Synthesis, Spectroscopic, Chemical Characterizations, Anticancer Capacities against HepG-2, Antibacterial and Antioxidant Activities of Cefotaxime Metal Complexes with Ca(II), Cr(III), Zn(II), Cu(II) and Se(IV)
Abstract
1. Introduction
2. Experimental Methods
2.1. Chemical Reagents
2.2. Synthesis of Cefotaxime Metal Complexes
2.3. Instruments
2.4. Antioxidant Activity
2.4.1. ORAC Assay
2.4.2. The Test of Metal Chelation
2.4.3. The Test of DPPH
2.4.4. ABTS Assay
2.4.5. FARAB Assay
2.5. Cell Culture
2.5.1. Cytotoxicity Assay
2.5.2. Cytotoxic Activity (IC50 Determination)
2.6. Antibacterial Activity against Bacillus Subtilis (ATCC 6633) and Escherichia coli (ATCC 8739)
2.7. Statistical Analysis
3. Results
3.1. Microanalytical and Physical Data
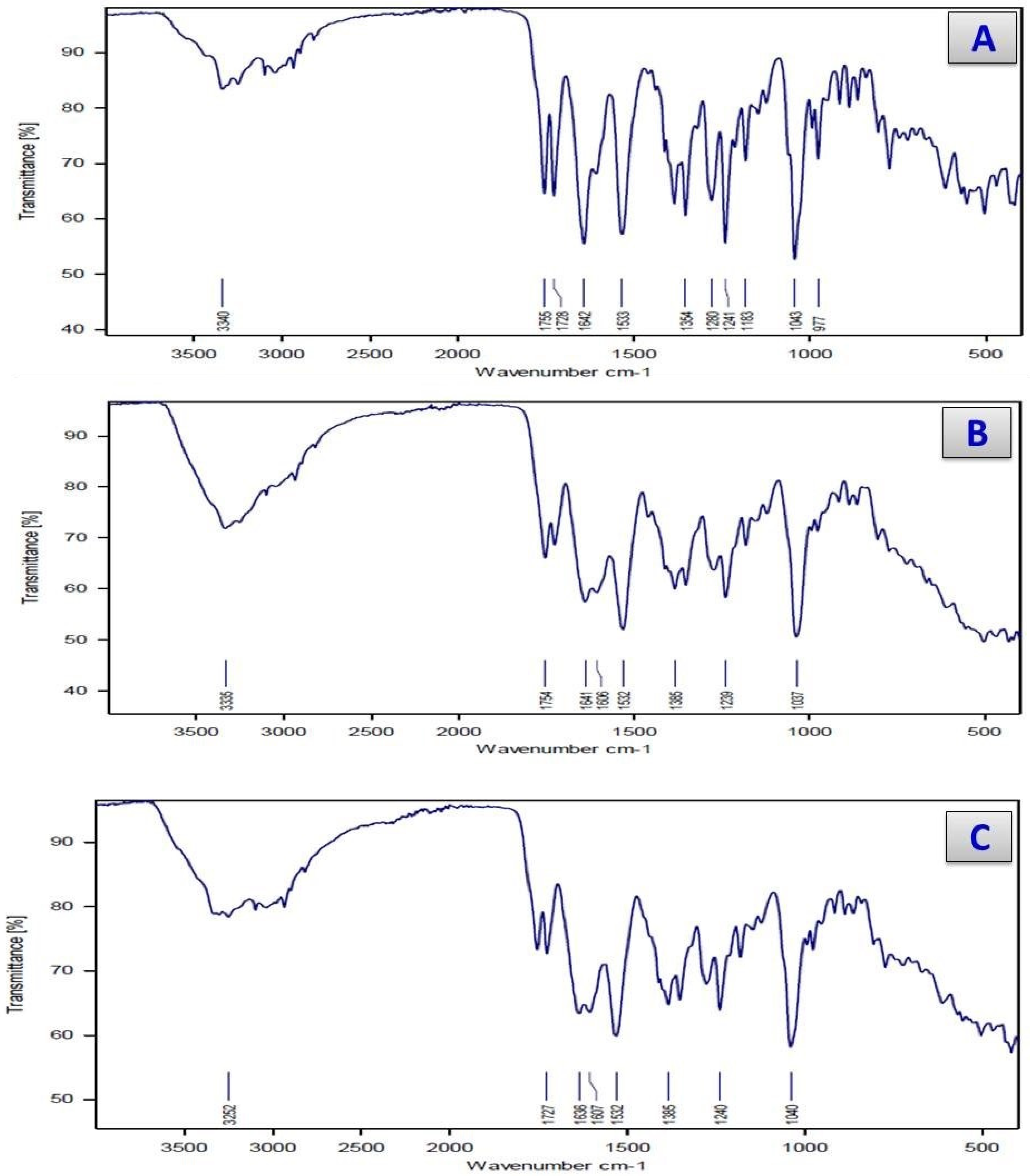
3.2. Infrared Spectra
3.3. UV–Vis Spectra
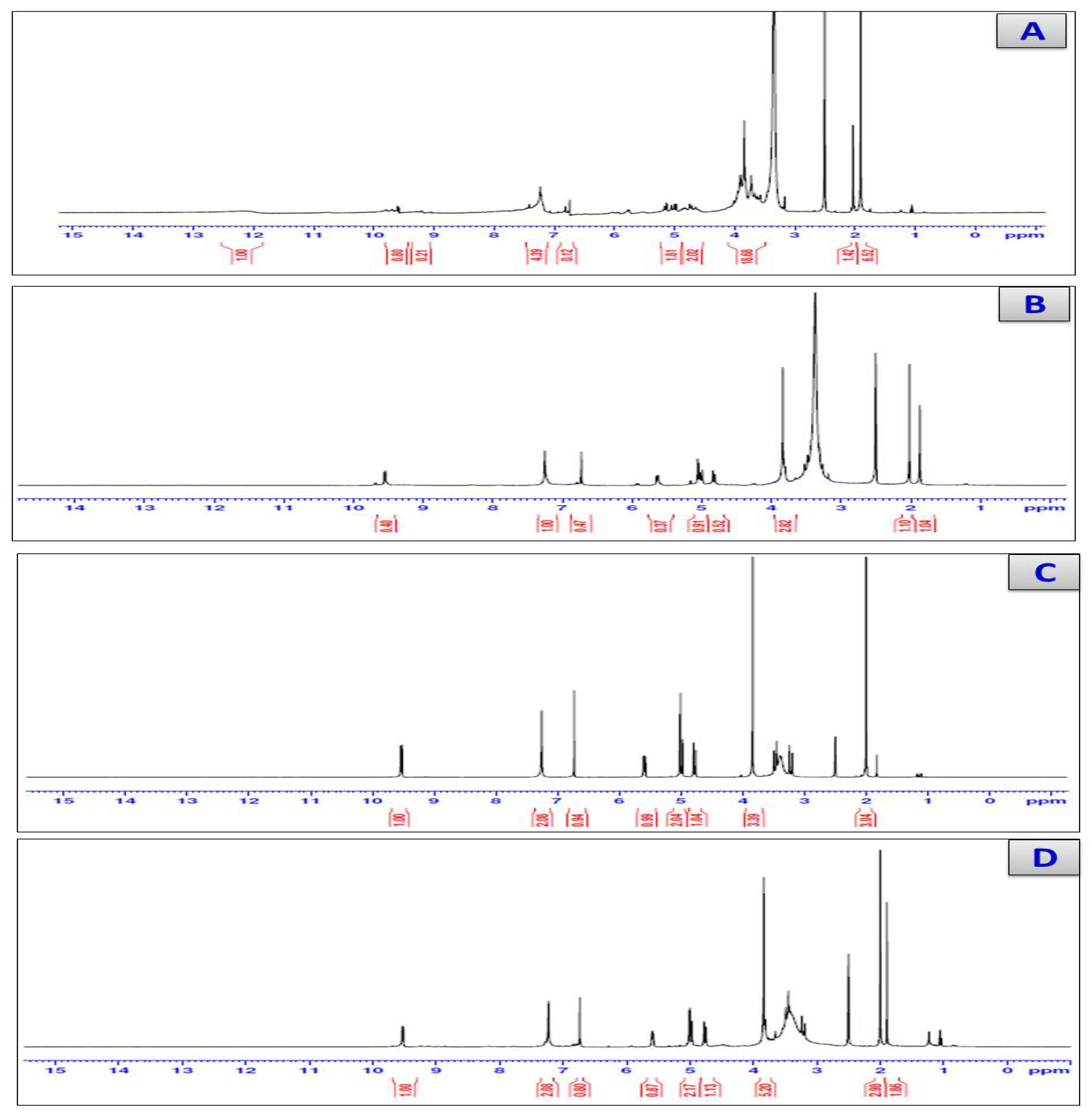
3.4. H-NMR Spectra
3.5. The Magnetic Measurements
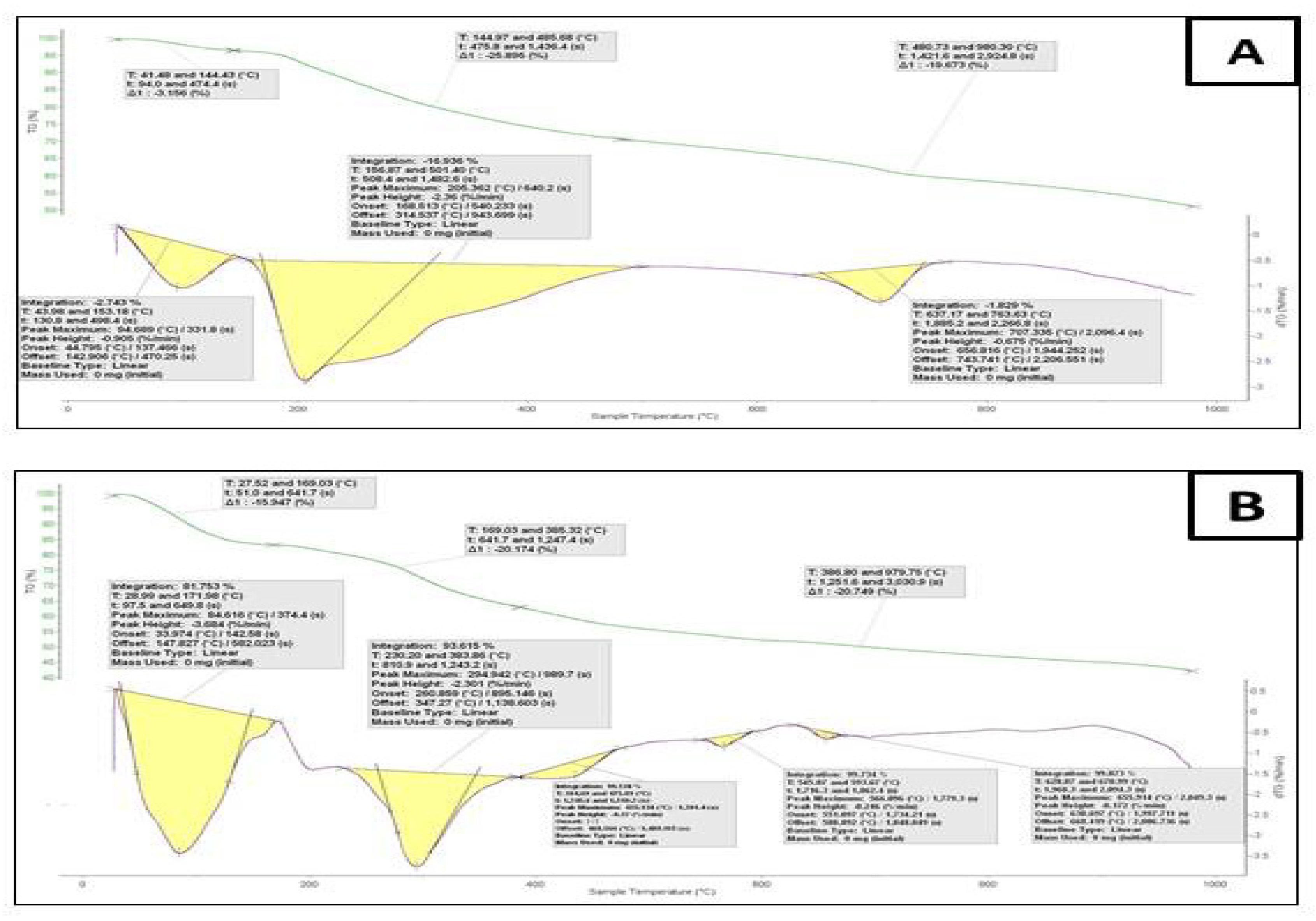
3.6. Thermogravimetric Analyses
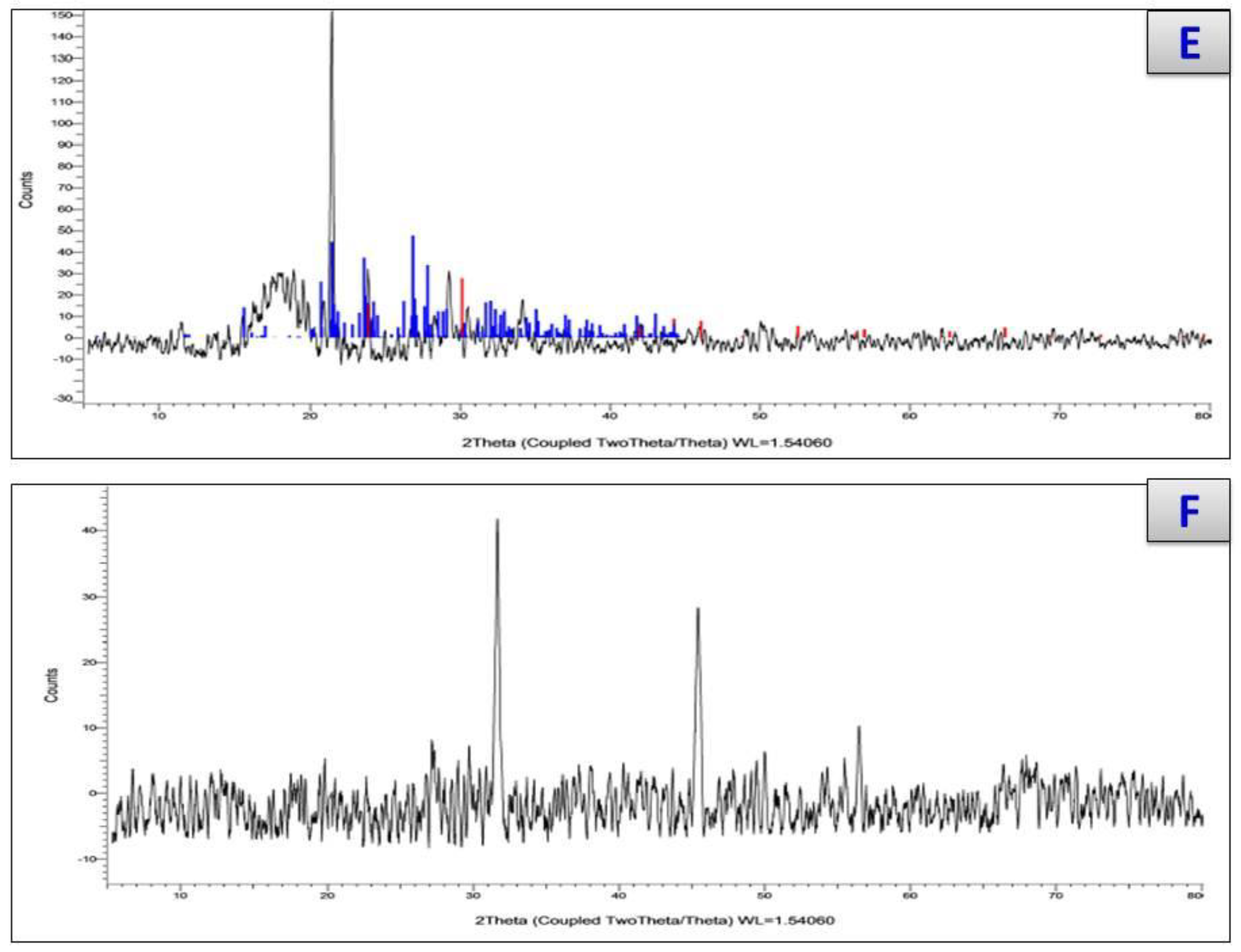
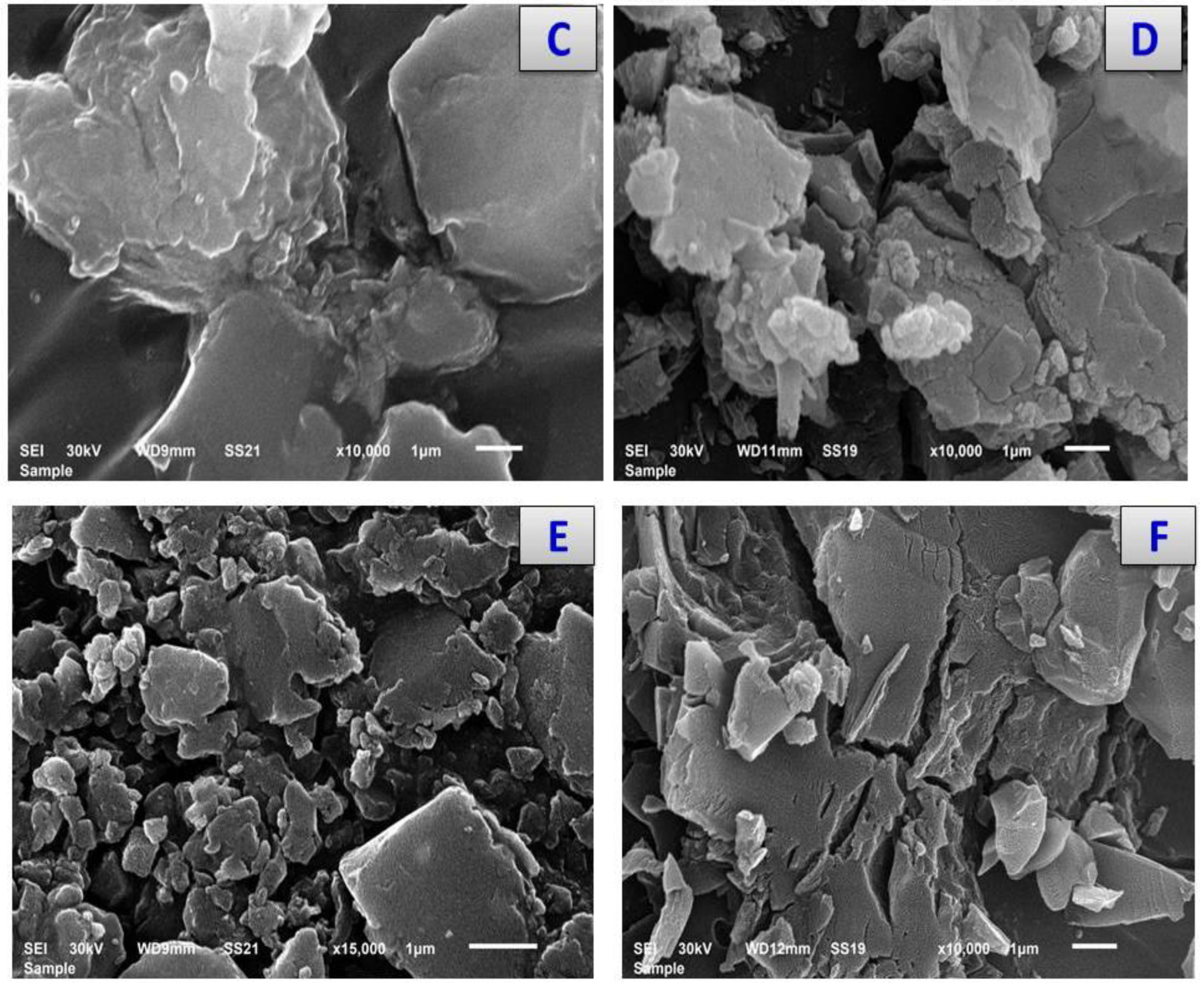
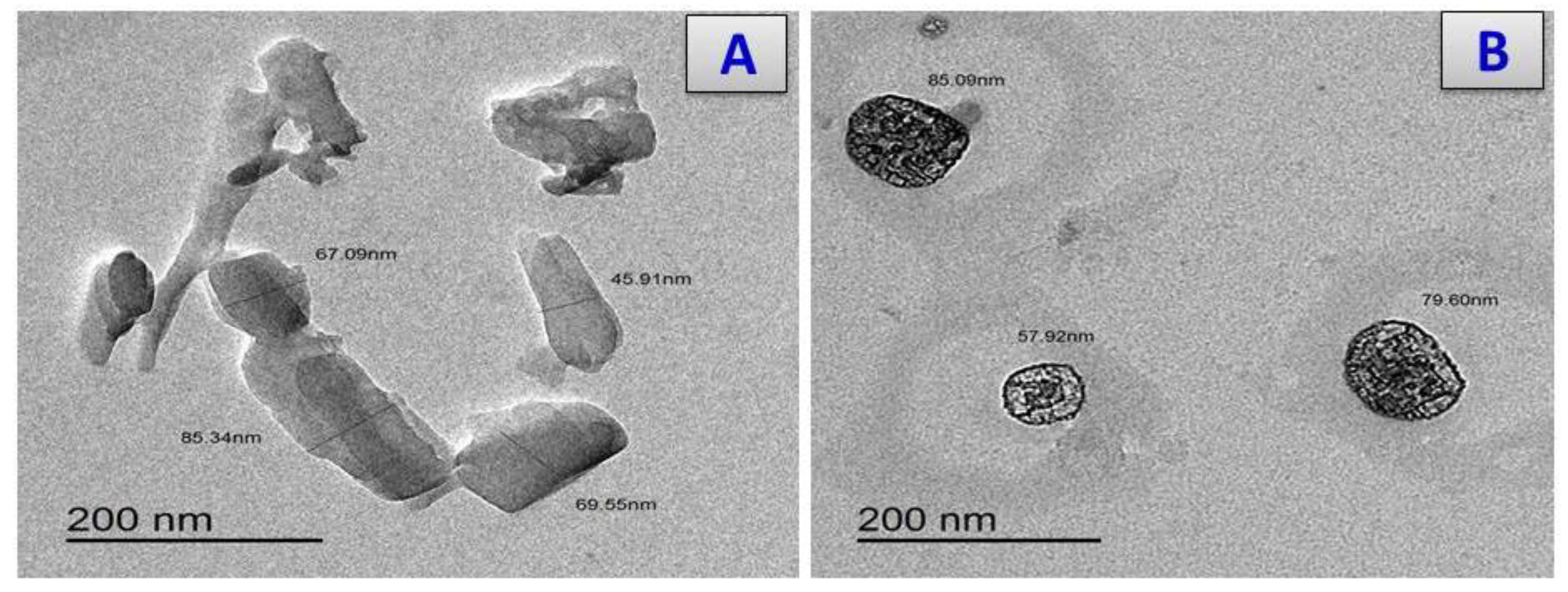
3.7. X-ray Diffraction and Scanning and Transmission Electron Microscopy
3.8. Structures of Cefotaxime Complexes
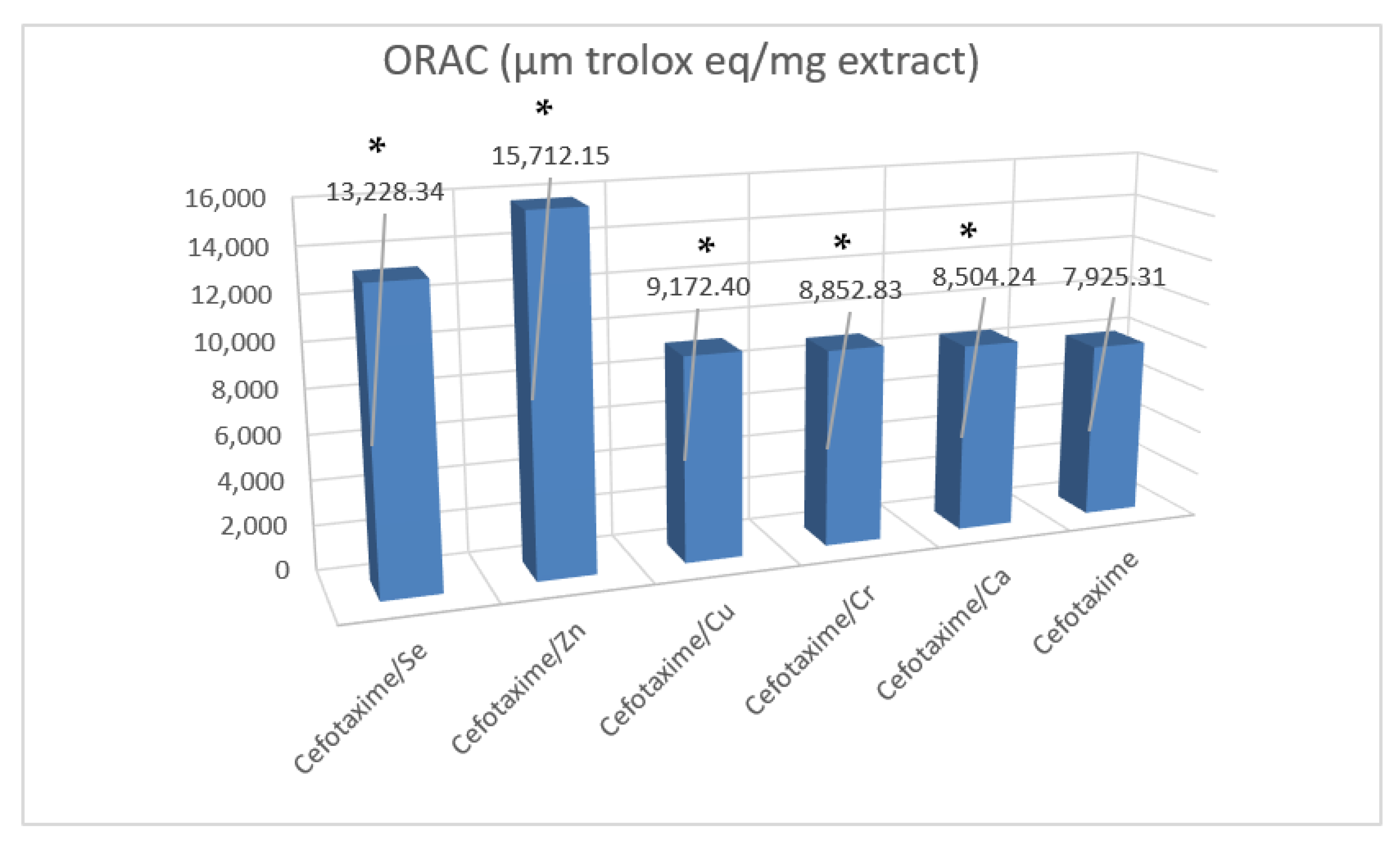
3.9. Antioxidant Activities of Cefotaxime Metal Complexes
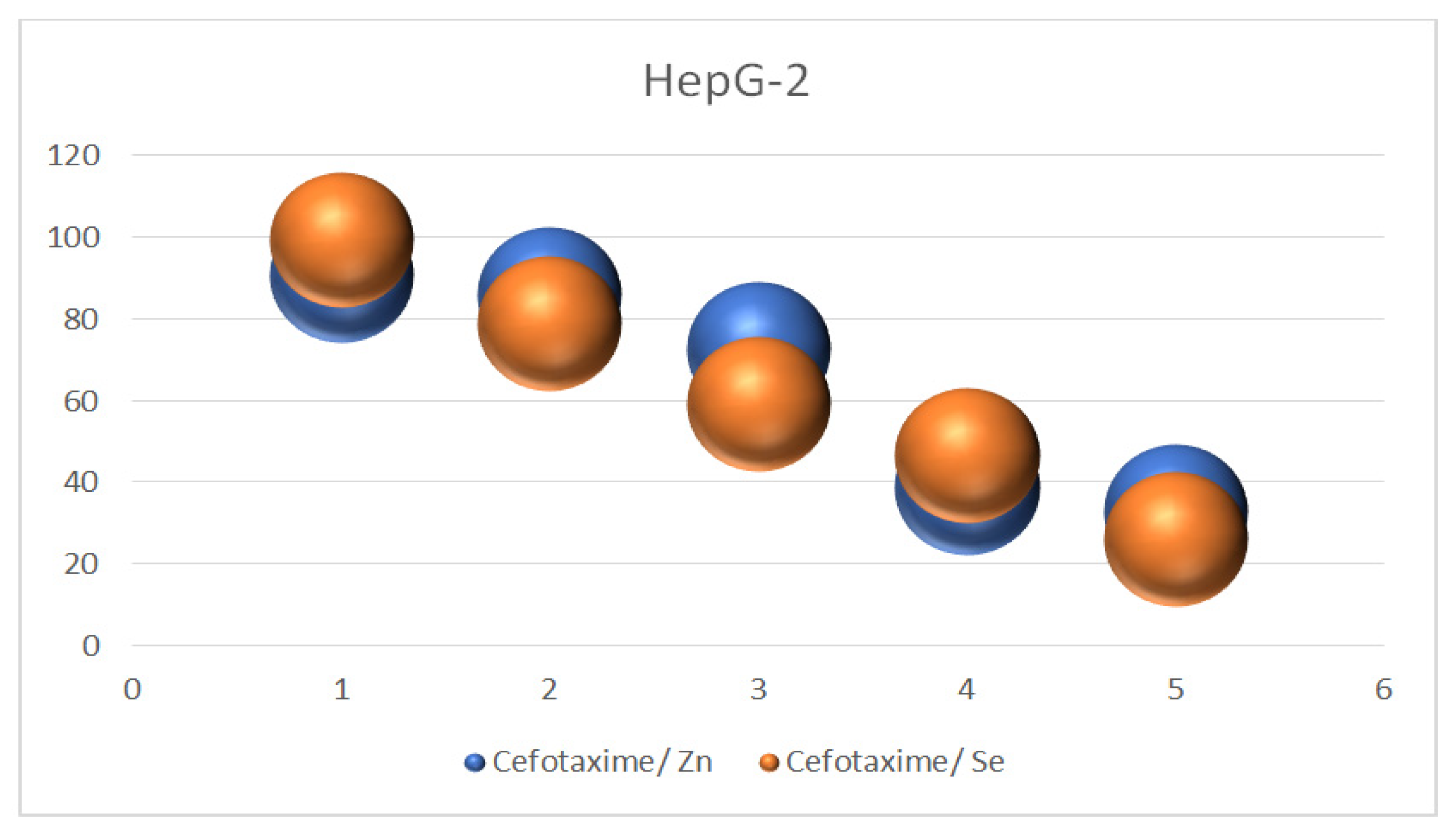
3.10. Screening of Cytotoxic Activity of Ceftox/Se and Ceftoax/Zn Metal Complexes
3.11. Anticancer Activity against HepG-2
3.12. Antibacterial Activity Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, K.P.; Foleno, B.D.; Lafredo, S.C.; LoCoco, J.M.; Isaacson, D.M. In vitro and in vivo antibacterial activities of FK037, a novel parenteral broad-spectrum cephalosporin. Antimicrob. Agents Chemother. 1993, 37, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gums, J.G.; Boatwright, D.W.; Camblin, M.; Halstead, D.C.; E Jones, M.; Sanderson, R. Differences between ceftriaxone and cefotaxime: Microbiological inconsistencies. Ann. Pharmacother. 2008, 42, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K. A review—Can metal ions be incorporated into drugs? Asian J. Res. Chem. 2009, 2, 14–18. [Google Scholar]
