Wastes and Their Effect on the Growth of Intestinal Bacteria Associated with Health. BioMed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I. Fungal Microbiota Dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step beyond Pre-and Probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Dausset, C.; Bornes, S.; Miquel, S.; Kondjoyan, N.; Angenieux, M.; Nakusi, L.; Veisseire, P.; Alaterre, E.; Bermúdez-Humarán, L.G.; Langella, P. Identification of Sulfur Components Enhancing the Anti-Candida Effect of Lactobacillus rhamnosus Lcr35. Sci. Rep. 2020, 10, 17074. [Google Scholar] [CrossRef]
- Allonsius, C.N.; Vandenheuvel, D.; Oerlemans, E.F.M.; Petrova, M.I.; Donders, G.G.G.; Cos, P.; Delputte, P.; Lebeer, S. Inhibition of Candida albicans Morphogenesis by Chitinase from Lactobacillus rhamnosus GG. Sci. Rep. 2019, 9, 2900. [Google Scholar] [CrossRef]
- Allonsius, C.N.; van den Broek, M.F.L.; De Boeck, I.; Kiekens, S.; Oerlemans, E.F.M.; Kiekens, F.; Foubert, K.; Vandenheuvel, D.; Cos, P.; Delputte, P.; et al. Interplay between Lactobacillus rhamnosus GG and Candida and the Involvement of Exopolysaccharides. Microb. Biotechnol. 2017, 10, 1753–1763. [Google Scholar] [CrossRef]
- García-Gamboa, R.; Basurto, R.I.O.; Santoyo, M.C.; Madrigal, J.B.; Álvarez, B.E.R.; Avila, M.G. In Vitro Evaluation of Prebiotic Activity, Pathogen Inhibition and Enzymatic Metabolism of Intestinal Bacteria in the Presence of Fructans Extracted from Agave: A Comparison Based on Polymerization Degree. LWT 2018, 92, 380–387. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; van Sinderen, D. Current Perspectives on Antifungal Lactic Acid Bacteria as Natural Bio-Preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Hassan, Z.; Sadon, S.K. Antifungal Activity of Lactobacillus fermentum Te007, Pediococcus pentosaceus Te010, Lactobacillus pentosus G004, and L. paracasi D5 on Selected Foods. J. Food Sci. 2011, 76, M493–M499. [Google Scholar] [CrossRef] [PubMed]
- Le Barz, M.; Anhê, F.F.; Varin, T.V.; Desjardins, Y.; Levy, E.; Roy, D.; Urdaci, M.C.; Marette, A. Probiotics as Complementary Treatment for Metabolic Disorders. Diabetes Metab. J. 2015, 39, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Pranckutė, R.; Kaunietis, A.; Kuisienė, N.; Čitavičius, D.J. Combining Prebiotics with Probiotic Bacteria Can Enhance Bacterial Growth and Secretion of Bacteriocins. Int. J. Biol. Macromol. 2016, 89, 669–676. [Google Scholar] [CrossRef] [PubMed]
- McDonough, L.D.; Mishra, A.A.; Tosini, N.; Kakade, P.; Penumutchu, S.; Liang, S.-H.; Maufrais, C.; Zhai, B.; Taur, Y.; Belenky, P. Candida albicans Isolates 529L and CHN1 Exhibit Stable Colonization of the Murine Gastrointestinal Tract. mBio 2021, 12, e02878-21. [Google Scholar] [CrossRef] [PubMed]
- García-Gamboa, R.; Domínguez-Simi, M.Á.; Gradilla-Hernández, M.S.; Bravo-Madrigal, J.; Moya, A.; González-Avila, M. Antimicrobial and Antibiofilm Effect of Inulin-Type Fructans, Used in Synbiotic Combination with Lactobacillus spp. against Candida albicans. Plant Foods Hum. Nutr. 2022, 77, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Femia, A.P.; Luceri, C.; Dolara, P.; Giannini, A.; Biggeri, A.; Salvadori, M.; Clune, Y.; Collins, K.J.; Paglierani, M.; Caderni, G. Antitumorigenic Activity of the Prebiotic Inulin Enriched with Oligofructose in Combination with the Probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on Azoxymethane-Induced Colon Carcinogenesis in Rats. Carcinogenesis 2002, 23, 1953–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Probiotic: | L. rhamnosus | P. acidilactici | |||
