Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus
Abstract
1. Introduction
2. Compounds with Diverse Structures
2.1. Polyphenol Compounds
2.2. Triterpenoids
2.3. Fatty Acid Compounds
2.4. Miscellaneous Compounds
3. Biological and Pharmacological Activities
3.1. Antitumor Activity
3.2. Antioxidant Activity
3.3. Antimicrobial Activity
3.4. Neurotrophic and Neuroprotective Activity
3.5. Enzyme Inhibitory Activity
3.6. Immunomodulatory Effects and Other Biological activities
4. Biosynthetic Progresses on Styrylpyrones
5. Discussions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Piątek, M. Inonotus hispidus (Bull.: Fr.) Karst. In Atlas of the Geographical Distribution of Fungi in Poland, Fascicle 1; Polish Academy of Sciences: Kraków, Poland, 2000; pp. 35–40. [Google Scholar]
- Qing-Jie, L. Study on the Active Substances and Quality Standards of Sanghuang. Ph.D. Thesis, Jilin Agricultural University, Changchun, China, 2017. [Google Scholar]
- Kirk, P.; Cannon, P.; Stalpers, J.; Minter, D.W. Dictionary of the Fungi, 10th ed.; CABI Publishing: Oxfordshire, UK, 2008. [Google Scholar]
- Zhang, F.; Xue, F.; Xu, H.; Yuan, Y.; Wu, X.; Zhang, J.; Fu, J. Optimization of Solid-State Fermentation Extraction of Inonotus hispidus Fruiting Body Melanin. Foods 2021, 10, 2893. [Google Scholar] [CrossRef]
- Wu, S.H.; Chang, C.C.; Wei, C.L.; Jiang, G.Z.; Cui, B.K. Sanghuangporus toxicodendri sp. nov. (Hymenochaetales, Basidiomycota) from China. MycoKeys 2019, 57, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Zan, L.-F.; Bao, H.-Y. Progress in Inonotus hispidus research. Acta Edulis. Fungi. 2011, 18, 78–82. [Google Scholar] [CrossRef]
- Wu, S.-H.; Dai, Y.-C.; Hattori, T.; Yu, T.W.; Wang, D.-M.; Parmasto, É.K.; Chang, H.-Y.; Shih, S.-Y. Species clarification for the medicinally valuable ‘sanghuang’ mushroom. Bota. Stu. 2012, 53, 135–149. [Google Scholar]
- Ikekawa, T.; Nakanishi, M.; Uehara, N.; Chihara, G.; Fukuoka, F. Antitumor action of some Basidiomycetes, especially Phllinus linteus. GaN 1968, 59, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.L.; Lewis, D.G.; Wilson, D.V. 983. Constituents of the higher fungi. Part I. Hispidin, a new 4-hydroxy-6-styryl-2-pyrone from polyporus hispidus (Bull.). Fr. J. Chem. Soc. 1961, 4995–5002. [Google Scholar] [CrossRef]
- Perrin, P.W.; Towers, G.H.N. Hispidin biosynthesis in cultures of Polyporus hispidus. Phytochemistry 1973, 12, 589–592. [Google Scholar] [CrossRef]
- Fiasson, J.-L. Distribution of styrylpyrones in the basidiocarps of various Hymenochaetaceae. Biochem. Syst. Ecol. 1982, 10, 289–296. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Jansen, R.; Pilgrim, H.; Liberra, K.; Lindequist, U. Hispolon, a yellow pigment from Inonotus hispidus. Phytochemistry 1996, 41, 927–929. [Google Scholar] [CrossRef]
