Effects of Levofloxacin, Aztreonam, and Colistin on Enzyme Synthesis by P. aeruginosa Isolated from Cystic Fibrosis Patients
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Intrinsic Activity (MICs)
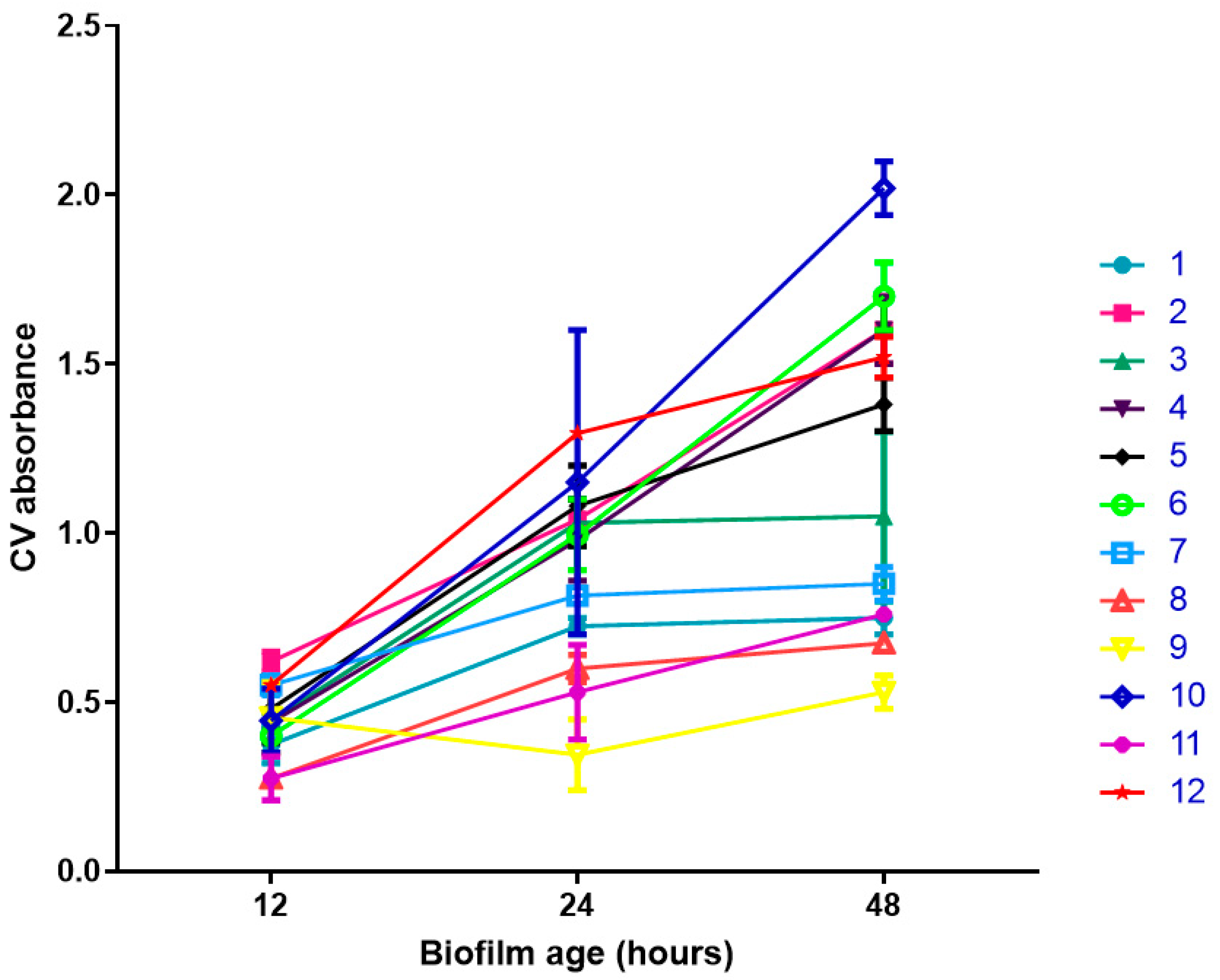
2.2. Biofilm Formation
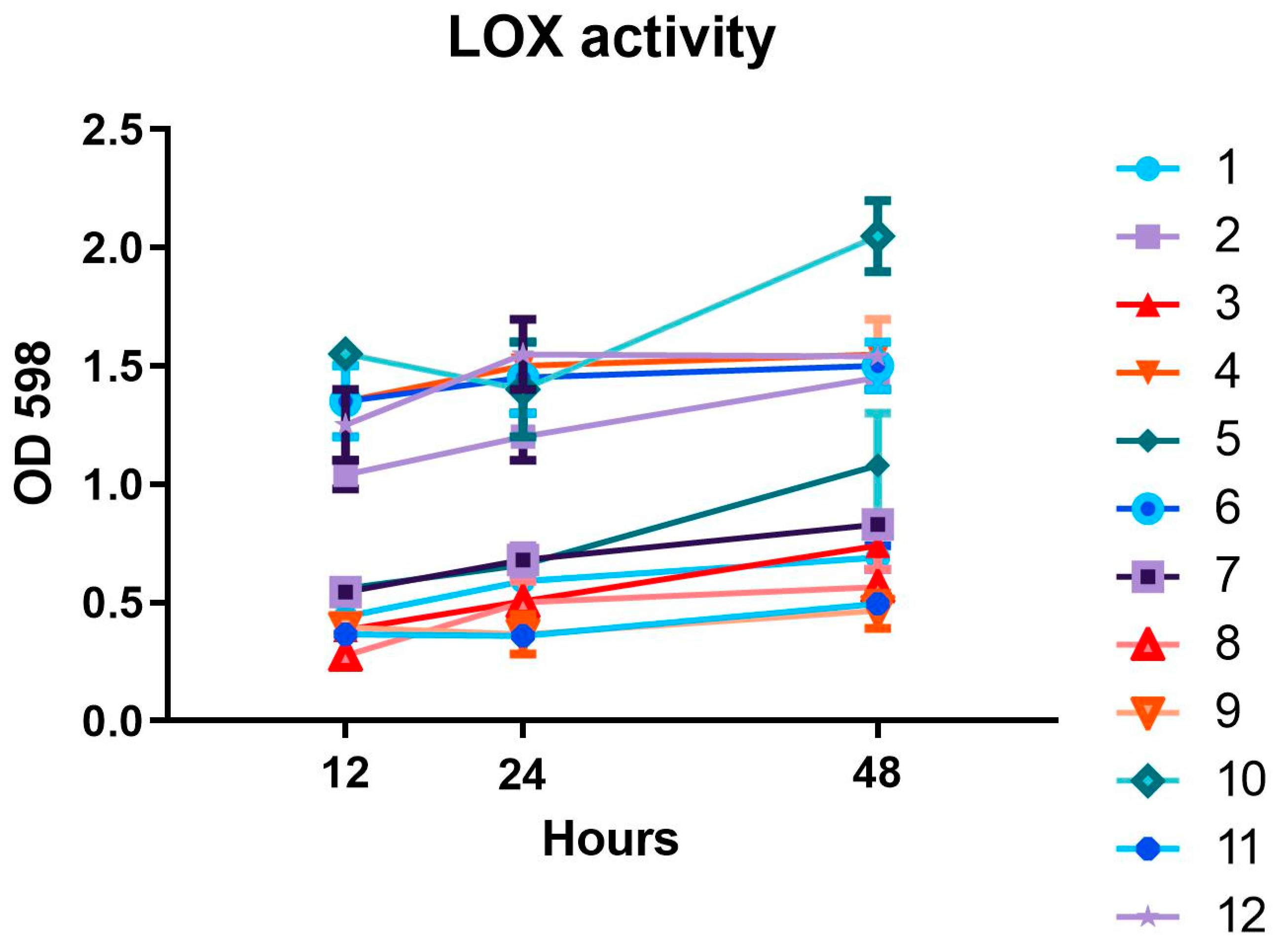
2.3. LOX Activity
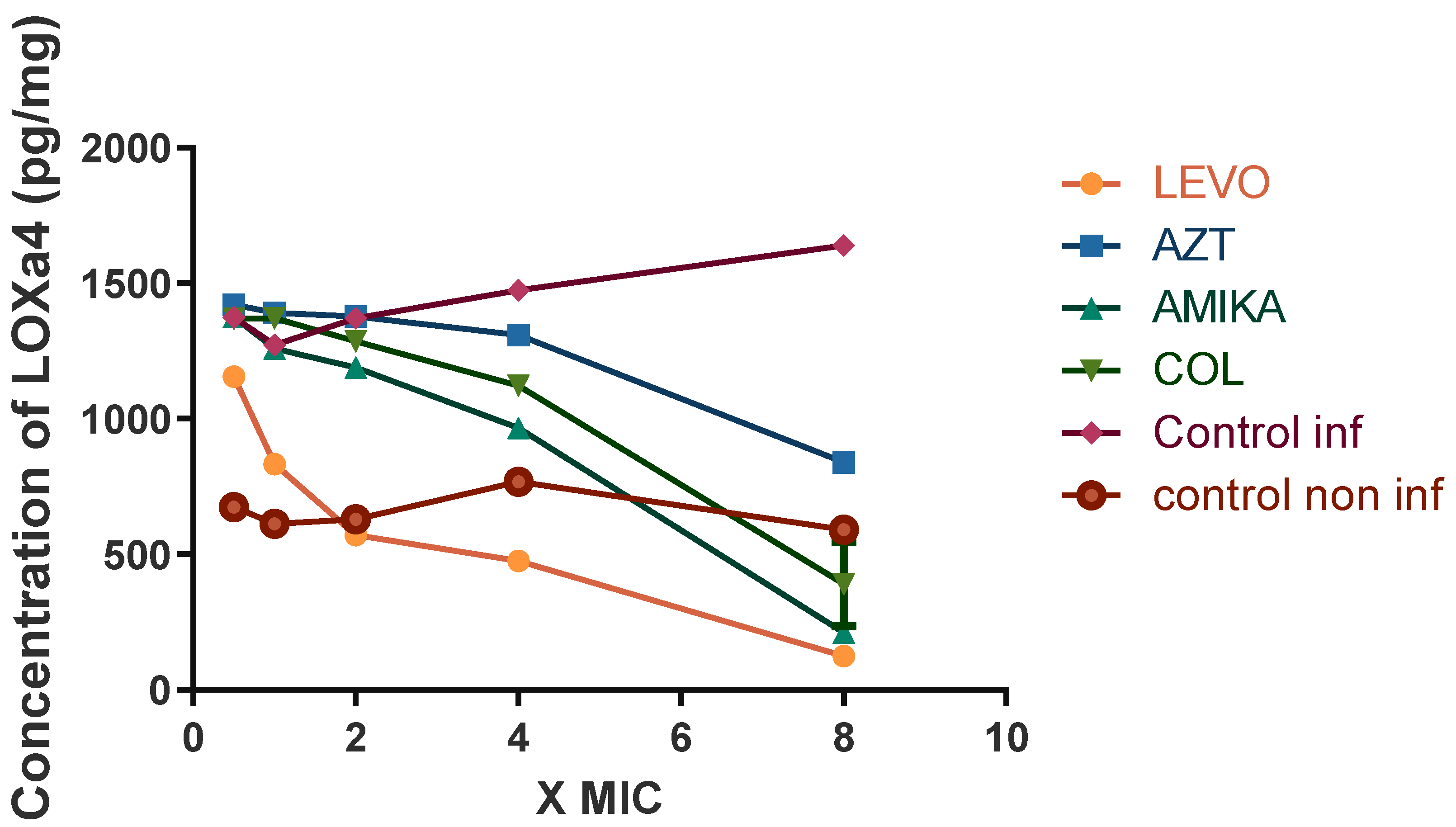
2.4. Effects of Antibiotics on LOX Activity
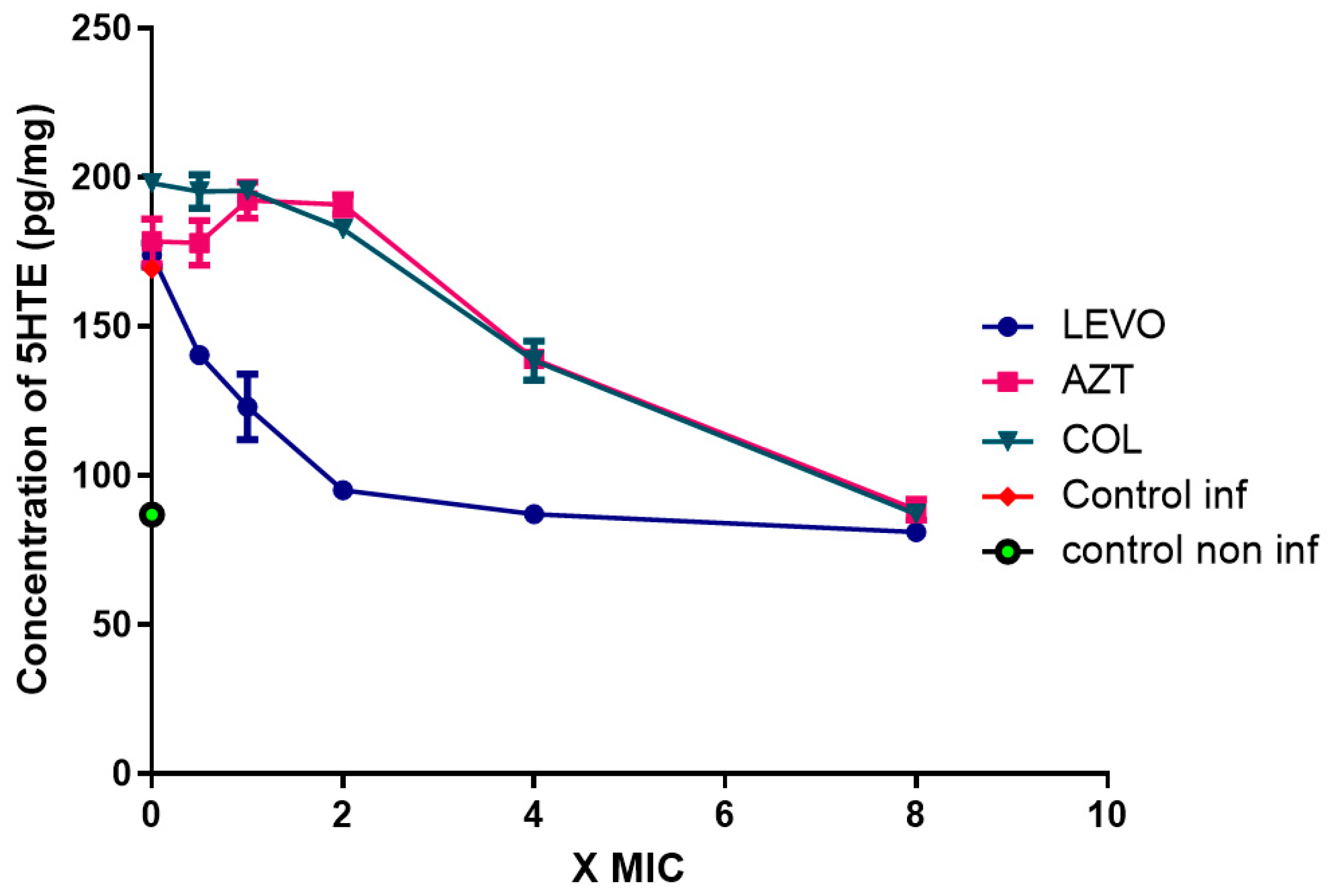
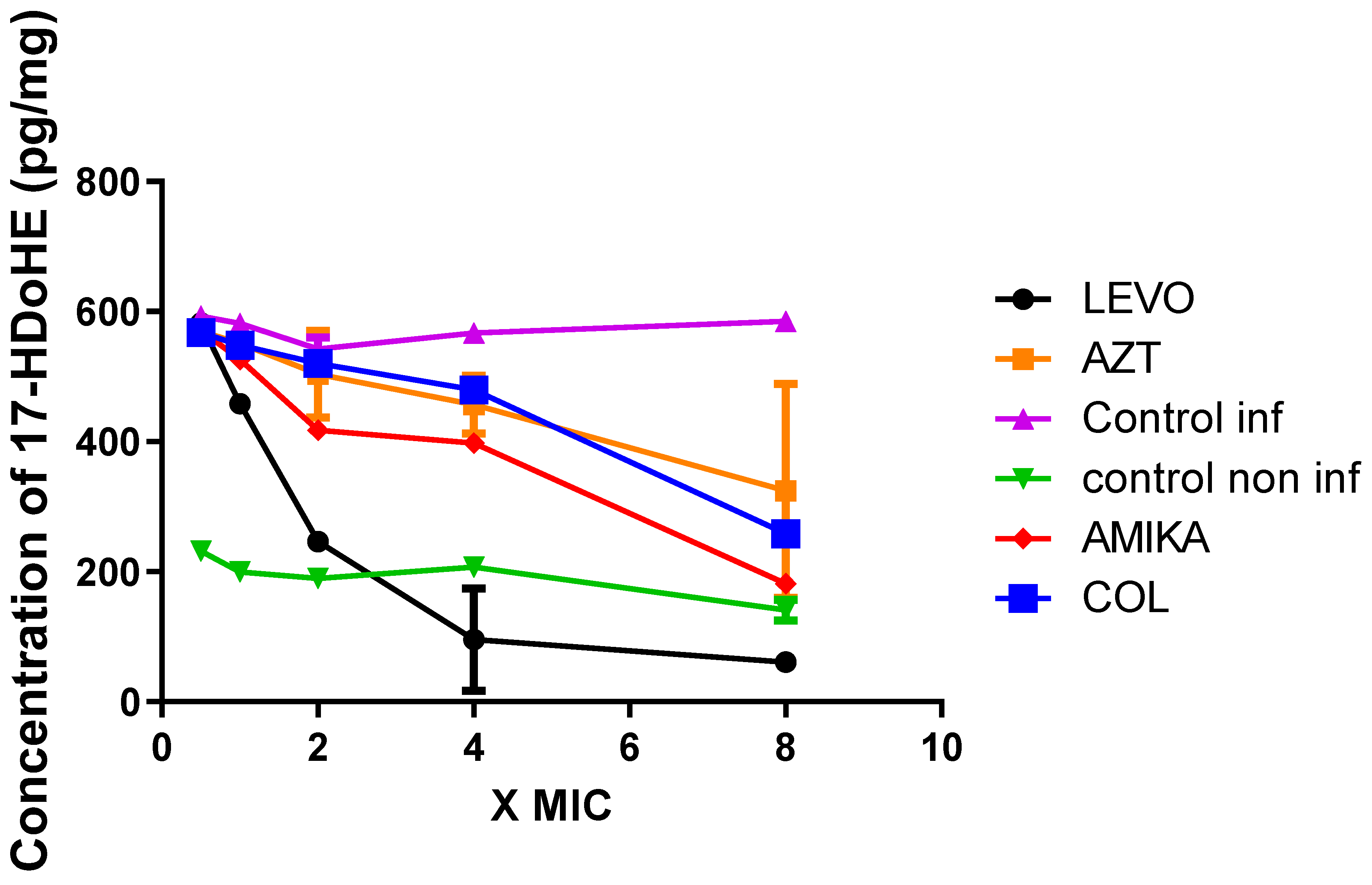
2.5. Production of 15-LOX-Dependent Metabolites in Lung Epithelial Cells Infected by P. aeruginosa
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Biofilm Quantification
4.3. Antibiotics
4.4. Lipoxygenase Assay
4.5. Culture and Infection of Pulmonary Epithelial Cells
4.6. Lipid Extraction and Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS)