|---|---|---|---|---|---|
| Cell Free Supernatant | Concentration Percentage (%) | Postbiotics Treatment | Carbon Source | Verification Code | |
| CFS | 50 | Without | Inulin | CFS-50%-W-I-rhamnosus | CFS-50%-W-I-P. acidilactici |
| 25 | CFS-25%-W-I-rhamnosus | CFS-25%-W-I-P. acidilactici | |||
| 12.5 | CFS-12.5%-W-I-rhamnosus | CFS-12.5%-W-I-P. acidilactici | |||
| 50 | Dextrose | CFS-50%-W-D-rhamnosus | CFS-50%-W-D-P. acidilactici | ||
| 25 | CFS-25%-W-D-rhamnosus | CFS-25%-W-D-P. acidilactici | |||
| 12.5 | CFS-12.5%-W-D-rhamnosus | CFS-12.5%-W-D-P. acidilactici | |||
| 50 | Neutralized | Inulin | CFS-50%-N-I-rhamnosus | CFS-50%-N-I-P. acidilactici | |
| 25 | CFS-25%-N-I-rhamnosus | CFS-25%-N-I-P. acidilactici | |||
| 12.5 | CFS-12.5%-N-I-rhamnosus | CFS-12.5%-N-I-P. acidilactici | |||
| 50 | Dextrose | CFS-50%-N-D-rhamnosus | CFS-50%-N-D-P. acidilactici | ||
| 25 | CFS-25%-N-D-rhamnosus | CFS-25%-N-D-P. acidilactici | |||
| 12.5 | CFS-12.5%-N-D-rhamnosus | CFS-12.5%-N-D-P. acidilactici | |||
| 50 | Thermal | Inulin | CFS-50%-T-I-rhamnosus | CFS-50%-T-I-P. acidilactici | |
| 25 | CFS-25%-T-I-rhamnosus | CFS-25%-T-I-P. acidilactici | |||
| 12.5 | CFS-12.5%-T-I-rhamnosus | CFS-12.5%-T-I-P. acidilactici | |||
| 50 | Dextrose | CFS-50%-T-D-rhamnosus | CFS-50%-T-D-P. acidilactici | ||
| 25 | CFS-25%-T-D-rhamnosus | CFS-25%-T-D-P. acidilactici | |||
| 12.5 | CFS-12.5%-T-D-rhamnosus | CFS-12.5%-T-D-P. acidilactici | |||
| L. rhamnosus | P. acidilactici | |||
|---|---|---|---|---|
| Lactate and SCFAs | Dextrose | Inulin | Dextrose | Inulin |
| Lactate | 8.24 ± 1.36 a | 5.87 ± 0.70 b | 9.02 ± 0.40 a | 5.48 ± 0.17 b |
| Acetic acid | 1.66 ± 0.37 b | 7.00 ± 1.26 a | 17.6 ± 2.54 a | 6.92 ± 0.76 b |
| Propionic acid | 0.21 ± 0.04 a | 0.23 ± 0.06 a | 0.66 ± 0.067 a | 0.20 ± 0.03 a |
| Butyric acid | 1.83 ± 0.23 a | 1.89 ± 0.20 a | 1.80 ± 0.04 a | 1.67 ±0.02 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Gamboa, R.; Domínguez-Simi, M.; Gradilla-Hernández, M.S.; Bravo, J.; Moya, A.; Ruiz-Álvarez, B.; González-Avila, M. Anticandidal and Antibiofilm Effect of Synbiotics including Probiotics and Inulin-Type Fructans. Antibiotics 2022, 11, 1135. https://doi.org/10.3390/antibiotics11081135
García-Gamboa R, Domínguez-Simi M, Gradilla-Hernández MS, Bravo J, Moya A, Ruiz-Álvarez B, González-Avila M. Anticandidal and Antibiofilm Effect of Synbiotics including Probiotics and Inulin-Type Fructans. Antibiotics. 2022; 11(8):1135. https://doi.org/10.3390/antibiotics11081135
Chicago/Turabian StyleGarcía-Gamboa, Ricardo, Miguel Domínguez-Simi, Misael S. Gradilla-Hernández, Jorge Bravo, Andrés Moya, Blanca Ruiz-Álvarez, and Marisela González-Avila. 2022. "Anticandidal and Antibiofilm Effect of Synbiotics including Probiotics and Inulin-Type Fructans" Antibiotics 11, no. 8: 1135. https://doi.org/10.3390/antibiotics11081135
APA StyleGarcía-Gamboa, R., Domínguez-Simi, M., Gradilla-Hernández, M. S., Bravo, J., Moya, A., Ruiz-Álvarez, B., & González-Avila, M. (2022). Anticandidal and Antibiofilm Effect of Synbiotics including Probiotics and Inulin-Type Fructans. Antibiotics, 11(8), 1135. https://doi.org/10.3390/antibiotics11081135