- Yousfi, M.; Djeridane, A.; Bombarda, I.; Chahrazed, H.; Duhem, B.; Gaydou, E.M. Isolation and Characterization of a New Hispolone Derivative from Antioxidant Extracts of Pistacia atlantica. Phytother. Res. 2009, 23, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, Y. Determination of trace elements in anti-influenza virus mushrooms. Biol. Trace Elem. Res. 2011, 143, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Lifeng, Z. Studies on the Chemical Constituents and Pharmacological Activities of Inonotus hispidus and Fomitiporia ellipsoidea. Ph.D. Thesis, Jilin Agricultural University, Changchun, China.
- Zan, L.-F.; Qin, J.-C.; Zhang, Y.-M.; Yao, Y.-H.; Bao, H.-Y.; Li, X. Antioxidant Hispidin Derivatives from Medicinal Mushroom Inonotus hispidus. Chem. Pharm. Bull. 2011, 59, 770–772. [Google Scholar] [CrossRef] [PubMed]
- Gruendemann, C.; Arnhold, M.; Meier, S.; Baecker, C.; Garcia-Kaeufer, M.; Grunewald, F.; Steinborn, C.; Klemd, A.M.; Wille, R.; Huber, R.; et al. Effects of Inonotus hispidus Extracts and Compounds on Human Immunocompetent Cells. Planta Med. 2016, 82, 1359–1367. [Google Scholar] [CrossRef]
- Ren, Q.; Lu, X.-Y.; Han, J.-X.; Aisa, H.A.; Yuan, T. Triterpenoids and phenolics from the fruiting bodies of Inonotus hispidus and their activations of melanogenesis and tyrosinase. Chin. Chem. Lett. 2017, 28, 1052–1056. [Google Scholar] [CrossRef]
- Kemami Wangun, H.V.; Hertweck, C. Squarrosidine and Pinillidine: 3,3′-Fused Bis(styrylpyrones) from Pholiota squarrosa and Phellinus pini. Eur. J. Org. Chem. 2007, 2007, 3292–3295. [Google Scholar] [CrossRef]
- Yang, S.; Bao, H.; Wang, H. Chemical components and anti-tumour compounds from Inonotus hispidus. Mycosystema 2019, 38, 127–133. [Google Scholar] [CrossRef]
- Kou, R.W.; Du, S.T.; Xia, B.; Zhang, Q.; Yin, X.; Gao, J.M. Phenolic and Steroidal Metabolites from the Cultivated Edible Inonotus hispidus Mushroom and Their Bioactivities. J. Agric. Food Chem. 2021, 69, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Shaojun, T.; Ping, L.; Wenhua, M.; Chenxia, S.; Shenglian, W.; Yi, Y.; Yuelin, H.; Jun, X. Isolation and Identification of Antitumor Metabolites from Fermentation Broth of Inonotus hispidus. Acta Edulis. Fungi 2021, 28, 109–116. [Google Scholar] [CrossRef]
- Yang, X. Studies on the Chemical Constituents of Xanthochrous hispidus. Master Thesis, Shandong University of Traditional Chinese Medicine, Jinan, China, 2008. [Google Scholar]
- Zhou, J.; Lin, X.; Liu, S.; Wang, Z.; Liu, D.; Huo, Y.; Li, D. Effects of Compound Elicitors on the Biosynthesis of Triterpenoids and Activity of Defense Enzymes from Inonotus hispidus (Basidiomycetes). Molecules 2022, 27, 2618. [Google Scholar] [CrossRef] [PubMed]
