Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils
Abstract
:1. Introduction
2. Results
2.1. Bacteriological Testing of Milk Samples
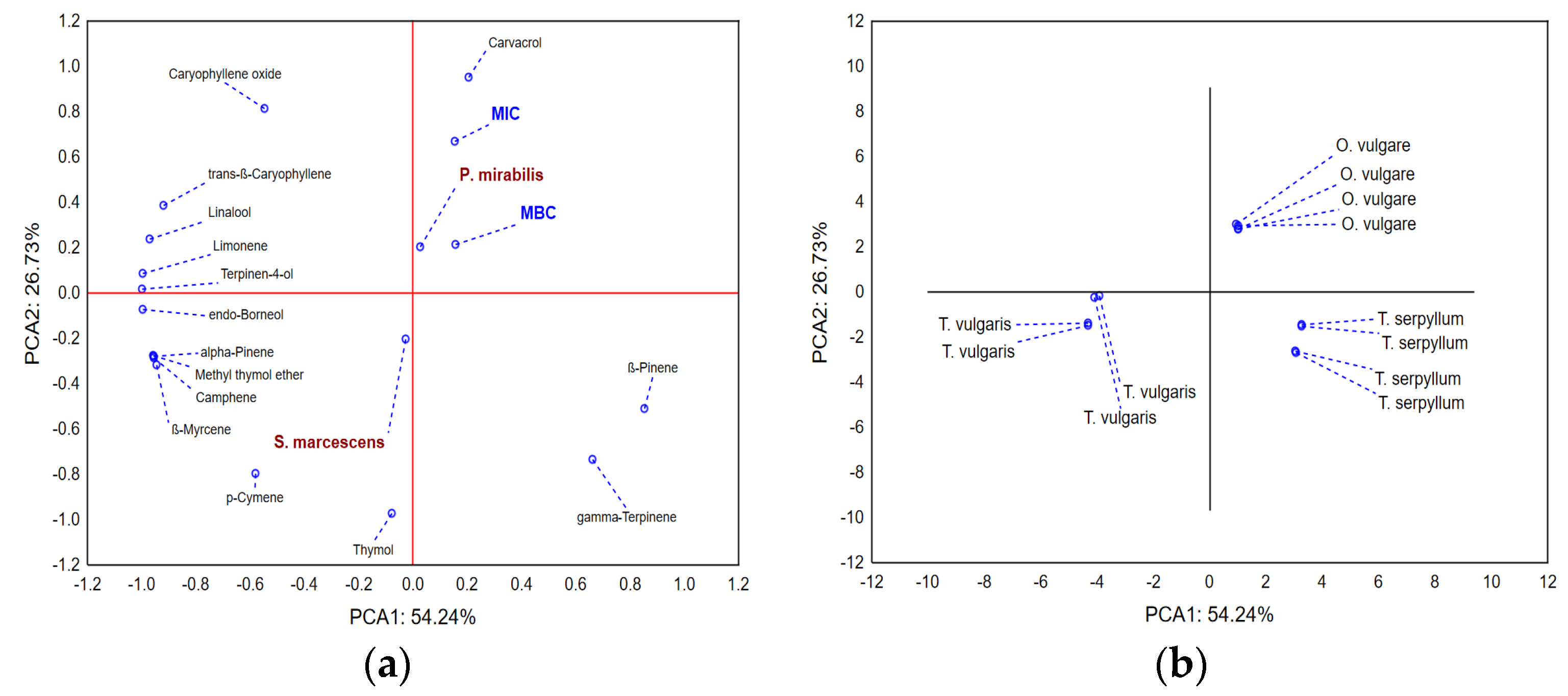
2.2. Chemical Composition of Selected EOs
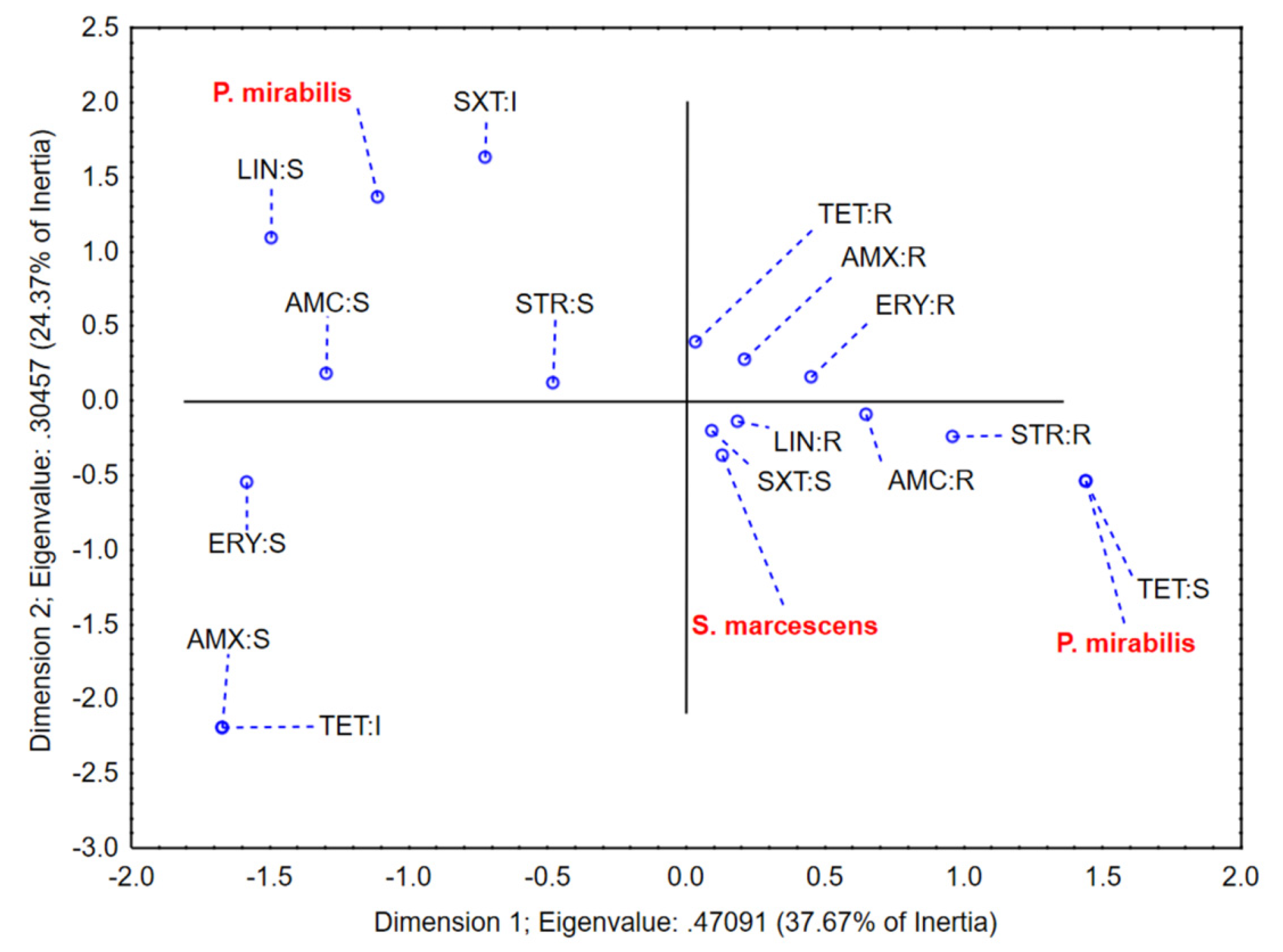
2.3. Antibiotic Susceptibility Testing of Mastitis-Associated Bacteria
2.4. EOs Effectiveness against Mastitis-Associated Bacteria
3. Discussion
4. Materials and Methods
4.1. Sampling Procedure
4.2. Essential Oils
4.3. Essential Oils Chemical Analysis
4.4. Antibiotic Susceptibility Testing of Mastitis-Associated Bacteria
4.5. The Determination of EOs’ Effectiveness against Mastitis-Associated Bacteria
4.6. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogožarski, D.; Dimitrijević, G.; Dobrosavljević, I. Participation of Diagnosed Mastitits in Cows in Milk Hygiene of Branicevo District in 2002. Arch. Vet.-Med. 2012, 4, 65–71. [Google Scholar] [CrossRef]
- Benić, M.; Maćešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Dufour, S.; Labrie, J.; Jacques, M. The Mastitis Pathogens Culture Collection. Microbiol. Resour. Announc. 2019, 8, e00133-19. [Google Scholar] [CrossRef]
- Gomes, F.; Saavedra, M.J.; Henriques, M. Bovine mastitis disease/pathogenicity: Evidence of the potential role of microbial biofilms. Pathog. Dis. 2016, 74, ftw006. [Google Scholar] [CrossRef] [PubMed]
- Bogni, C.; Odierno, L.; Raspanti, C.; Giraudo, J.; Larriestra, A.; Reinoso, E.; Lasagno, M.; Ferrari, M.; Ducrós, E.; Frigerio, C. War against mastitis: Current concepts on controlling bovine mastitis pathogens. In Science against Microbial Pathogens: Communicafing Current Research and Technological Advances; Mendez-Vilas, A., Ed.; World Scientific: Singapore, 2011; pp. 483–494. [Google Scholar]
- Manrique, L.E.T.; Villate-Hernández, J.R.; Andrade-Becerra, R.J. Bacterial and fungal infectious etiology causing mastitis in dairy cows in the highlands of Boyacá (Colombia). Rev. Fac. Med. Vet.-Zootec. 2019, 66, 208–218. [Google Scholar] [CrossRef]
- Cervinkova, D.; Vlkova, H.; Borodacova, I.; Makovcova, J.; Babak, V.; Lorencova, A.; Vrtkova, I.; Marosevic, D.; Jaglic, Z. Prevalence of mastitis pathogens in milk from clinically healthy cows. Vet. Med. 2013, 58, 567–575. [Google Scholar] [CrossRef]
- Milanov, D.; Prunić, B.; Košarčić, S.; Potkonjak, A. Less Common Aetiological Agent of Bovine Mastitis: -Serratia marcescens-. Arch. Vet. Med. 2012, 5, 3–17. [Google Scholar] [CrossRef]