- Anacona, J.R.; Osorio, I. Synthesis and antibacterial activity of copper (II) complexes with sulphathiazole and cephalosporin ligands. Trans. Met. Chem. 2008, 33, 517–521. [Google Scholar] [CrossRef]
- Masoud, M.S.; Ali, A.E.; Elasala, G.S. Synthesis, spectral, computational and thermal analysis studies of metallocefotaxime antibiotics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 363–377. [Google Scholar] [CrossRef]
- Reiss, A.; Chifiriuc, M.C.; Amzoiu, E.; Spînu, C.I. Transition metal (II) complexes with cefotaxime-derived Schiff base: Synthesis, characterization, and antimicrobial studies. Bioinorg. Chem. Appl. 2014, 17, 926287. [Google Scholar] [CrossRef]
- Williams, D.R. The Metals of Life; Van Nostrand Reinhold: London, UK, 1971. [Google Scholar]
- Sorenson, J.R.J. Copper chelates as possible active forms of the antiarthritic agents. J. Med. Chem. 1976, 19, 135. [Google Scholar] [CrossRef]
- Brown, D.H.; Smith, W.E.; Teape, J.W. Antiinflammatory effects of some copper complexes. J. Med. Chem. 1980, 23, 729. [Google Scholar] [CrossRef]
- Sorenson, J.R.J.; Nraign, J.O. (Eds.) Copper in the Environment; Wiley-Interscience: New York, NY, USA, 1981; Part 2, Chapter 5. [Google Scholar]
- Anacona, J.R.; Toledo, C. Synthesis and antibacterial activity of metal complexes of ciprofloxacin. Transit. Met. Chem. 2001, 26, 228–231. [Google Scholar] [CrossRef]
- Anacona, J.R.; Alvarez, P. Synthesis and antibacterial activity of metal complexes of cefazolin. Transit. Met. Chem. 2002, 27, 856–860. [Google Scholar] [CrossRef]
- Anacona, J.R.; Serrano, J. Synthesis and antibacterial activity of metal complexes of cephalothin. J. Coord. Chem. 2003, 56, 313. [Google Scholar] [CrossRef]
- Anacona, J.R.; Rodriguez, I. Synthesis and antibacterial activity of cephalexin metal complexes. J. Coord. Chem. 2004, 57, 1263. [Google Scholar] [CrossRef]