- Politi, M.; Silipo, A.; Siciliano, T.; Tebano, M.; Flamini, G.; Braca, A.; Jiménez-Barbero, J. Current analytical methods to study plant water extracts: The example of two mushrooms species, Inonotus hispidus and Sparassis crispa. Phytochem. Anal. 2007, 18, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fushiya, S.; Matsuda, M.; Yamada, S.; Nozoe, S. New opine type amino acids from a poisonous mushroom, Clitocybe acromelalga. Tetrahedron 1996, 52, 877–886. [Google Scholar] [CrossRef]
- Yang, S.; Bao, H.; Wang, H.; Li, Q. Anti-tumour Effect and Pharmacokinetics of an Active Ingredient Isolated from Inonotus hispidus. Biol. Pharm. Bull. 2019, 42, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Fan, N.-W.; Chang, H.-S.; Cheng, M.-J.; Hsieh, S.-Y.; Liu, T.-W.; Yuan, G.-F.; Chen, I.-S. Secondary Metabolites from the Endophytic Fungus Xylaria cubensis. Helv. Chim. Acta 2014, 97, 1689–1699. [Google Scholar] [CrossRef]
- Ali, N.A.A.; Mothana, R.A.A.; Lesnau, A.; Pilgrim, H.; Lindequist, U. Antiviral activity of Inonotus hispidus. Fitoterapia 2003, 74, 483–485. [Google Scholar] [CrossRef]
- Zan, L.; Liang, R.; Bao, H. Antioxidant and Antimicrobial Activities of Inonotus hispidus Extracts. North. Hortic. 2015, 5, 151–155. Available online: https://kns.cnki.net/kcms/detail/detail.aspx?FileName=BFYY201505047&DbName=CJFQ2015 (accessed on 10 July 2022).
- Benarous, K.; Bombarda, I.; Iriepa, I.; Moraleda, I.; Gaetan, H.; Linani, A.; Tahri, D.; Sebaa, M.; Yousfi, M. Harmaline and hispidin from Peganum harmala and Inonotus hispidus with binding affinity to Candida rugosa lipase: In silico and in vitro studies. Bioorg. Chem. 2015, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Benarous, K.; Benali, F.Z.; Bekhaoua, I.C.; Yousfi, M. Novel potent natural peroxidases inhibitors with in vitro assays, inhibition mechanism and molecular docking of phenolic compounds and alkaloids. J. Biomol. Struct Dyn. 2021, 39, 7168–7180. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J.; Shen, D.A.; Shao, C.X.; Jun, X.U.; Yang, Y.; Jin, L.; Wu, S.-L. Classification and identification of a wild Inonotus hispidus and the antitumor activity of the fermentation supernatant. J. South. Agric. 2019, 50, 1671–1679. [Google Scholar] [CrossRef]
- Li, Z.; Bao, H. Anti-tumor effect of Inonotus hispidus petroleum ether extract in H22 tumor-bearing mice and analysis its mechanism by untargeted metabonomic. J. Ethnopharmacol. 2022, 285, 114898. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bao, H. Deciphering key regulators of Inonotus hispidus petroleum ether extract involved in anti-tumor through whole transcriptome and proteome analysis in H22 tumor-bearing mice model. J. Ethnopharmacol. 2022, 296, 115468. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Bao, H.Y.; Bau, T.; Li, Y. Antitumor Effect of Solid State Fermentation Powder of Inonotus hispidus on H22 Bearing Mice. Zhong Yao Cai 2016, 39, 389–394. [Google Scholar] [CrossRef]