- Bannerman, D.D.; Paape, M.J.; Goff, J.P.; Kimura, K.; Lippolis, J.D.; Hope, J.C. Innate immune response to intramammary infection with Serratia marcescens and Streptococcus uberis. Vet.-Res. 2004, 35, 681–700. [Google Scholar] [CrossRef]
- Friman, M.J.; Eklund, M.H.; Pitkälä, A.H.; Rajala-Schultz, P.J.; Rantala, M.H.J. Description of two Serratia marcescens associated mastitis outbreaks in Finnish dairy farms and a review of literature. Acta Vet. Scand. 2019, 61, 54. [Google Scholar] [CrossRef]
- Pinzón-Sánchez, C.; Ruegg, P. Risk factors associated with short-term post-treatment outcomes of clinical mastitis. J. Dairy Sci. 2011, 94, 3397–3410. [Google Scholar] [CrossRef]
- Drzewiecka, D. Significance and Roles of Proteus spp. Bacteria in Natural Environments. Microb. Ecol. 2016, 72, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Algammal, A.M.; Hashem, H.R.; Alfifi, K.J.; Hetta, H.F.; Sheraba, N.S.; Ramadan, H.; El-Tarabili, R.M. atpD gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci. Rep. 2021, 11, 9476. [Google Scholar] [CrossRef]
- Djebala, S.; Evrard, J.; Gregoire, F.; Bayrou, C.; Gille, L.; Eppe, J.; Casalta, H.; Frisée, V.; Moula, N.; Sartelet, A.; et al. Antimicrobial Susceptibility Profile of Several Bacteria Species Identified in the Peritoneal Exudate of Cows Affected by Parietal Fibrinous Peritonitis after Caesarean Section. Vet.-Sci. 2021, 8, 295. [Google Scholar] [CrossRef] [PubMed]
- Zappa, V.; Bolaños, C.A.D.; De Paula, C.L.; Callefe, J.L.R.; Alves, A.C.; De Morais, A.B.C.; Guerra, S.T.; Cabrini, M.C.; Melville, P.A.; Ribeiro, M.G. Antimicrobial multiple resistance index, minimum inhibitory concentrations, and extended-spectrum beta-lactamase producers of Proteus mirabilis and Proteus vulgaris strains isolated from domestic animals with various clinical manifestations of infection. Semin. Ciências Agrárias 2017, 38, 775. [Google Scholar] [CrossRef]
- Hogan, J.; Smith, K.L. Coliform mastitis. Vet. Res. 2003, 34, 507–519. [Google Scholar] [CrossRef]
- Duse, A.; Persson-Waller, K.; Pedersen, K. Microbial Aetiology, Antibiotic Susceptibility and Pathogen-Specific Risk Factors for Udder Pathogens from Clinical Mastitis in Dairy Cows. Animals 2021, 11, 2113. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumar, S.; Khurana, R. Essential oils and mastitis in dairy animals: A review. Haryana Vet. 2020, 59, 1–9. [Google Scholar]