- Liang, Z.; Cheng, L.; Zhong, G.Y.; Liu, R.H. Antioxidant and Antiproliferative activities of Twenty-Four Vitis vinifera Grapes. PLoS ONE 2014, 9, e105146. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I. DPPH free radical scavenging activity of two extracts from Agelanthus sosoneifolius (Loranthaceae) Leaves. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 29–34. [Google Scholar]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution of total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FARAP) as a measure of “antioxidant power”: The FARAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Algehani, R.A.; Abou Khouzam, R.; Hegazy, G.A.; Alamoudi, A.A.; El-Halawany, A.M.; El Dine, R.S.; Ajabnoor, G.A.; Al-Abbasi, F.A.; Baghdadi, M.A.; Elsayed, I.; et al. Colossolactone-G synergizes the anticancer properties of 5-fluorouracil and gemcitabine against colorectal cancer cells. Biomed. Pharmacother. 2021, 140, 111730. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S.; Bazaid, S.A.; Shohayeb, M.M.; El-Sayed, M.M.; El-Wakil, E.A. Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. Eur. J. Med. Plants 2012, 2, 93–112. [Google Scholar] [CrossRef]
- Houdkova, M.; Chaure, A.; Doskocil, I.; Havlik, J.; Kokoska, L. New Broth Macrodilution Volatilization Method for Antibacterial Susceptibility Testing of Volatile Agents and Evaluation of Their Toxicity Using Modified MTT Assay In Vitro. Molecules 2021, 26, 4179. [Google Scholar] [CrossRef]
- Liu, D.; Kwasniewska, K. An improved agar plate method for rapid assessment of chemical inhibition to microbial populations. Bull. Environ. Contam. Toxicol. 1981, 27, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Geary, W.J. The Use of Conductivity Measurements in Organic Solvents for the Characterisation of Coordination Compounds. Coord. Chem. Rev. 1971, 7, 81. [Google Scholar] [CrossRef]
- Maeda, Y.; Okawara, R.J. Vibrational Spectra of Organometallics: Theoretical and Experimental Data. Organometal. Chem. 1967, 10, 247. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; John Wiley: New York, NY, USA, 1986. [Google Scholar]
- Franchini, G.C.; Giusti, A.; Preti, C.; Tassi, L.; Zannini, P. Coordinating ability of methylpiperidine dithiocarbamates towards platinum group metals. Polyhedron 1985, 4, 1553–1558. [Google Scholar] [CrossRef]