4.7. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dp, N.; Jf, C. Inflammation and its genesis in cystic fibrosis. Pediatr. Pulmonol. 2015, 50 (Suppl. 40), S39–S56. [Google Scholar] [CrossRef]
- Gifford, A.M.; Chalmers, J.D. The role of neutrophils in cystic fibrosis. Curr. Opin. Hematol. 2014, 21, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J.F. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015, 14, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Cohen, T.S.; Prince, A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat. Med. 2012, 18, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Flume, P.A.; VanDevanter, D.R.; Morgan, E.E.; Dudley, M.N.; Loutit, J.S.; Bell, S.C.; Kerem, E.; Fischer, R.; Smyth, A.R.; Aaron, S.D.; et al. A phase 3, multi-center, multinational, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of levofloxacin inhalation solution (APT-1026) in stable cystic fibrosis patients. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2016, 15, 495–502. [Google Scholar] [CrossRef]
- Elborn, J.S.; Flume, P.A.; Cohen, F.; Loutit, J.; VanDevanter, D.R. Safety and efficacy of prolonged levofloxacin inhalation solution (APT-1026) treatment for cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2016, 15, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Geller, D.E.; Flume, P.A.; Staab, D.; Fischer, R.; Loutit, J.S.; Conrad, D.J. Mpex 204 Study Group Levofloxacin inhalation solution (MP-376) in patients with cystic fibrosis with Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2011, 183, 1510–1516. [Google Scholar] [CrossRef] [PubMed]
- Morello, E.; Pérez-Berezo, T.; Boisseau, C.; Baranek, T.; Guillon, A.; Bréa, D.; Lanotte, P.; Carpena, X.; Pietrancosta, N.; Hervé, V.; et al. Pseudomonas aeruginosa Lipoxygenase LoxA Contributes to Lung Infection by Altering the Host Immune Lipid Signaling. Front. Microbiol. 2019, 10, 1826. [Google Scholar] [CrossRef] [PubMed]