- Yang, S.; Bao, H.; Wang, H.; Li, Z.; Han, C. Bacteriostatic and Antioxidative Effects of Volatile Oil from Inonotus hispidus. J. Jilin Agric. Univ. 2020, 42, 148–153. [Google Scholar] [CrossRef]
- Shomali, N.; Onar, O.; Alkan, T.; Demirtaş, N.; Akata, I.; Yildirim, Ö. Investigation of the Polyphenol Composition, Biological Activities, and Detoxification Properties of Some Medicinal Mushrooms from Turkey. Turk. J. Pharm. Sci. 2019, 16, 155–160. [Google Scholar] [CrossRef]
- Benarous, K.; Djeridane, A.; Kameli, A.; Yousfi, M. Inhibition of Candida rugosa Lipase by Secondary Metabolites Extracts of Three Algerian Plants and their Antioxydant Activities. Curr. Enzym. Inhib. 2013, 9, 75–82. [Google Scholar] [CrossRef]
- Ying, Y.-M.; Zhang, L.-Y.; Zhang, X.; Bai, H.-B.; Liang, D.-E.; Ma, L.-F.; Shan, W.-G.; Zhan, Z.-J. Terpenoids with alpha-glucosidase inhibitory activity from the submerged culture of Inonotus obliquus. Phytochemistry 2014, 108, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ding, Y.; Yang, Y.; Xie, M.; Wang, S.; Chen, C.; Wang, H.; Li, Y. α-glucosidase Inhibition and Antioxidant Activities of Different Polar Extracts of Inonotus hispidus. Edible Fungi China 2021, 40, 37–41. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Yan, J.; Xu, K.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int. J. Biol. Macromol. 2019, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-K.; Yun, B.-S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.C.; Menezes, R.; Stewart, D. Polyphenols as New Leads in Drug Discovery: Biological Activity and Mechanisms. Curr. Pharm. Des. 2018, 24, 2041–2042. [Google Scholar] [CrossRef]
- Sarfraz, A.; Rasul, A.; Sarfraz, I.; Shah, M.A.; Hussain, G.; Shafiq, N.; Masood, M.; Adem, S.; Sarker, S.D.; Li, X. Hispolon: A natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Env. Res. 2020, 190, 110017. [Google Scholar] [CrossRef] [PubMed]
- Nambudiri, A.M.D.; Vance, C.P.; Towers, G.H.N. Effect of light on enzymes of phenylpropanoid metabolism and hispidin biosynthesis in Polyporus hispidus. Biochem. J. 1973, 134, 891–897. [Google Scholar] [CrossRef][Green Version]
- Kemami Wangun, H.V.; Härtl, A.; Tam Kiet, T.; Hertweck, C. Inotilone and related phenylpropanoid polyketides from Inonotus sp. and their identification as potent COX and XO inhibitors. Org. Biom. Chem. 2006, 4, 2545–2548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, R.; Zhang, J.; Bu, Q.; Wang, W.; Liu, Y.; Li, Q.; Guo, Y.; Zhang, L.; Yang, Y. The integration of metabolome and proteome reveals bioactive polyphenols and hispidin in ARTP mutagenized Phellinus baumii. Sci. Rep. 2019, 9, 16172. