Should We Consider Them as a Threat? Antimicrobial Resistance, Virulence Potential and Genetic Diversity of Campylobacter spp. Isolated from Varsovian Dogs
Abstract
1. Introduction
2. Results
2.1. Occurrence of Campylobacter spp.
2.2. Detection of Virulence Factor Genes
2.3. Phenotypes and Genotypes of the Antimicrobial Resistance
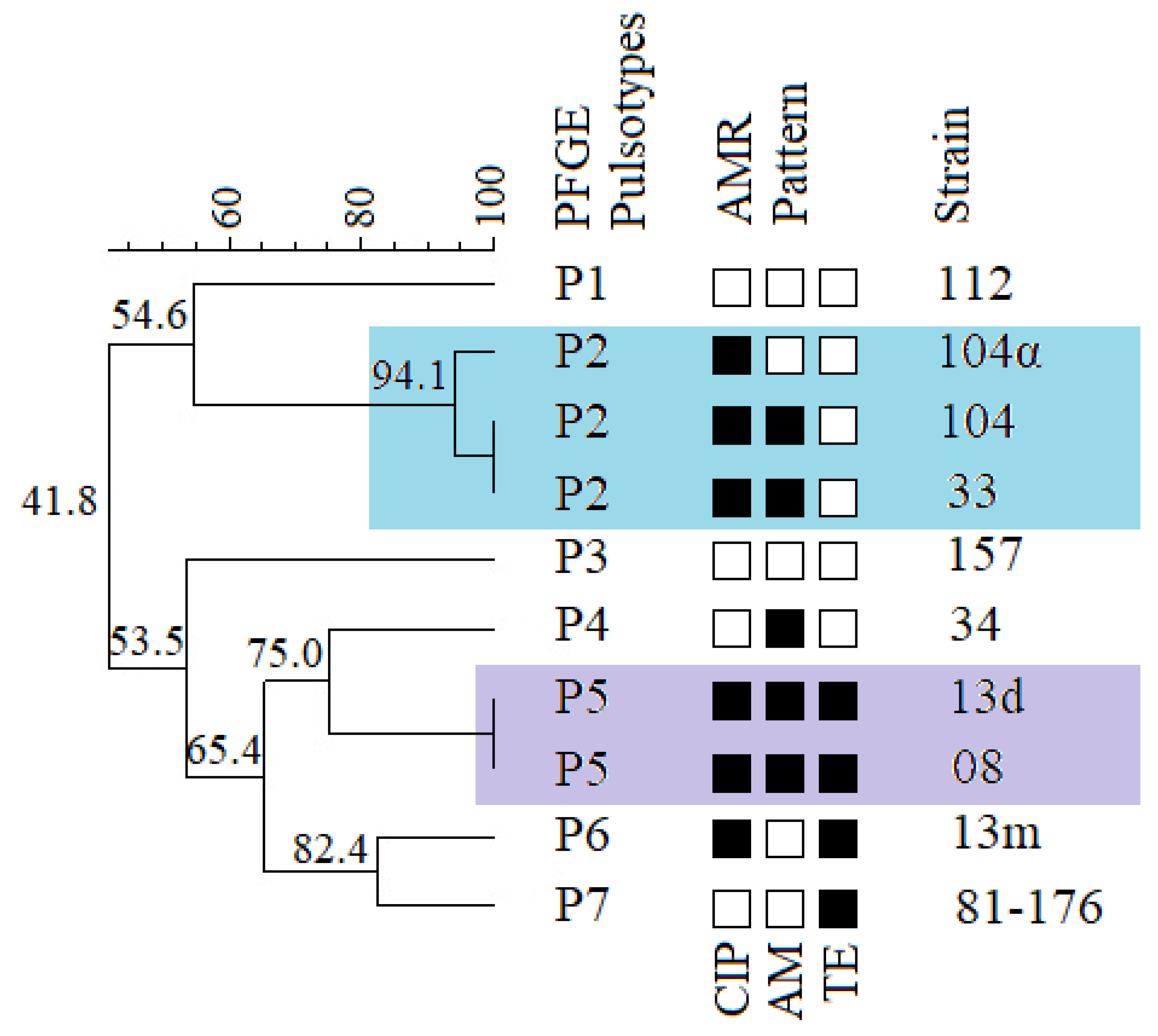
2.4. Genetic Diversity of Campylobacter jejuni
3. Discussion
4. Materials and Methods
4.1. Sampling and Shipment
4.2. Isolation and Identification of Campylobacter spp.
4.3. Virulence Factor Genes Occurrence
4.4. Antimicrobial Susceptibility Testing
4.5. Investigation of Antimicrobial Resistance Mechanisms
4.6. Pulsed-Field Gel Electrophoresis (PFGE)
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar] [CrossRef]
- Kaakoush, N.O.; Castaño-Rodríguez, N.; Mitchell, H.M.; Man, S.M. Global Epidemiology of Campylobacter Infection. Clin. Microbiol. Rev. 2015, 28, 687–720. [Google Scholar] [CrossRef]
- Costa, D.; Iraola, G. Pathogenomics of emerging Campylobacter species. Clin. Microbiol. Rev. 2019, 32, e00072-18. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M.; Rosendal, T.; Engvall, E.O.; Ohlson, A.; Lindberg, A. Prevalence of thermophilic Campylobacter species in Swedish dogs and characterization of C. jejuni isolates. Acta Vet. Scand 2015, 57, 19. [Google Scholar] [CrossRef] [PubMed]
- Acke, E. Campylobacteriosis in dogs and cats: A review. New Zeal Vet. J. 2018, 66, 221–228. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Szczepańska, B.; Klawe, J.J.; Spica, D.; Chudzińska, M. Prevalence of Campylobacter jejuni and Campylobacter coli species in cats and dogs from Bydgoszcz (Poland) region. Pol. J. Vet. Sci. 2013, 16, 115–120. [Google Scholar] [CrossRef]
- Thépault, A.; Rose, V.; Queguiner, M.; Chemaly, M.; Rivoal, K. Dogs and Cats: Reservoirs for Highly Diverse Campylobacter jejuni and a Potential Source of Human Exposure. Animals 2020, 10, 838. [Google Scholar] [CrossRef]
- Mughini Gras, L.; Smid, J.H.; Wagenaar, J.A.; Koene, M.G.; Havelaar, A.H.; Friesema, I.H.; French, N.P.; Flemming, C.; Galson, J.D.; Graziani, C.; et al. Increased risk for Campylobacter jejuni and C. coli infection of pet origin in dog owners and evidence for genetic association between strains causing infection in humans and their pets. Epidemiology Infect. 2013, 141, 2526–2535. [Google Scholar] [CrossRef]
- Chaban, B.; Ngeleka, M.; Hill, J.E. Detection and quantification of 14 Campylobacter species in pet dogs reveals an increase in species richness in feces of diarrheic animals. BMC Microbiol. 2010, 10, 73. [Google Scholar] [CrossRef]
- Acke, E.; Whyte, P.; Jones, B.R.; McGill, K.; Collins, J.D.; Fanning, S. Prevalence of termophilic Campylobacter species in cats and dogs in two animal shelters in Ireland. Vet. Rec. 2006, 158, 51–54. [Google Scholar] [CrossRef]