- Starkey, M.; Hickman, J.H.; Ma, L.; Zhang, N.; De Long, S.; Hinz, A.; Palacios, S.; Manoil, C.; Kirisits, M.J.; Starner, T.D.; et al. Pseudomonas aeruginosa rugose small-colony variants have adaptations that likely promote persistence in the cystic fibrosis lung. J. Bacteriol. 2009, 191, 3492–3503. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Rao, G.N. Emerging role of 12/15-Lipoxygenase (ALOX15) in human pathologies. Prog. Lipid Res. 2019, 73, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Cole, B.K.; Lieb, D.C.; Dobrian, A.D.; Nadler, J.L. 12- and 15-lipoxygenases in adipose tissue inflammation. Prostaglandins Other Lipid Mediat. 2013, 104–105, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Kühn, H.; O’Donnell, V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006, 45, 334–356. [Google Scholar] [CrossRef] [PubMed]
- Namgaladze, D.; Snodgrass, R.G.; Angioni, C.; Grossmann, N.; Dehne, N.; Geisslinger, G.; Brüne, B. AMP-activated protein kinase suppresses arachidonate 15-lipoxygenase expression in interleukin 4-polarized human macrophages. J. Biol. Chem. 2015, 290, 24484–24494. [Google Scholar] [CrossRef] [PubMed]
- Feltenmark, S.; Gautam, N.; Brunnström, Å.; Griffiths, W.; Backman, L.; Edenius, C.; Lindbom, L.; Björkholm, M.; Claesson, H.-E. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc. Natl. Acad. Sci. USA 2008, 105, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, R.; Reddy, M.A.; Malik, K.U.; Fatima, S.; Khan, B.V. Signaling mechanisms of nuclear factor-kappab-mediated activation of inflammatory genes by 13-hydroperoxyoctadecadienoic acid in cultured vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Kippelen, P. Airway injury as a mechanism for exercise-induced bronchoconstriction in elite athletes. J. Allergy Clin. Immunol. 2008, 122, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.T.; Lainšček, D.; Gesslbauer, B.; Jarc-Jovičić, E.; Hyötyläinen, T.; Ilc, N.; Lakota, K.; Tomšič, M.; van de Loo, F.A.J.; Bochkov, V.; et al. Synergy between 15-lipoxygenase and secreted PLA2 promotes inflammation by formation of TLR4 agonists from extracellular vesicles. Proc. Natl. Acad. Sci. USA 2020, 117, 25679–25689. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.S.; Johndrow, J.E.; Esper, L.; Dias, A.; Bafica, A.; Serhan, C.N.; Aliberti, J. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat. Med. 2006, 12, 330–334. [Google Scholar] [CrossRef] [PubMed]
- M07: Dilution AST for Aerobically Grown Bacteria—CLSI. Available online: https://clsi.org/standards/products/microbiology/documents/m07/ (accessed on 5 August 2022).