- Queiroga, M.C.; Coelho, M.P.; Arantes, S.M.; Potes, M.E.; Martins, M.R. Antimicrobial Activity of Essential Oils of Lamiaceae Aromatic Spices Towards Sheep mastitis-Causing Staphylococcus aureus and Staphylococcus epidermidis. J. Essent. Oil Bear. Plants 2018, 21, 1155–1165. [Google Scholar] [CrossRef]
- Szweda, P.; Zalewska, M.; Pilch, J.; Kot, B.; Milewski, S. Essential oils as potential anti-staphylococcal agents. Acta Vet.-Beogr. 2018, 68, 95–107. [Google Scholar]
- Kovačević, Z.; Radinović, M.; Čabarkapa, I.; Kladar, N.; Božin, B. Natural Agents against Bovine Mastitis Pathogens. Antibiotics 2021, 10, 205. [Google Scholar] [CrossRef]
- Kovačević, Z.; Kladar, N.; Čabarkapa, I.; Radinović, M.; Maletić, M.; Erdeljan, M.; Božin, B. New Perspective of Origanum vulgare L. and Satureja montana L. Essential Oils as Bovine Mastitis Treatment Alternatives. Antibiotics 2021, 10, 1460. [Google Scholar] [CrossRef] [PubMed]
- Barreiros, Y.; de Meneses, A.C.; Alves, J.L.F.; Mumbach, G.D.; Ferreira, F.A.; Machado, R.A.F.; Bolzan, A.; de Araujo, P.H.H. Xanthan gum-based film-forming suspension containing essential oils: Production and in vitro antimicrobial activity evaluation against mastitis-causing microorganisms. LWT 2022, 153, 112470. [Google Scholar] [CrossRef]
- Tomanić, D.; Božin, B.; Čabarkapa, I.; Kladar, N.; Radinović, M.; Maletić, M.; Kovačević, Z. Chemical Composition, Antioxidant and Antibacterial Activity of Two Different Essential Oils Against Mastitis Associated Pathogens. Acta Vet. 2022, 72, 45–58. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157:H7, Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Velebit, B.; Matekalo-Sverak, V.; Petrović, Z.; Lakićević, B.; Janković, V.; Lilić, S.; Vranić, D. Ispitivanje antimikrobne aktivnosti cinamaldehida i karvakrola na mikroorganizme prenosive hranom. Meat Technol. 2012, 53, 166–172. [Google Scholar]
- Bradley, A.J.; Green, M.J. Adaptation of Escherichia coli to the Bovine Mammary Gland. J. Clin. Microbiol. 2001, 39, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wang, Y.J.; Qin, Y.; Vallverdú, R.G.; García, J.M.; Sun, W.; Li, S.; Cao, Z. Prevalence of Bovine Mastitis Pathogens in Bulk Tank Milk in China. PLoS ONE 2016, 11, e0155621. [Google Scholar] [CrossRef]