- Leahy, A.M.; Cummings, K.J.; Rodriguez-Rivera, L.D.; Hamer, S.A.; Lawhon, S.D. Faecal Campylobacter shedding among dogs in animal shelters across Texas. Zoonoses Public Hlth. 2017, 64, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Karama, M.; Cenci-Goga, B.T.; Prosperi, A.; Etter, E.; El-Ashram, S.; McCrindle, C.; Ombui, J.N.; Kalake, A. Prevalence and risk factors associated with Campylobacter spp. occurrence in healthy dogs visiting four rural community veterinary clinics in South Africa. Onderstepoort J. Vet. Res. 2019, 86, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Torkan, S.; Vazirian, B.; Khamesipour, F.; Dida, G.O. Prevalence of thermotolerant Campylobacter species in dogs and cats in Iran. Vet. Med. Sci. 2018, 4, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Olkkola, S.; Kovanen, S.; Roine, J.; Hänninen, M.L.; Hielm-Björkman, A.; Kivistö, R. Population Genetics and Antimicrobial Susceptibility of Canine Campylobacter Isolates Collected before and after a Raw Feeding Experiment. PLoS ONE 2015, 10, e0132660. [Google Scholar] [CrossRef]
- LaLonde-Paul, D.; Cummings, K.J.; Rodriguez-Rivera, L.D.; Wu, J.; Lawhon, S.D. Ciprofloxacin resistance among Campylobacter jejuni isolates obtained from shelter dogs in Texas. Zoonoses Public Hlth. 2019, 66, 337–342. [Google Scholar] [CrossRef]
- Pölzler, T.; Stüger, H.P.; Lassnig, H. Prevalence of most common human pathogenic Campylobacter spp. in dogs and cats in Styria, Austria. Vet. Med. Sci. 2018, 4, 115–125. [Google Scholar] [CrossRef]
- Santaniello, A.; Varriale, L.; Dipineto, L.; Borrelli, L.; Pace, A.; Fioretti, A.; Menna, L.F. Presence of Campylobacter jejuni and C. coli in Dogs under Training for Animal-Assisted Therapies. Int. J. Environ. Res. Public Health 2021, 18, 3717. [Google Scholar] [CrossRef]
- Runesvärd, E.; Wikström, C.; Fernström, L.L.; Hansson, I. Presence of pathogenic bacteria in faeces from dogs fed raw meat-based diets or dry kibble. Vet. Rec. 2020, 5, e002231. [Google Scholar] [CrossRef]
- Bojanić, K.; Midwinter, A.C.; Marshall, J.C.; Rogers, L.E.; Biggs, P.J.; Acke, E. Isolation of Campylobacter spp. from Client-Owned Dogs and Cats, and Retail Raw Meat Pet Food in the Manawatu, New Zealand. Zoonoses Public Hlth. 2017, 64, 438–449. [Google Scholar] [CrossRef]
- Marks, S.L.; Rankin, S.C.; Byrne, B.A.; Weese, J.S. Enteropathogenic bacteria in dogs and cats: Diagnosis, epidemiology, treatment, and control. J. Vet. Intern. Med. 2011, 25, 1195–1208. [Google Scholar] [CrossRef]
- Queen, E.V.; Marks, S.L.; Farver, T.B. Prevalence of selected bacterial and parasitic agents in feces from diarrheic and healthy control cats from northern California. J. Vet. Intern. Med. 2012, 26, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.G.; Melo, R.T.; Fonseca, B.B.; Martins, P.A.; Ferreira, F.A.; Araujo, M.B.J.; Rossi, D.A. Occurrence and characterization of Campylobacter spp. isolates in dogs, cats and children. Pesquisa Vet. Brasil 2015, 35, 365–370. [Google Scholar] [CrossRef]