- Deschamps, J.D.; Ogunsola, A.F.; Jameson, J.B.; Yasgar, A.; Flitter, B.A.; Freedman, C.J.; Melvin, J.A.; Nguyen, J.V.M.H.; Maloney, D.J.; Jadhav, A.; et al. Biochemical and Cellular Characterization and Inhibitor Discovery of Pseudomonas aeruginosa 15-Lipoxygenase. Biochemistry 2016, 55, 3329–3340. [Google Scholar] [CrossRef] [PubMed]

| Levofloxacin | Amikacin | Aztreonam | Colistin | |
|---|---|---|---|---|
| Strain 1 | 8 | 8 | <0.25 | >64 |
| Strain 2 | 4 | 1 | <0.25 | 8 |
| Strain 3 | 4 | 2 | <0.25 | 0.5 |
| Strain 4 | <0.25 | <0.25 | <0.25 | <0.25 |
| Strain 5 | <0.25 | 1 | 4 | 0.5 |
| Strain 6 | <0.25 | 1 | 4 | 0.5 |
| Strain 7 | 0.25 | 1 | 4 | 1 |
| Strain 8 | 0.25 | <0.25 | <0.25 | <0.25 |
| Strain 9 | 4 | 1 | 16 | <0.25 |
| Strain 10 | 8 | 4 | 32 | <0.25 |
| Strain 11 | 8 | 8 | 8 | 16 |
| Strain 12 | 16 | 32 | 8 | 2 |
| Bonferroni’s Multiple Comparison Test | Mean Difference | t | p < 0.05 | 95% CI |
|---|---|---|---|---|
| Levofloxacin vs. aztreonam | −0.7200 | 4.237 | Yes | −1.273 to −0.1673 |
| Levofloxacin vs. amikacin | −0.4800 | 2.824 | No | −1.033 to 0.07266 |
| Levofloxacin vs. colistin | −0.6680 | 3.931 | Yes | −1.221 to −0.1153 |
| Levofloxacin vs. control | −1.180 | 6.943 | Yes | −1.733 to −0.6273 |
| Aztreonam vs. amikacin | 0.2400 | 1.412 | No | −0.3127 to 0.7927 |
| Aztreonam vs. colistin | 0.05200 | 0.3060 | No | −0.5007 to 0.6047 |
| Aztreonam vs. control | −0.4600 | 2.707 | No | −1.013 to 0.09266 |
| Amikacin vs. colistin | −0.1880 | 1.106 | No | −0.7407 to 0.3647 |
| Amikacin vs. control | −0.7000 | 4.119 | Yes | −1.253 to −0.1473 |
| Colistin vs. control | −0.5120 | 3.013 | No | −1.065 to 0.04066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pani, A.; Lucini, V.; Dugnani, S.; Schianchi, A.; Scaglione, F. Effects of Levofloxacin, Aztreonam, and Colistin on Enzyme Synthesis by P. aeruginosa Isolated from Cystic Fibrosis Patients. Antibiotics 2022, 11, 1114. https://doi.org/10.3390/antibiotics11081114
Pani A, Lucini V, Dugnani S, Schianchi A, Scaglione F. Effects of Levofloxacin, Aztreonam, and Colistin on Enzyme Synthesis by P. aeruginosa Isolated from Cystic Fibrosis Patients. Antibiotics. 2022; 11(8):1114. https://doi.org/10.3390/antibiotics11081114
Chicago/Turabian StylePani, Arianna, Valeria Lucini, Silvana Dugnani, Alice Schianchi, and Francesco Scaglione. 2022. "Effects of Levofloxacin, Aztreonam, and Colistin on Enzyme Synthesis by P. aeruginosa Isolated from Cystic Fibrosis Patients" Antibiotics 11, no. 8: 1114. https://doi.org/10.3390/antibiotics11081114
APA StylePani, A., Lucini, V., Dugnani, S., Schianchi, A., & Scaglione, F. (2022). Effects of Levofloxacin, Aztreonam, and Colistin on Enzyme Synthesis by P. aeruginosa Isolated from Cystic Fibrosis Patients. Antibiotics, 11(8), 1114. https://doi.org/10.3390/antibiotics11081114