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Zhong, S.; Du, X.; Cao, Y.; Wang, W.; Sun, Y.; Tian, Y.; Zhu, J.; Chen, J.; Xuan, L.; et al. Whole-genome sequence of Phellinus gilvus (mulberry Sanghuang) reveals its unique medicinal values. J. Adv. Res. 2020, 24, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Angulo-Sanchez, L.T.; López-Peña, D.; Torres-Moreno, H.; Gutiérrez, A.; Gaitán-Hernández, R.; Esqueda, M. Biosynthesis, Gene Expression, and Pharmacological Properties of Triterpenoids of Ganoderma Species (Agaricomycetes): A Review. Int. J. Med. Mushrooms 2022, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Zöll, M.; Peintner, U.; Rollinger, J.M. European medicinal polypores—A modern view on traditional uses. J. Ethnopharmacol. 2014, 154, 564–583. [Google Scholar] [CrossRef]
- Floris, B.; Galloni, P.; Conte, V.; Sabuzi, F. Tailored Functionalization of Natural Phenols to Improve Biological Activity. Biomolecules 2021, 11, 1325. [Google Scholar] [CrossRef]
- Dasgupta, A.; Acharya, K. Chapter 10—Bioactive terpenoids from mushrooms. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 145–154. [Google Scholar]
- Duan, Y.; Han, H.; Qi, J.; Gao, J.-m.; Xu, Z.; Wang, P.; Zhang, J.; Liu, C. Genome sequencing of Inonotus obliquus reveals insights into candidate genes involved in secondary metabolite biosynthesis. BMC Genom. 2022, 23, 314. [Google Scholar] [CrossRef]
- Yingce, D.; Dan, S.; Lin, W.; Xiaoning, Z.; Chunlei, W.; Chengwei, L. Research Progress of Small Molecule Chemical Components and Pharmacological Values of Inonotus obliquus. J. Fungal Res. 2022, 20, 1–14. [Google Scholar] [CrossRef]
- Agger, S.; Lopez-Gallego, F.; Schmidt-Dannert, C. Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus. Mol. Microbiol. 2009, 72, 1181–1195. [Google Scholar] [CrossRef]
- Wawrzyn, G.T.; Quin, M.B.; Choudhary, S.; López-Gallego, F.; Schmidt-Dannert, C. Draft Genome of Omphalotus olearius Provides a Predictive Framework for Sesquiterpenoid Natural Product Biosynthesis in Basidiomycota. Chem. Biol. 2012, 19, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Quin, M.B.; Flynn, C.M.; Wawrzyn, G.T.; Choudhary, S.; Schmidt-Dannert, C. Mushroom Hunting by Using Bioinformatics: Application of a Predictive Framework Facilitates the Selective Identification of Sesquiterpene Synthases in Basidiomycota. ChemBioChem 2013, 14, 2480–2491. [Google Scholar] [CrossRef] [PubMed]
- Nagamine, S.; Liu, C.; Nishishita, J.; Kozaki, T.; Sogahata, K.; Sato, Y.; Minami, A.; Ozaki, T.; Schmidt-Dannert, C.; Maruyama, J.-I.; et al. Ascomycete Aspergillus oryzae Is an Efficient Expression Host for Production of Basidiomycete Terpenes by Using Genomic DNA Sequences. Appl. Environ. Microbio. 2019, 85, e00409–e00419. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Ma, K.; Yang, Y.; Wang, K.; Chen, B.; Huang, Y.; Han, J.; Bao, L.; Liu, X.-B.; Yang, Z.; et al. Bioactive Sesquiterpenes from the Edible Mushroom Flammulina velutipes and Their Biosynthetic Pathway Confirmed by Genome Analysis and Chemical Evidence. J. Org. Chem. 2016, 81, 9867–9877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, X.; Orban, A.; Shukal, S.; Birk, F.; Too, H.