- Hadjikostas, C.C.; Katsoulos, G.A.; Shakhatreh, S.K. Synthesis and spectral studies of some new palladium(II) and 8 International Journal of Inorganic Chemistry platinum(II) dithiocarbimato complexes. Reactions of bases with the corresponding N-alkyldithiocarbamates. Inorg. Chim. Acta 1987, 133, 129–132. [Google Scholar] [CrossRef]
- Castillo, M.; Criado, J.J.; Macias, B.; Vaquero, M.V. Chemistry of dithiocarbamate derivatives of amino acids. I. Study of some dithiocarbamate derivatives of linear α-amino acids and their nickel(II) complexes. Inorg. Chim. Acta 1986, 124, 127–132. [Google Scholar] [CrossRef]
- Oztürk, O.F.; Şekerci, M.; Ozdemir, E. Synthesis of 5, 6-O-Cyclohexylidene-l-amino-3-azahexane and Its Co (II), Ni (II), and Cu (II) Complexes. Russ. J. Coord. Chem. 2005, 31, 687–690. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Qahl, S.H.; Alaryani, F.S.; Hamza, R.Z. Synthesis, Spectroscopic Studies for Five New Mg (II), Fe (III), Cu (II), Zn (II) and Se (IV) Ceftriaxone Antibiotic Drug Complexes and Their Possible Hepatoprotective and Antioxidant Capacities. Antibiotics 2022, 11, 547. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Figgis, B.N. Introduction to Ligand Fields; John Wiley: New York, NY, USA, 1976. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley: Reading, MA, USA, 1972; p. 102. [Google Scholar]
- Anacona, J.R.; Da Silva, G. Synthesis and antibacterial activity of cefotaxime metal complexes. J. Chil. Chem. Soc. 2005, 50, 447–450. [Google Scholar] [CrossRef]
- Chin, N.-X.; Gu, J.-W.; Fang, W.; Neu, H.C. In vitro activity and 𝛽-lactamase stability of GR69153, a new long-acting cephalosporin. Antimicrob. Agents Chemother. 1991, 35, 259–266. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Malki, N.A.; Alharthi, S.; Alharthy, S.A.; Albogami, B.; El-Megharbel, S.M. Chemical Characterization of Taif Rose (Rosa damascena) Methanolic Extract and Its Physiological Effect on Liver Functions, Blood Indices, Antioxidant Capacity, and Heart Vitality against Cadmium Chloride Toxicity. Antioxidants 2022, 11, 1229. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Alaryani, F.S.; Alotaibi, R.E.; Al-Harthi, M.A.; Alotaibi, G.S.; Al-Subaie, N.A.; Al-Talhi, A.A.; Al-Bogami, B.; Al-Baqami, N.M.; El-Megharbel, S.M.; et al. Efficacy of Prednisolone/Zn Metal Complex and Artemisinin Either Alone or in Combination on Lung Functions after Excessive Exposure to Electronic Cigarettes Aerosol with Assessment of Antibacterial Activity. Crystals 2022, 12, 972. [Google Scholar] [CrossRef]