- Szczepanska, B.; Andrzejewska, M.; Spica, D.; Klawe, J.J. Prevalence and antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from children and environmental sources in urban and suburban areas. BMC Microbiol. 2017, 17, 80. [Google Scholar] [CrossRef] [PubMed]
- Selwet, M.; Cłapa, T.; Galbas, M.; Słomski, R.; Porzucek, F. The prevalence of Campylobacter spp. and occurrence of virulence genes isolated from dogs. Pol. J. Microbiol. 2015, 64, 73–76. [Google Scholar] [CrossRef]
- Igwaran, A.; Okoh, A.I. Human campylobacteriosis: A public health concern of global importance. Heliyon 2019, 5, e02814. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Klawe, J.J.; Szczepańska, B.; Śpica, D. Occurence of virulence genes among Campylobacter jejuni and Campylobacter coli isolates from domestic animals and children. Pol. J. Vet. Sci. 2011, 14, 207–211. [Google Scholar] [CrossRef][Green Version]
- Bang, D.D.; Borck, B.; Nielsen, E.M.; Scheutz, F.; Pedersen, K.; Madsen, M. Detection of seven virulence and toxin genes of Campylobacter jejuni isolates from Danish turkeys by PCR and cytolethal distending toxin production of the isolates. J. Food Protect. 2004, 67, 2171–2177. [Google Scholar] [CrossRef]
- Selwet, M. The Prevalence of Virulence Genes and Multidrug Resistance in Thermophilic Campylobacter Spp. Isolated from Dogs. Open Life Sci. 2019, 14, 681–687. [Google Scholar] [CrossRef]
- Andrzejewska, M.; Szczepańska, B.; Śpica, D.; Klawe, J.J. Prevalence, Virulence, and Antimicrobial Resistance of Campylobacter spp. in Raw Milk, Beef, and Pork Meat in Northern Poland. Foods 2019, 8, 420. [Google Scholar] [CrossRef]
- Wysok, B.; Wojtacka, J.; Hänninen, M.L.; Kivistö, R. Antimicrobial Resistance and Virulence-Associated Markers in Campylobacter Strains from Diarrheic and Non-diarrheic Humans in Poland. Front. Microbiol. 2020, 11, 1799. [Google Scholar] [CrossRef]
- Kreling, V.; Falcone, F.H.; Kehrenberg, C.; Hensel, A. Campylobacter sp.: Pathogenicity factors and prevention methods-new molecular targets for innovative antivirulence drugs? Appl. Microbiol. Biotechnol. 2020, 104, 10409–10436. [Google Scholar] [CrossRef] [PubMed]
- Selwet, M.; Galbas, M. Monitoring of selected genes in Campylobacter jejuni and Campylobacter coli isolates from domestic animals. Bull. Veter-Inst. Pulawy 2012, 56, 507–511. [Google Scholar] [CrossRef][Green Version]
- Montgomery, M.P.; Robertson, S.; Koski, L.; Salehi, E.; Stevenson, L.M.; Silver, R.; Sundararaman, P.; Singh, A.; Joseph, L.A.; Weisner, M.B.; et al. Multidrug-Resistant Campylobacter jejuni Outbreak Linked to Puppy Exposure—United States, 2016–2018. MMWR. Morb. Mortal. Wkly. Rep. 2012, 67, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J.; Ricci, V.; Pumbwe, L.; Everett, M.J.; Griggs, D.J. Fluoroquinolone resistance in Campylobacter species from man and animals: Detection of mutations in topoisomerase genes. J. Antimicrob. Chemoth. 2003, 51, 19–26. [Google Scholar] [CrossRef]