-P.; Rühl, M. Agrocybe aegerita Serves as a Gateway for Identifying Sesquiterpene Biosynthetic Enzymes in Higher Fungi. ACS Chem. Biol. 2020, 15, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Su, D.; Keyhani, N.O.; Wang, C.; Shen, L.; Qiu, J. Influence of selenium on the mycelia of the shaggy bracket fungus, Inonotus hispidus. J. Sci. Food Agric. 2022, 102, 3762–3770. [Google Scholar] [CrossRef]
- Hou, R.; Liu, X.; Xiang, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Characterization of the physicochemical properties and extraction optimization of natural melanin from Inonotus hispidus mushroom. Food Chem. 2019, 277, 533–542. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Xu, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus. Int. J. Biol. Macromol. 2019, 123, 468–476. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A Review on Antimicrobial Activity of Mushroom (Basidiomycetes) Extracts and Isolated Compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.-J.; Lim, K.-T. Mushroom-Derived Bioactive Molecules as Immunotherapeutic Agents: A Review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Liu, Y.H.; Chen, J.X. Pleuromutilin and its derivatives-the lead compounds for novel antibiotics. Mini Rev. Med. Chem. 2012, 12, 53–61. [Google Scholar] [CrossRef] [PubMed]
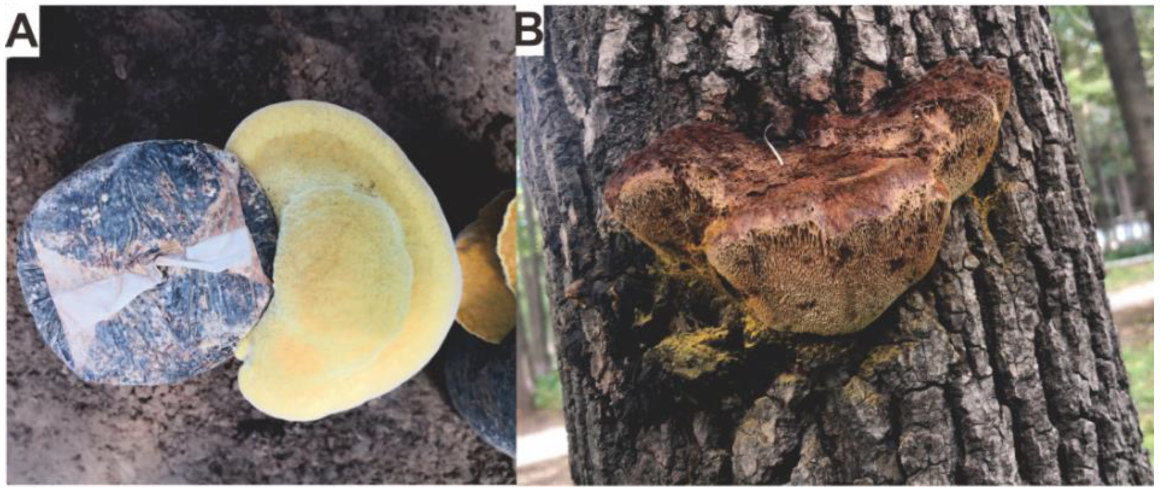
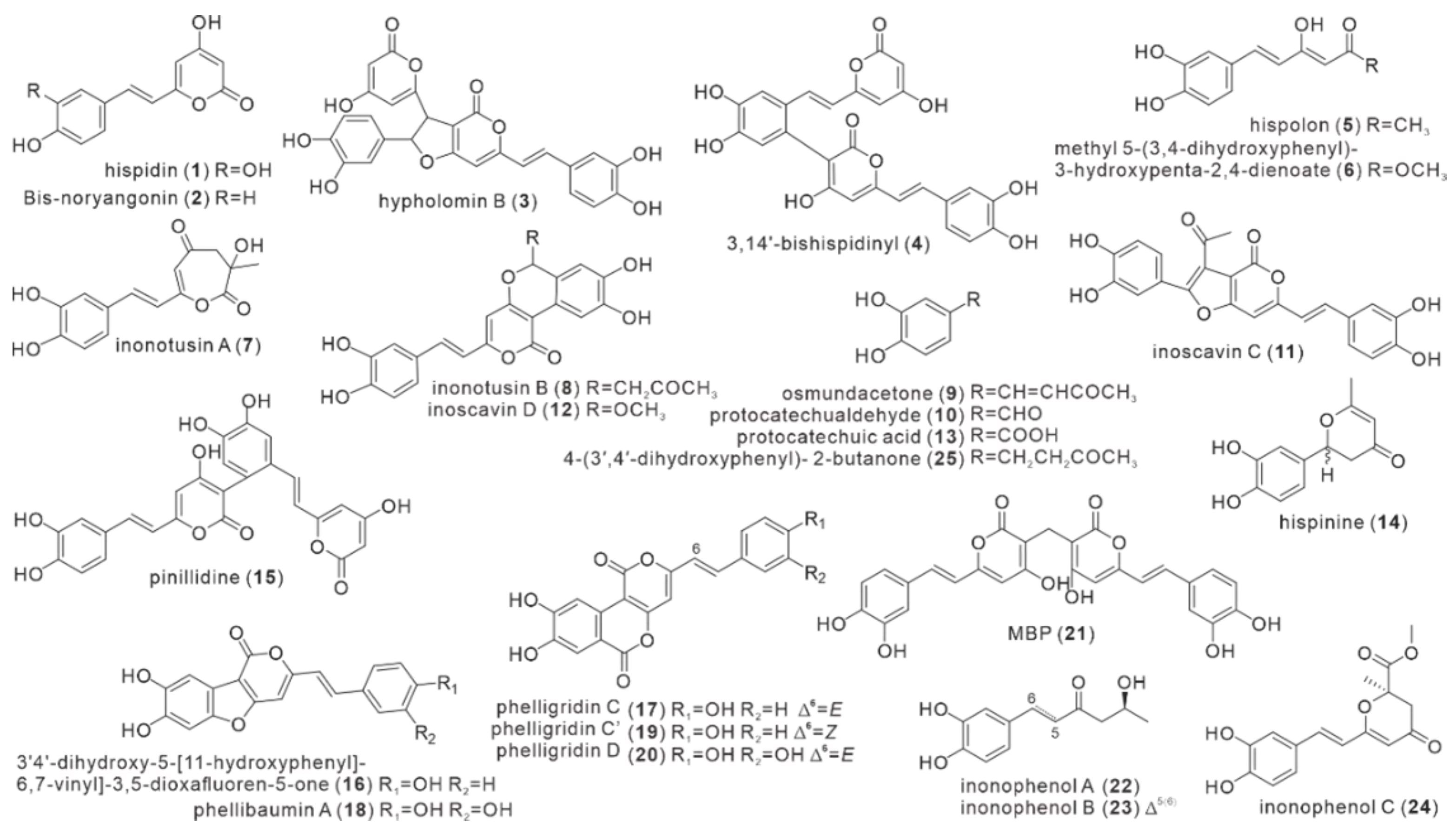
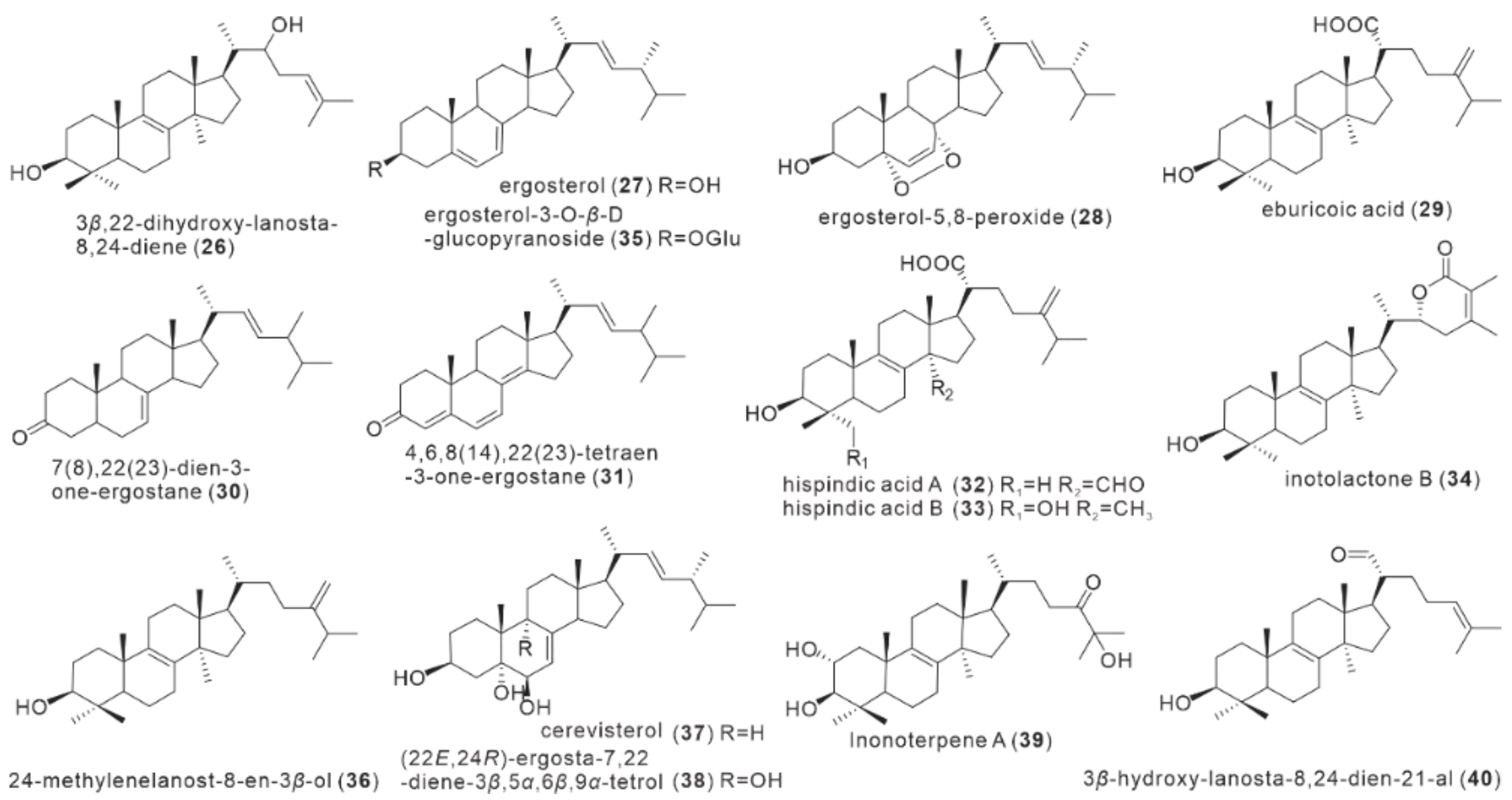

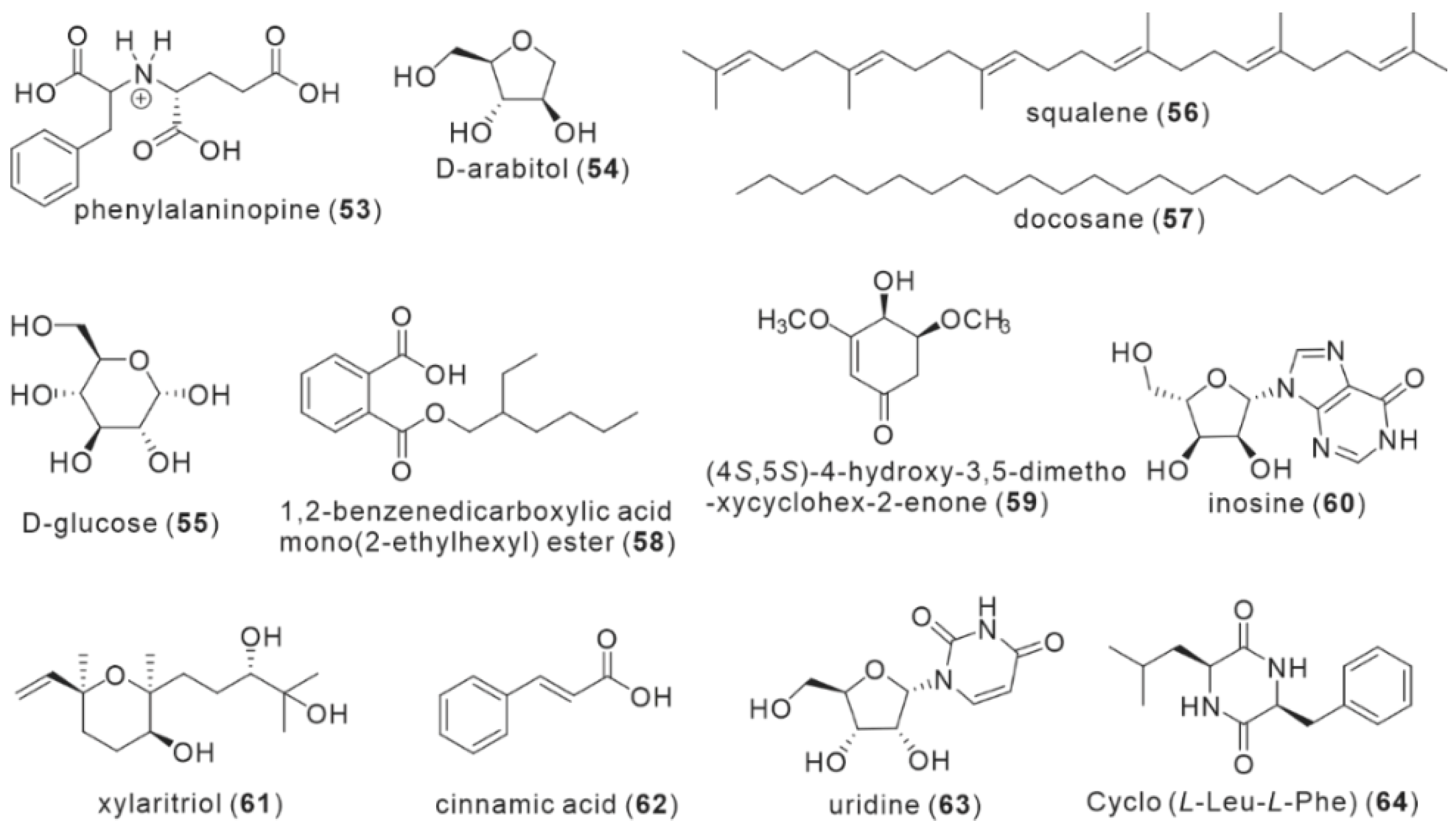
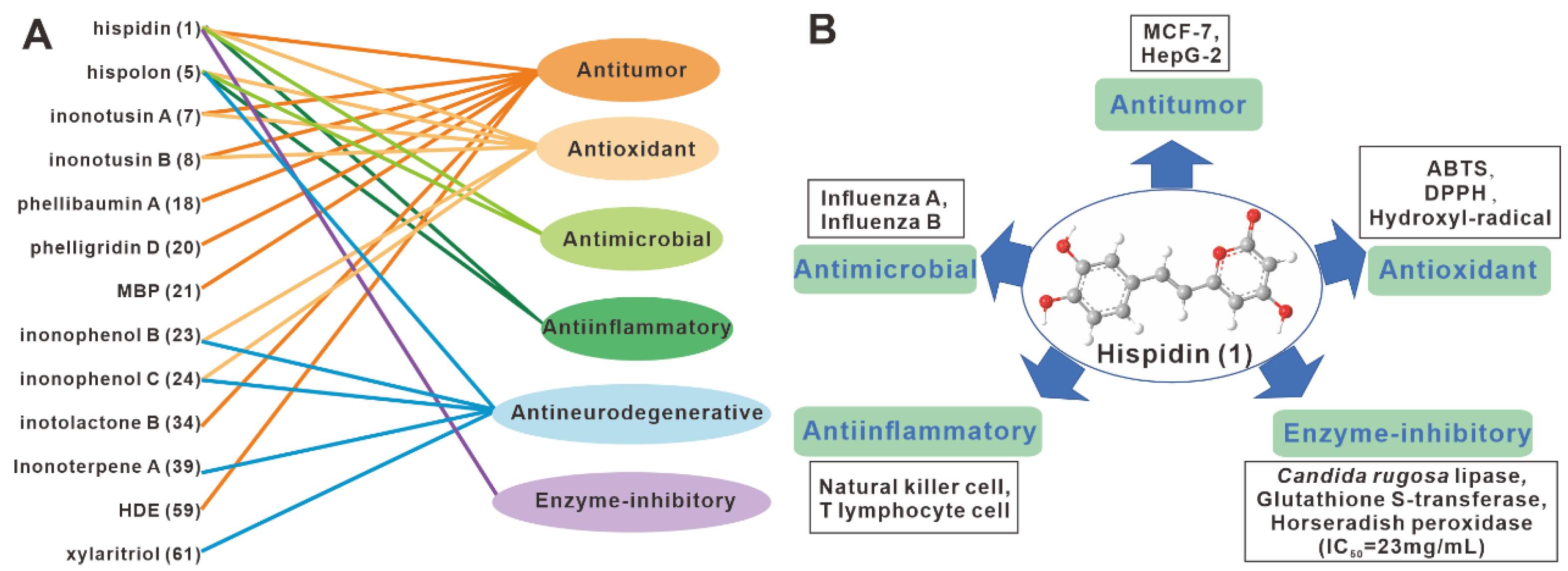
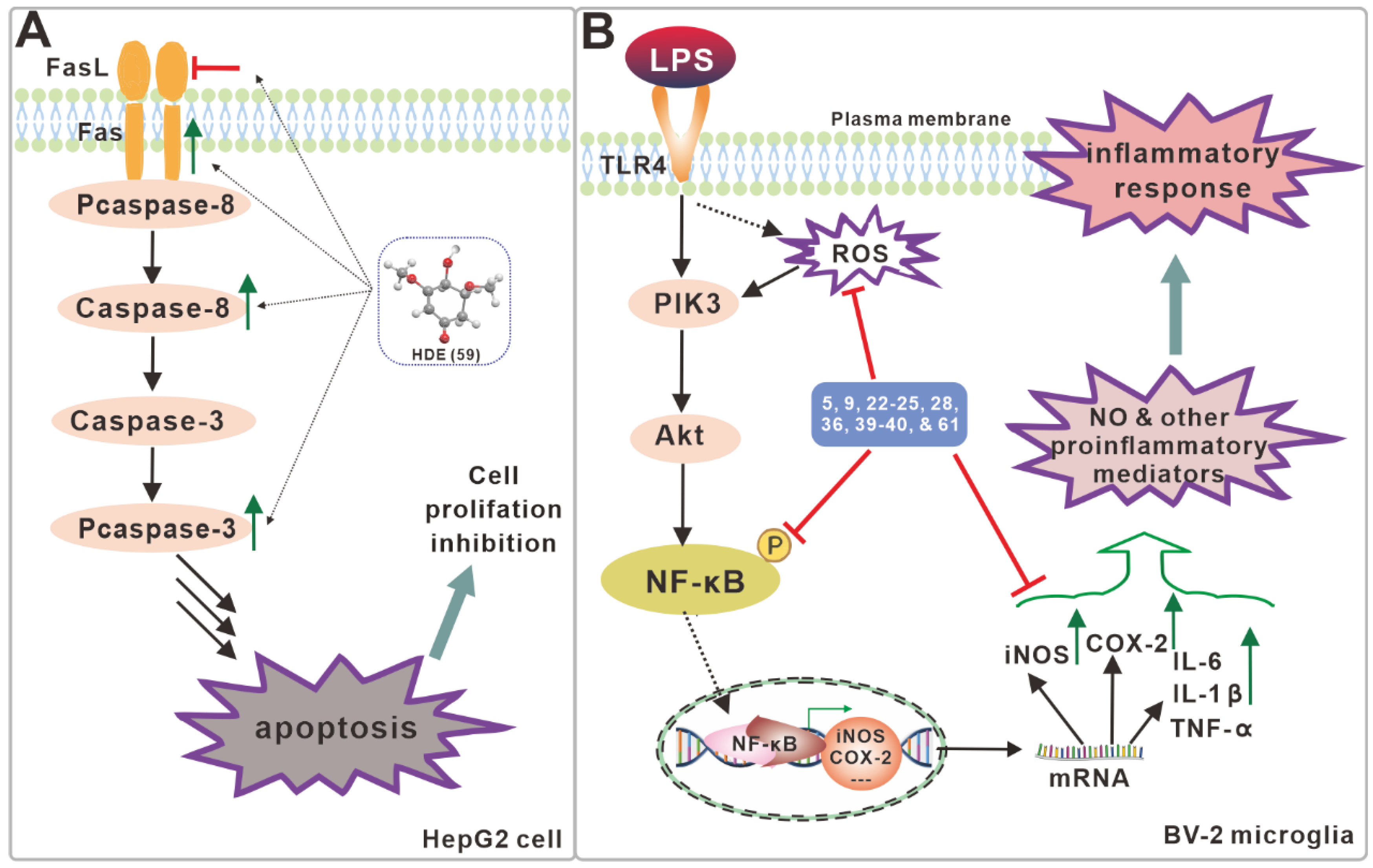

| No. | Name | Resource | Method | Activity | Reference |
|---|---|---|---|---|---|
| 1 | hispidin | fruiting body, mycelium | EtOH, MeOH | Antitumor, ABTS, DPPH, influenza A and B viruses, Enzyme-inhibitory, antineurodegenerative | Edwards et al. [9]. Zan et al. [15]. Ali, N.A.A. et al. [29]. Zan et al. [16]. Zan et al. [30]. Benarous, K. et al. [31]. Benarous, K. et al. [32]. Gruendemann, C. et al. [17]. |
| 2 | Bis-noryangonin | mycelium | NA | NA | Perrin, P.W.et al. [10]. |
| 3 | hypholomin B | fruiting body | NA | NA | Fiasson et al. [11]. |
| 4 | 3,14’-bishispidinyl | fruiting body | MeOH | NA | Fiasson et al. [11]. Ren et al. [18]. |
| 5 | hispolon | fruiting body mycelium | EtOH, MeOH EAC | DPPH, influenza A and B viruses, PC-12 cell, BV-2 microglia, ntineurodegenerative | Nasser Ali et al. [12]. Zan et al. [15]. Tang et al. [22]. Kou et al. [21]. Ali, N.A.A. et al. [29]. Gruendemann, C. et al. [17]. |
| 6 | methyl 5-(3,4-dihydroxyphenyl)-3-hydroxypenta-2,4-dienoate | fruiting body | MeOH | NA | Yousfi et al. [13]. |
| 7 | inonotusin A | fruiting body | MeOH | MCF-7, ABTS | Zan et al. [15]. Zan et al. [16]. |