- Abed, A.; Menshawy, A.; Zeinhom, M.; Hossain, D.; Khalifa, E.; Wareth, G.; Awad, M. Subclinical Mastitis in Selected Bovine Dairy Herds in North Upper Egypt: Assessment of Prevalence, Causative Bacterial Pathogens, Antimicrobial Resistance and Virulence-Associated Genes. Microorganisms 2021, 9, 1175. [Google Scholar] [CrossRef]
- Di Guardo, G.; Battisti, A.; Agrimi, U.; Forletta, R.; Reitano, M.E.; Calderini, P. Pathology of Serratia marcescens Mastitis in Cattle. J. Vet.-Med. Ser. B 1997, 44, 537–546. [Google Scholar] [CrossRef]
- Abdullah, A.H.; Nadhom, B.N.; Al-Ammiri, H.H. Isolation and Identification of Serratia marcescens from Bovine Mastitis infections in Iraq and their Susceptibility to Antibiotics. J. Entomol. Zool. Stud. 2017, 5, 489–492. [Google Scholar]
- Lopes, T.S.; Fussieger, C.; Rizzo, F.A.; Silveira, S.; Lunge, V.R.; Streck, A.F. Species identification and antimicrobial susceptibility profile of bacteria associated with cow mastitis in southern Brazil. Pesqui. Vet. Bras. 2022, 42, e06958. [Google Scholar] [CrossRef]
- Ohnishi, M.; Sawada, T.; Hirose, K.; Sato, R.; Hayashimoto, M.; Hata, E.; Yonezawa, C.; Kato, H. Antimicrobial susceptibilities and bacteriological characteristics of bovine Pseudomonas aeruginosa and Serratia marcescens isolates from Mastitis. Vet.-Microbiol. 2011, 154, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Parmar, B.; Pal, M.; Dhami, A.; Patel, J. Investigation on bovine mastitis caused by Staphylococcus Aureus. Int. J. Cow Sci. 2006, 2, 52–53. [Google Scholar]
- Kasa, G.; Tegegne, B.; Tadesse, B. Isolation and Identification of Major Pathogenic Bacteria from Clinical Mastitic Cows in Asella Town, Ethiopia. Vet.-Med. Int. 2020, 2020, 6656755. [Google Scholar] [CrossRef]
- Sumathi, B.; Veeregowda, B.; Amitha, R.G. Prevalence and antibiogram profile of bacterial isolates from clinical bovine mastitis. Vet. World 2008, 1, 237–238. [Google Scholar]
- Verma, H.; Rawat, S.; Sharma, N.; Jaiswal, V.; Singh, R.; Harshit, V. Prevalence, bacterial etiology and antibiotic susceptibility pattern of bovine mastitis in Meerut. J. Entomol. Zool. Stud. 2018, 6, 706–709. [Google Scholar]
- Ajose, D.J.; Oluwarinde, B.O.; Abolarinwa, T.O.; Fri, J.; Montso, K.P.; Fayemi, O.E.; Aremu, A.O.; Ateba, C.N. Combating Bovine Mastitis in the Dairy Sector in an Era of Antimicrobial Resistance: Ethno-veterinary Medicinal Option as a Viable Alternative Approach. Front. Vet.-Sci. 2022, 9, 800322. [Google Scholar] [CrossRef]