- Anacona, J.R.; Estacio, J. Synthesis and antibacterial activity of cefixime metal complexes. Transit. Met. Chem. 2006, 31, 227–231. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hamza, R.Z. Synthesis, spectroscopic characterizations, conductometric titration and investigation of potent antioxidant activities of gallic acid complexes with Ca (II), Cu (II), Zn(III), Cr(III) and Se (IV) metal ions. J. Mol. Liq. 2022, 358, 119196. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Yasi, H.M.; Ali, E.F.; Fawzy, M.A.; Abdelkader, T.G.; Galal, T.M. Chemical Characterization of Taif Rose (Rosa damascena Mill var. trigentipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2). Crystals 2022, 12, 460. [Google Scholar]
- Hamza, R.Z.; Al-Eisa, R.A.; El-Shenawy, N.S. Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats. Biology 2022, 11, 450. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Al-Thubaiti, E.H.; Qahl, S.H.; Al-Eisa, R.A.; Hamza, R.Z. Synthesis and Spectroscopic Characterization of Dapagliflozin/Zn (II), Cr (III) and Se (IV) Novel Complexes That Ameliorate Hepatic Damage, Hyperglycemia and Oxidative Injury Induced by Streptozotocin-Induced Diabetic Male Rats and Their Antibacterial Activity. Crystals 2022, 12, 304. [Google Scholar]
- Alhumaidha, K.A.; El-Awdan, S.A.; El-Iraky, W.I.; Ezz-El-Din, S. Protective effects of ursodeoxycholic acid on ceftriaxone-induced hepatic injury in rats. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 45–50. [Google Scholar] [CrossRef][Green Version]
- Andrade, R.; Lopez-Vega, M.; Robles, M.; Cueto, I.; Lucena, M.I. Idiosyncratic drug hepatotoxicity: A 2008 update. Expert Rev. Clin. Pharmacol. 2008, 1, 261–276. [Google Scholar] [CrossRef]