- Beckmann, L.; Müller, M.; Luber, P.; Schrader, C.; Bartelt, E.; Klein, G. Analysis of gyrA mutations in quinolone-resistant and -susceptible Campylobacter jejuni isolates from retail poultry and human clinical isolates by non-radioactive single-strand conformation polymorphism analysis and DNA sequencing. J. Appl. Microbiol. 2004, 96, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Chatur, Y.A.; Brahmbhatt, M.N.; Modi, S.; Nayak, J.B. Fluoroquinolone resistance and detection of topoisomerase gene mutation in Campylobacter jejuni isolated from animal and human sources. Int. J. Curr. Microbiol. 2014, 3, 773–783. [Google Scholar]
- Connell, S.R.; Tracz, D.M.; Nierhaus, K.H.; Taylor, D.E. Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob. Agents Chemother. 2003, 47, 3675–3681. [Google Scholar] [CrossRef]
- Gibreel, A.; Tracz, D.M.; Nonaka, L.; Ngo, T.M.; Connell, S.R.; Taylor, D.E. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet(O)-mediated tetracycline resistance. Antimicrob. Agents Chemother. 2004, 48, 3442–3450. [Google Scholar] [CrossRef]
- Rozynek, E.; Dzierzanowska-Fangrat, K.; Korsak, D.; Konieczny, P.; Wardak, S.; Szych, J.; Jarosz, M.; Dzierzanowska, D. Comparison of antimicrobial resistance of Campylobacter jejuni and Campylobacter coli isolated from humans and chicken carcasses in Poland. J. Food Protect. 2008, 71, 602–607. [Google Scholar] [CrossRef]
- Wardak, S.; Szych, J.; Zasada, A.A.; Gierczynski, R. Antibiotic resistance of Campylobacter jejuni and Campylobacter coli clinical isolates from Poland. Antimicrob. Agents Chemother. 2007, 51, 1123–1125. [Google Scholar] [CrossRef] [PubMed]
- Woźniak-Biel, A.; Bugla-Płoskońska, G.; Kielsznia, A.; Korzekwa, K.; Tobiasz, A.; Korzeniowska-Kowal, A.; Wieliczko, A. High Prevalence of Resistance to Fluoroquinolones and Tetracycline Campylobacter Spp. Isolated from Poultry in Poland. Microb. Drug Resist. 2018, 24, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Wysok, B.; Wojtacka, J.; Wiszniewska-Łaszczych, A.; Szteyn, J. Antimicrobial Resistance and Virulence Properties of Campylobacter Spp. Originating from Domestic Geese in Poland. Animals 2020, 10, 742. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, C.; Ye, Y.; Liu, Y.; Wang, A.; Li, Y.; Zhou, X.; Pan, H.; Zhang, J.; Xu, X. Molecular Identification of Multidrug-Resistant Campylobacter Species from Diarrheal Patients and Poultry Meat in Shanghai, China. Front. Microbiol. 2018, 9, 1642. [Google Scholar] [CrossRef] [PubMed]
- Bakhshi, B.; Kalantar, M.; Rastegar-Lari, A.; Fallah, F. PFGE genotyping and molecular characterization of Campylobacter spp. isolated from chicken meat. Iran. J. Vet. Res. 2016, 17, 177–183. [Google Scholar] [PubMed]