- Pascu, C.; Herman, V.; Iancu, I.; Costinar, L. Etiology of Mastitis and Antimicrobial Resistance in Dairy Cattle Farms in the Western Part of Romania. Antibiotics 2022, 11, 57. [Google Scholar] [CrossRef]
- Zargoosh, Z.; Ghavam, M.; Bacchetta, G.; Tavili, A. Effects of ecological factors on the antioxidant potential and total phenol content of Scrophularia striata Boiss. Sci. Rep. 2019, 9, 16021. [Google Scholar] [CrossRef]
- Türkmen, M.; Kara, M.; Maral, H.; Soylu, S. Determination of chemical component of essential oil of Origanum dubium plants grown at different altitudes and antifungal activity against Sclerotinia sclerotiorum. J. Food Process. Preserv. 2021, 46, e15787. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the Volatile Composition of Essential Oils of Some Lamiaceae Spices and the Antimicrobial and Antioxidant Activities of the Entire Oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef] [PubMed]
- Kosakowska, O.; Węglarz, Z.; Bączek, K. Yield and quality of ‘Greek oregano’ (Origanum vulgare L. subsp hirtum) herb from organic production system in temperate climate. Ind. Crop. Prod. 2019, 141, 111782. [Google Scholar] [CrossRef]
- EDQM. European Pharmacopoeia 10.3; The European Directorate for the Quality of Medicines & HealthCare, Council of Europe: Brussels, Belgium, 2020; pp. 1648–1650. [Google Scholar]
- Pyörälä, S. Treatment of mastitis during lactation. Ir. Vet. J. 2009, 62, S40–S44. [Google Scholar] [CrossRef]
- Vakanjac, S.; Pavlović, V.; Magaš, V.; Pavlović, M.; Đurić, M.; Maletić, M.; Nedić, S.; Sočo, I. Investigations of efficacy of intramammary applied antimicrobials and glucocorticosteroides in the treatment of subclinical and clinical mastitis in cows. Vet. Glas. 2013, 67, 15–27. [Google Scholar] [CrossRef]
- Lavor, U.L.; Guimarães, F.F.; Salina, A.; Mioni, M.S.; Langoni, H. Bacterial identification, somatic cell count, antimicrobial profile and toxigenic Staphylococcus strains search from mastitic cow milk samples on small farms properties. Pesqui. Veterinária Bras. 2019, 39, 715–722. [Google Scholar] [CrossRef]
- Vidović, J.; Stojanović, D.; Cagnardi, P.; Kladar, N.; Horvat, O.; Ćirković, I.; Bijelić, K.; Stojanac, N.; Kovačević, Z. Farm Animal Veterinarians’ Knowledge and Attitudes toward Antimicrobial Resistance and Antimicrobial Use in the Republic of Serbia. Antibiotics 2022, 11, 64. [Google Scholar] [CrossRef]