- Al Hagbani, T.; Rizvi, S.M.D.; Hussain, T.; Mehmood, K.; Rafi, Z.; Moin, A.; Abu Lila, A.S.; Alshammari, F.; Khafagy, E.-S.; Rahamathulla, M.; et al. Cefotaxime Mediated Synthesis of Gold Nanoparticles: Characterization and Antibacterial Activity. Polymers 2022, 14, 771. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Supuran, C.T. In-vitro antibacterial and cytotoxic activity of cobalt (ii), copper (ii), nickel (ii) and zinc (ii) complexes of the antibiotic drug cephalothin (keflin). J. Enzym. Inhib. Med. Chem. 2005, 20, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Pervez, H.; Khan, K.M.; Rauf, A.; Maharvi, G.M.; Supuran, C.T. Antifungal cobalt(II), copper(II), nickel(II) and zinc(II) complexes of furanyl-thiophenyl-, pyrrolyl-, salicylyl- and pyridyl-derived cephalexins. J. Enzym. Inhib. Med. Chem. 2004, 19, 85–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iqbal, M.S.; Bukhari, I.H.; Arif, M. Preparation, characterization and biological evaluation of copper(II) and zinc(II) complexes with Schiff bases derived from amoxicillin and cephalexin. Appl. Organomet. Chem. 2005, 19, 864–869. [Google Scholar] [CrossRef]









| Complexes | M. Wt | Elemental Analysis (%) | Λm (Ω−1cm2mol−1) | ||||
|---|---|---|---|---|---|---|---|
| C | H | N | S | M+n | |||
| [Ca(Cefotax) Cl]·H2O | 565.50 | (33.95) 33.88 | (3.53) 3.46 | (12.37) 12.64 | (11.31) 11.74 | (7.08) 6.89 | 18 |
| [Cr(Cefotax) (H2O) (Cl)2]·H2O | 612 | (31.37) 31.84 | (3.26) 3.85 | (11.43) 11.23 | (10.45) 10.92 | (8.49) 8.35 | 23 |
| [Cu(Cefotax) Cl]·3H2O | 607 | (31.63) 31.63 | (3.62) 3.64 | (11.53) 11.83 | (10.54) 10.71 | (10.46) 11.03 | 16 |
| [Zn(Cefotax) Cl]·2H2O | 589 | (32.59) 32.72 | (3.39) 3.89 | (11.88) 11.29 | (10.86) 10.45 | (11.10) 11.51 | 17 |
| [Se(Cefotax) (Cl)3]·2H2O | 675.50 | (28.42) 28.59 | (2.96) 3.31 | (10.36) 10.83 | (9.47) 9.93 | (11.69) 11.32 | 24 |
| Assignments | Complexes | |||||
|---|---|---|---|---|---|---|
| Cefotax | Ca(II) | Cr(III) | Cu(II) | Zn(II) | Se(IV) | |
| ν(O-H), COOH | 3360 | -- | -- | -- | -- | -- |
| ν(O-H), H2O | -- | 3335 | 3360 | 3334 | 3345 | 3365 |
| ν(N-H), NH2 | 3330 | 3310 | 3252 | 3316 | 3208 | 3230 |
| ν(C-H); Aromatic | 3075 | 3064 | 3070 | 3082 | 3071 | 3067 |
| ν(C-H); Aliphatic | 2938 | 2945 | 2941 | 2925 | 2936 | 2929 |