| 8 | inonotusin B | fruiting body | MeOH | Antitumor, ABTS | Zan et al. [15]. Zan et al. [16]. |
| 9 | osmundacetone | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Zan et al. [15]. Kou et al. [21]. |
| 10 | protocatechualdehyde | fruiting body | MeOH | NA | Zan et al. [15]. |
| 11 | inoscavin C | fruiting body | MeOH | NA | Zan et al. [15]. |
| 12 | inoscavin D | fruiting body | MeOH | NA | Zan et al. [15]. |
| 13 | protocatechuic acid | fruiting body | MeOH | NA | Zan et al. [15]. |
| 14 | hispinine | fruiting body | MeOH | NA | Ren et al. [18]. |
| 15 | pinillidine | fruiting body | MeOH | NA | Ren et al. [18]. |
| 16 | 3’4’-dihydroxy-5-[11-hydroxyphenyl]-6,7-vinyl]-3,5-dioxafluoren-5-one | fruiting body | EtOH | mouse macrophage cell | Li et al. [2]. |
| 17 | phelligridin C | fruiting body | EtOH | NA | Li et al. [2]. |
| 18 | phellibaumin A | fruiting body | EtOH MeOH | mouse macrophage cell | Li et al. [2]. Yang et al. [20]. |
| 19 | phelligridin C’ | fruiting body | EtOH | NA | Li et al. [2]. |
| 20 | phelligridin D | fruiting body | EtOH | mouse macrophage cell | Li et al. [2]. |
| 21 | MBP | fruiting body | MeOH | HepG2, MCF-7, Hela and A549 cells | Yang et al. [20]. |
| 22 | inonophenol A | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 23 | inonophenol B | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 24 | inonophenol C | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 25 | 4-(3′,4′-dihydroxyphenyl)-2-butanone | fruiting body | MeOH | DPPH, PC-12 cell, BV-2 microglia | Kou et al. [21]. |
| 26 | 3β, 22-dihydroxy-lanosta-8,24-diene | fruiting body | EtOH | U937, Hela, QRH-7701 | Yang et al. [23]. |
| 27 | ergosterol | fruiting body mycelium | EtOH, MeOH EAC | NA | Yang et al. [23]. Zan et al. [15]. Tang et al. [22]. |
| 28 | ergosterol-5,8-peroxide | fruiting body | EtOH, MeOH | BV-2 microglia | Yang et al. [23]. Zan et al. [15]. Kou et al. [21]. |
| 29 | eburicoic acid | fruiting body | EtOH, MeOH | NA | Yang et al. [23]. Zan et al. [15]. |
| 30 | 7(8),22(23)-dien-3-one-ergostane | fruiting body | MeOH | NA | Zan et al. [15]. |
| 31 | 4,6,8(14),22(23)-tetraen-3-one-ergostane | fruiting body | MeOH | NA | Zan et al. [15]. |
| 32 | hispindic acid A | fruiting body | MeOH | NA | Ren et al. [18]. |
| 33 | hispindic acid B | fruiting body | MeOH | NA | Ren et al. [18]. |
| 34 | inotolactone B | fruiting body | MeOH | B16 melanoma cell | Ren et al. [18]. |
| 35 | ergosterol-3-O-β-D-glucopyranoside | fruiting body | MeOH | BV-2 microglia | Ren et al. [18]. Kou et al. [21]. |
| 36 | 24-methylenelanost-8-en-3β-ol | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 37 | cerevisterol | fruiting body | MeOH | NA | Kou et al. [21]. |
| 38 | (22E, 24R)-ergosta-7,22-diene-3β,5α,6β,9α-tetrol | fruiting body | MeOH | NA | Kou et al. [21]. |