- Burović, J. Izolacija bakterijskih patogena kod klinički manifestnih mastitisa mliječnih goveda i njihova antimikrobna osjetljivost u zeničkoj regiji u 2017. godini. Vet. Stanica 2020, 51, 47–52. [Google Scholar] [CrossRef]
- Sun, Y.; Wen, S.; Zhao, L.; Xia, Q.; Pan, Y.; Liu, H.; Wei, C.; Chen, H.; Ge, J.; Wang, H. Association among biofilm formation, virulence gene expression, and antibiotic resistance in Proteus mirabilis isolates from diarrhetic animals in Northeast China. BMC Vet.-Res. 2020, 16, 176. [Google Scholar] [CrossRef]
- Kayitsinga, J.; Schewe, R.; Contreras, G.; Erskine, R. Antimicrobial treatment of clinical mastitis in the eastern United States: The influence of dairy farmers’ mastitis management and treatment behavior and attitudes. J. Dairy Sci. 2017, 100, 1388–1407. [Google Scholar] [CrossRef]
- Lopes, T.S.; Fontoura, P.S.; Oliveira, A.; Rizzo, F.A.; Silveira, S.; Streck, A.F. Use of plant extracts and essential oils in the control of bovine mastitis. Res. Vet.-Sci. 2020, 131, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Cerioli, M.F.; Moliva, M.V.; Cariddi, L.N.; Reinoso, E.B. Effect of the Essential Oil of Minthostachys verticillata (Griseb.) Epling and Limonene on Biofilm Production in Pathogens Causing Bovine Mastitis. Front. Vet.-Sci. 2018, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Tanhaeian, A.; Sekhavati, M.H.; Moghaddam, M. Antimicrobial activity of some plant essential oils and an antimicrobial-peptide against some clinically isolated pathogens. Chem. Biol. Technol. Agric. 2020, 7, 13. [Google Scholar] [CrossRef]
- Fratini, F.; Casella, S.; Leonardi, M.; Pisseri, F.; Ebani, V.V.; Pistelli, L.; Pistelli, L. Antibacterial activity of essential oils, their blends and mixtures of their main constituents against some strains supporting livestock mastitis. Fitoterapia 2014, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.; Skandamis, P.; Coote, P.; Nychas, G.-J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Protocol; American Society for Microbiology (ASM): Washington, DC, USA, 2009. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals, 3rd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- M02-A12; Performance Standards for Antimicrobial Disk Susceptibility Tests. CLSI: Wayne, PA, USA, 2012.