| ν(C=O), lactam | 1775 | 1720 | 1728 | 1715 | 1733 | 1740 |
| ν(C=O)ester + ν(C=O)amide | 1642 | 1650 | 1645 | 1630 | 1640 | 1646 |
| νas (COO−) | 1598 | 1606 | 1607 | 1600 | 1612 | 1604 |
| νs (COO−) | 1380 | 1385 | 1385 | 1384 | 1390 | 1375 |
| ∆ν = νas (COO−)– νs (COO−) | 218 | 221 | 222 | 216 | 222 | 229 |
| ν (M-O) | -- | 640 530 | 618 518 | 624 523 | 618 525 | 614 536 |
| ν (M-N) | -- | 470 | 476 | 495 | 480 | 475 |
| Compound | π → π* | n → π* |
|---|---|---|
| Cefotax | 273,320 | 350 |
| Ca(II) | 285,322 | 391 |
| Cr(III) | 280,327 | 405 |
| Zn(II) | 288,331 | 407 |
| Se(IV) | 286,340 | 410 |
| 1H-NMR Assignments | δ(ppm) | |||
|---|---|---|---|---|
| Cefotaxime | Ca(II) | Zn(II) | Se(VI) | |
| 1H; COOH | 12 | - | - | - |
| 6H; 2CH3 | 1.851 | 1.864 | 1.894 | 1.892 |
| 2H; S-CH2 | 3.127 | 3.129 | 3.131 | 3.165 |
| 2H; O-CH2 | 4.741 | 4.807 | 4.762 | 4.745 |
| 1H; CO-CH | 4.956 | 4.989 | 4.975 | 4.964 |
| 1H; N-CH | 5.770 | 5.617 | 5.575 | 5.589 |
| 2H; NH2 | 7.459 | 7.249 | 7.255 | 7.229 |
| 1H; NH | 9.534 | 9.535 | 9.538 | 9.532 |
| 2H; H2O | - | 3.831 | 3.836 | 3.808 |
| Test Name | ORAC | Metal Chelation | ABTS | FARAB | DPPH | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Name | (µM Trolox eq/mg) | (µM EDTA eq/mg) | (µM Trolox eq/mg) | (µM Trolox eq/mg) | (µM Trolox eq/mg) | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| cefotaxime | 7925.31 e | 363.96 | 2.99 e | 0.59 | 288.52 d | 12.26 | 34.04 e | 3.72 | 19.10 d | 0.29 |
| cefotaxime/Ca | 8504.24 d | 378.36 | 2.98 e | 0.34 | 296.02 c | 4.58 | 24.05 f | 1.01 | 21.02 c | 3.25 |
| cefotaxime/Cr | 8852.83 d | 471.45 | 6.42 d | 0.84 | 320.02 ab | 3.25 | 106.02 d | 9.58 | 14.25 e | 2.40 |
| cefotaxime/Cu | 9172.406 c | 532.59 | 10.41 c | 2.96 | 289.14 d | 4.69 | 110.87 c | 10.69 | 15.05 e | 1.69 |
| cefotaxime/Zn | 15,712.15 a | 354.84 | 15.42 a | 2.52 | 278.02 e | 5.02 | 179.68 a | 4.58 | 27.02 b | 1.58 |
| cefotaxime/Se | 13,228.34 b | 734.90 | 13.24 b | 0.24 | 303.33 b | 2.64 | 116.32 b | 11.16 | 32.82 a | 2.05 |
| HepG-2 | ||||
|---|---|---|---|---|
| Viability % | Cefotaxime/Se (µg/mL) | Cefotaxime/Zn (µg/mL) | ||
| Mean | SD | Mean | SD | |