| 39 | Inonoterpene A | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 40 | 3β-hydroxy-lanosta-8,24-dien-21-al | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 41 | hexadecanoic acid | fruiting body | EtOH, MeOH | NA | Yang et al. [23]. Zan et al. [15]. |
| 42 | octadecanoic acid | fruiting body | EtOH | NA | Yang et al. [23]. |
| 43 | (E)-9-hexadecenoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 44 | hexadecanoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 45 | hexadecanoic acid ethyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 46 | 10,13-octadecadienoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 47 | ethyl oleate | fruiting body | MeOH | NA | Zan et al. [15]. |
| 48 | linoleic acid ethyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 49 | 9,12-octadecadienoic acid (Z, Z)-methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 50 | Isopropyl linoleate | fruiting body | MeOH | NA | Zan et al. [15]. |
| 51 | cis-erucic acid | fruiting body | MeOH | NA | Zan et al. [15]. |
| 52 | cis-9,17 octadecadienoic acid methyl ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 53 | phenylalaninopine | fruiting body | Water | NA | Politi et al. [25]. |
| 54 | D-arabitol | fruiting body | EtOH | NA | Yang et al. [23]. |
| 55 | D-glucose | fruiting body | EtOH | NA | Yang et al. [23]. |
| 56 | squalene | fruiting body | MeOH | NA | Zan et al. [15]. |
| 57 | docosane | fruiting body | MeOH | NA | Zan et al. [15]. |
| 58 | 1,2-benzenedicarboxylic acid mono(2-ethylhexyl) ester | fruiting body | MeOH | NA | Zan et al. [15]. |
| 59 | HDE | fruiting body | MeOH | HepG2, McF-7, Hela, A549 and H22 cells | Yang et al. [27]. |
| 60 | inosine | fruiting body | MeOH | NA | Yang et al. [20]. |
| 61 | xylaritriol | fruiting body | MeOH | BV-2 microglia | Kou et al. [21]. |
| 62 | cinnamic acid | mycelium | NA | NA | Tang et al. [22]. |
| 63 | uridine | mycelium | NA | NA | Tang et al. [22]. |
| 64 | Cyclo (L-Leu-L-Phe) | mycelium | NA | NA | Tang et al. [22]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.-x.; Feng, X.-l.; Liu, C.; Gao, J.-m.; Qi, J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus. Antibiotics 2022, 11, 1097. https://doi.org/10.3390/antibiotics11081097
Wang Z-x, Feng X-l, Liu C, Gao J-m, Qi J. Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus. Antibiotics. 2022; 11(8):1097. https://doi.org/10.3390/antibiotics11081097
Chicago/Turabian StyleWang, Zhen-xin, Xi-long Feng, Chengwei Liu, Jin-ming Gao, and Jianzhao Qi. 2022. "Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus" Antibiotics 11, no. 8: 1097. https://doi.org/10.3390/antibiotics11081097
APA StyleWang, Z.-x., Feng, X.-l., Liu, C., Gao, J.-m., & Qi, J. (2022). Diverse Metabolites and Pharmacological Effects from the Basidiomycetes Inonotus hispidus. Antibiotics, 11(8), 1097. https://doi.org/10.3390/antibiotics11081097