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]


| Peack No. | Compound | RI * | O. vulgare | T. serpyllum | T. vulgaris |
|---|---|---|---|---|---|
| Monoterpene Hydrocarbons | 4.04 | 25.78 | 11.26 | ||
| 1 | α-Pinene | 937 | 0.18 | 0.19 | 1.47 |
| 2 | Camphene | 952 | 0.14 | 0.16 | 1.83 |
| 3 | β-Pinene | 978 | 0.67 | 2.37 | 0.17 |
| 4 | β-Myrcene | 991 | 0.24 | 0.32 | 1.73 |
| 6 | α-Phellandrene | 1005 | 0.08 | 0.11 | 0.15 |
| 7 | α-Terpinene | 1017 | 0.45 | 0.15 | 0.63 |
| 9 | Limonene | 1030 | 0.79 | 0.17 | 1.82 |
| 11 | γ-Terpinene | 1060 | 1.49 | 22.31 | 3.46 |
| Aromatic Monoterpene Hydrocarbons | 4.52 | 16.66 | 23.83 | ||
| 8 | p-Cymene | 1025 | 4.52 | 16.66 | 23.83 |
| Oxygenated Monoterpenes | 2.49 | 1.57 | 6.49 | ||
| 10 | 1,8-Cineole | 1032 | 0.37 | 0.17 | 0.84 |
| 12 | Linalool | 1099 | 1.08 | - | 2.14 |
| 13 | Camphor | 1145 | 0.07 | 0.57 | 0.27 |
| 14 | endo-Borneol | 1167 | 0.39 | - | 1.73 |
| 15 | Terpinen-4-ol | 1177 | 0.47 | 0.11 | 1.28 |
| 16 | Isomenthol | 1183 | - | 0.63 | - |
| 14 | α-Terpineol | 1189 | 0.11 | 0.01 | 0.19 |
| 20 | Carvone | 1242 | - | - | - |
| 23 | Geranyl acetate | 1382 | - | - | - |
| 24 | Bornyl acetate | 1285 | - | 0.08 | 0.04 |
| Aromatic Oxygenated Monoterpenes | 83.81 | 55.78 | 51.7 | ||
| 18 | Isothymol methyl ether | 1230 | - | - | 0.83 |
| 19 | Methyl thymol ether | 1235 | - | - | 1.25 |
| 21 | Thymol | 1291 | 4.87 | 55.11 | 46.37 |
| 22 | Carvacrol | 1299 | 78.94 | 0.67 | 3.25 |
| Sesquiterpene Hydrocarbons | 2.88 | 0.14 | 4.94 | ||
| 25 | α-Cubebene | 1351 | 0.01 | - | 0.09 |
| 26 | β-Cubenene | 1388 | - | - | 0.01 |
| 27 | trans-β-Caryophyllene | 1419 | 2.49 | 0.09 | 3.86 |
| 28 | Aromandendrene | 1440 | - | - | - |
| 29 | cis-β-Famesene | 1443 | - | - | - |
| 30 | Humulene | 1454 | 0.11 | 0.05 | 0.57 |
| 31 | allo-Aromandendrene | 1461 | - | - | - |
| 32 | γ-Muurolene | 1477 | - | - | - |
| 33 | β-Selinene | 1486 | - | - | - |
| 34 | β-Bisabolene | 1509 | - | - | - |
| 35 | γ-Cadinene | 1513 | - | - | - |
| 36 | δ-Cadinene | 1524 | 0.27 | - | 0.41 |
| Oxygenated Sesquiterpenes | 1.37 | 0 | 1.05 | ||
| 37 | Caryophyllene oxide | 1581 | 1.37 | - | 1.05 |
| Aliphatic Compunds | 0.02 | 0 | 0 | ||
| 5 | 3-Octanol | 994 | 0.02 | - | - |
| Total of Identified Compounds | 99.13 | 99.93 | 99.27 | ||