| 10 μg/mL | 96.52 | 2.05 | 94.70 | 1.42 |
| 100 μg/mL | 81.07 | 1.06 | 83.25 | 0.11 |
| 200 μg/mL | 61.11 | 2.60 | 65.71 | 2.41 |
| 300 μg/mL | 48.52 | 1.80 | 41.72 | 2.01 |
| 500 μg/mL | 28.02 | 2.68 | 30.82 | 1.97 |
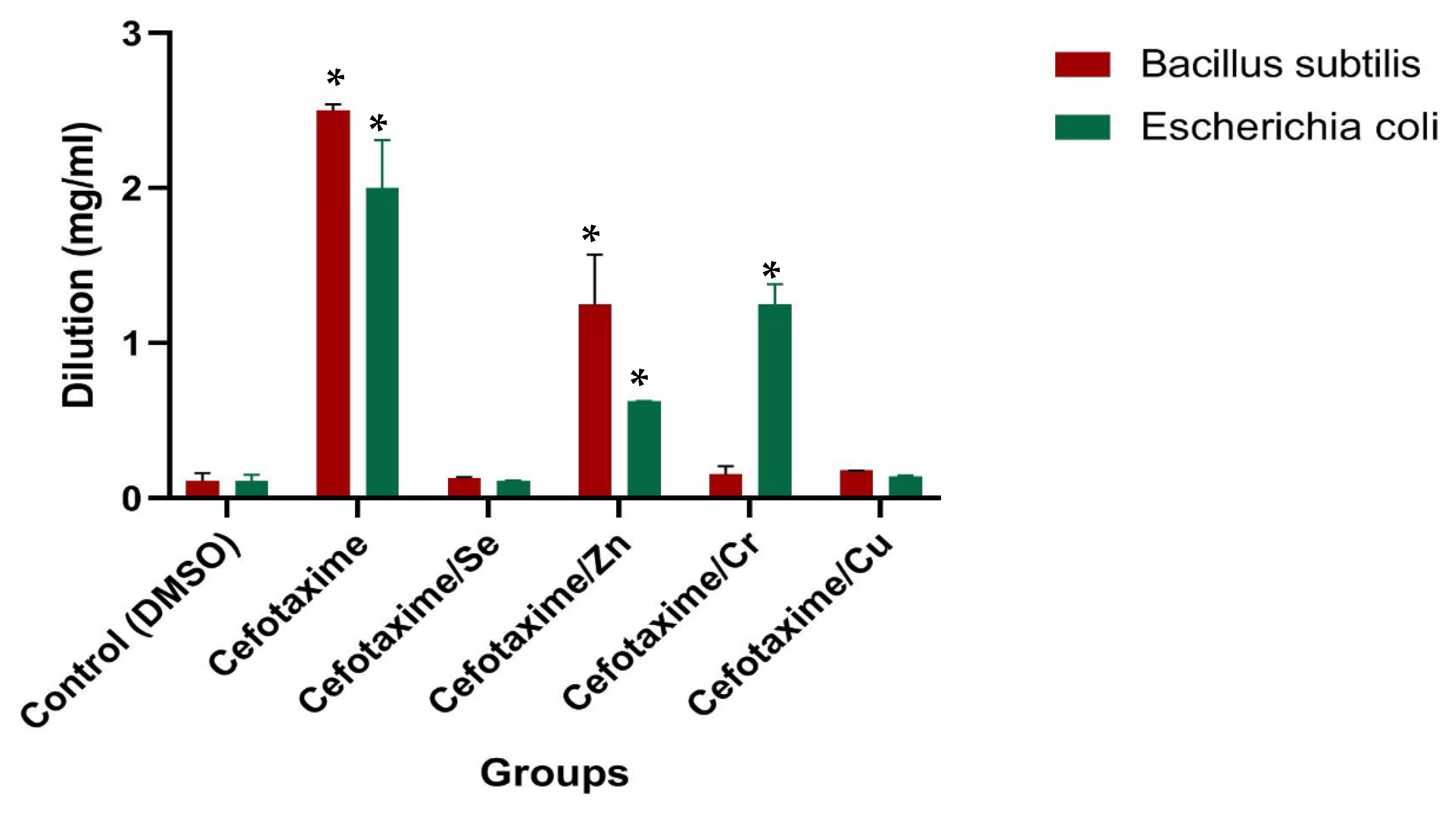
| Sample | Dilution (mg/mL) | |
|---|---|---|
| Bacillus subtilis (G+) | Escherichia coli (G−) | |
| Control (DMSO) | 0.0 ± 0.00 e | 0.0 ± 0.00 f |
| Cefotaxime | 2.5 ± 0.04 a | 2.0 ± 0.31 a |
| Cefotaxime/Se | 0.03 ± 0.007 d | 0.009 ± 0.004 e |
| Cefotaxime/Zn | 1.25 ± 0.32 b | 0.625 ± 0.001 c |
| Cefotaxime/Cr | 0.156 ± 0.05 c | 1.25 ± 0.13 b |
| Cefotaxime/Cu | 0.078 ± 0.001 c | 0.039 ± 0.007 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Thubaiti, E.H.; El-Megharbel, S.M.; Albogami, B.; Hamza, R.Z. Synthesis, Spectroscopic, Chemical Characterizations, Anticancer Capacities against HepG-2, Antibacterial and Antioxidant Activities of Cefotaxime Metal Complexes with Ca(II), Cr(III), Zn(II), Cu(II) and Se(IV). Antibiotics 2022, 11, 967. https://doi.org/10.3390/antibiotics11070967
Al-Thubaiti EH, El-Megharbel SM, Albogami B, Hamza RZ. Synthesis, Spectroscopic, Chemical Characterizations, Anticancer Capacities against HepG-2, Antibacterial and Antioxidant Activities of Cefotaxime Metal Complexes with Ca(II), Cr(III), Zn(II), Cu(II) and Se(IV). Antibiotics. 2022; 11(7):967. https://doi.org/10.3390/antibiotics11070967
Chicago/Turabian StyleAl-Thubaiti, Eman H., Samy M. El-Megharbel, Bander Albogami, and Reham Z. Hamza. 2022. "Synthesis, Spectroscopic, Chemical Characterizations, Anticancer Capacities against HepG-2, Antibacterial and Antioxidant Activities of Cefotaxime Metal Complexes with Ca(II), Cr(III), Zn(II), Cu(II) and Se(IV)" Antibiotics 11, no. 7: 967. https://doi.org/10.3390/antibiotics11070967
APA StyleAl-Thubaiti, E. H., El-Megharbel, S. M., Albogami, B., & Hamza, R. Z. (2022). Synthesis, Spectroscopic, Chemical Characterizations, Anticancer Capacities against HepG-2, Antibacterial and Antioxidant Activities of Cefotaxime Metal Complexes with Ca(II), Cr(III), Zn(II), Cu(II) and Se(IV). Antibiotics, 11(7), 967. https://doi.org/10.3390/antibiotics11070967