| Bacterial Strain | AMX | AMP | CRO | ENR | ERY | GEN | LIN | NEO | PEN | STR | TET | AMC | NB | SXT | CLO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. mirabilis_1 | R | R | S | S | S | S | S | S | R | S | R | S | R | S | R |
| P. mirabilis_2 | R | R | S | S | R | S | R | S | R | S | R | S | R | I | R |
| P. mirabilis_3 | R | R | S | S | R | S | R | S | R | R | S | R | R | S | R |
| S. marcescens_1 | S | R | S | S | S | S | R | S | R | S | I | S | R | S | R |
| S. marcescens_2 | R | R | S | S | R | S | R | S | R | R | R | R | R | S | R |
| S. marcescens_3 | R | R | S | S | R | S | R | S | R | S | R | R | R | S | R |
| S. marcescens_4 | R | R | S | S | R | S | R | S | R | S | R | R | R | S | R |
| S. marcescens_5 | R | R | S | S | R | S | R | S | R | R | R | R | R | S | R |
| S. marcescens_6 | R | R | S | S | R | S | R | S | R | S | R | R | R | S | R |
| Sample(mg/mL) | TS **Average ± SD | TV ***Average ± SD | OV ****Average ± SD | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MBC | |
| P. mirabilis | 3.125 ± 1.35 | 6.25 ± 2.7 | 3.125 ± 0.00 | 6.25 ± 2.7 | 3.125 ± 1.35 | 3.125 ± 1.35 |
| S. marcescens | 1.56 ± 0.96 * | 3.125 ± 1.91 * | 1.56 ± 0.96 * | 3.125 ± 1.91 * | 3.125 ± 1.91 | 6.25 ± 3.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomanić, D.; Božin, B.; Kladar, N.; Stanojević, J.; Čabarkapa, I.; Stilinović, N.; Apić, J.; Božić, D.D.; Kovačević, Z. Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils. Antibiotics 2022, 11, 1077. https://doi.org/10.3390/antibiotics11081077
Tomanić D, Božin B, Kladar N, Stanojević J, Čabarkapa I, Stilinović N, Apić J, Božić DD, Kovačević Z. Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils. Antibiotics. 2022; 11(8):1077. https://doi.org/10.3390/antibiotics11081077
Chicago/Turabian StyleTomanić, Dragana, Biljana Božin, Nebojša Kladar, Jovan Stanojević, Ivana Čabarkapa, Nebojša Stilinović, Jelena Apić, Dragana D. Božić, and Zorana Kovačević. 2022. "Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils" Antibiotics 11, no. 8: 1077. https://doi.org/10.3390/antibiotics11081077
APA StyleTomanić, D., Božin, B., Kladar, N., Stanojević, J., Čabarkapa, I., Stilinović, N., Apić, J., Božić, D. D., & Kovačević, Z. (2022). Environmental Bovine Mastitis Pathogens: Prevalence, Antimicrobial Susceptibility, and Sensitivity to Thymus vulgaris L., Thymus serpyllum L., and Origanum vulgare L. Essential Oils. Antibiotics, 11(8), 1077. https://doi.org/10.3390/antibiotics11081077