- Di Giannatale, E.; Calistri, P.; Di Donato, G.; Decastelli, L.; Goffredo, E.; Adriano, D.; Mancini, M.E.; Galleggiante, A.; Neri, D.; Antoci, S.; et al. Thermotolerant Campylobacter spp. in chicken and bovine meat in Italy: Prevalence, level of contamination and molecular characterization of isolates. PLoS ONE 2019, 14, e0225957. [Google Scholar] [CrossRef]
- Di Donato, G.; Marotta, F.; Nuvoloni, R.; Zilli, K.; Neri, D.; Di Sabatino, D.; Calistri, P.; Di Giannatale, E. Prevalence, Population Diversity and Antimicrobial Resistance of Campylobacter coli Isolated in Italian Swine at Slaughterhouse. Microorganisms 2020, 8, 222. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Shao, Y.; Hu, Y.; Lou, H.; Chen, X.; Wu, Y.; Mei, L.; Zhou, B.; Zhang, X.; et al. Molecular Characterization and Antibiotic Resistant Profiles of Campylobacter Species Isolated from Poultry and Diarrheal Patients in Southeastern China 2017–2019. Front. Microbiol. 2020, 11, 1244. [Google Scholar] [CrossRef]
- Marotta, F.; Garofolo, G.; Di Donato, G.; Aprea, G.; Platone, I.; Cianciavicchia, S.; Alessiani, A.; Di Giannatale, E. Population Diversity of Campylobacter jejuni in Poultry and Its Dynamic of Contamination in Chicken Meat. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Di Giannatale, E.; Di Serafino, G.; Zilli, K.; Alessiani, A.; Sacchini, L.; Garofolo, G.; Aprea, G.; Marotta, F. Characterization of antimicrobial resistance patterns and detection of virulence genes in Campylobacter isolates in Italy. Sensors 2014, 14, 3308–3322. [Google Scholar] [CrossRef]
- On, S.L.W.; Jordan, P.J. Evaluation of 11 PCR Assays for Species-Level Identification of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 2003, 41, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Linton, D.; Owen, R.J.; Stanley, J. Rapid identification by PCR of the genus Campylobacter and of five Campylobacter species enteropathogenic for man and animals. Res. Microbiol. 1996, 147, 707–718. [Google Scholar] [CrossRef]
- Klena, J.D.; Parker, C.T.; Knibb, K.; Ibbitt, J.C.; Devane, P.M.; Horn, S.T.; Miller, W.G.; Konkel, M.E. Differentiation of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis by a multiplex PCR developed from the nucleotide sequence of the lipid A gene lpxA. J. Clin. Microbiol. 2004, 42, 5549–5557. [Google Scholar] [CrossRef]
- Nachamkin, I.; Bohachick, K.; Patton, C.M. Flagellin gene typing of Campylobacter jejuni by restriction fragment length polymorphism analysis. J. Clin. Microbiol. 1993, 31, 1531–1536. [Google Scholar] [CrossRef]
- Goon, S.; Kelly, J.F.; Logan, S.M.; Ewing, C.P.; Guerry, P. Pseudaminic acid, the major modification on Campylobacter flagellin, is synthesized via the Cj1293 gene. Mol. Microbiol. 2003, 50, 659–671. [Google Scholar] [CrossRef]
- Hickey, T.E.; McVeigh, A.L.; Scott, D.A.; Michielutti, R.A.; Bixby, A.; Carroll, S.A.; Bourgeois, A.L.; Guerry, P. Campylobacter jejuni cytolethal distending toxin mediates release of interleukin-8 from intestinal epithelial cells. Infect. Immun. 2000, 68, 6535–6541. [Google Scholar] [CrossRef]
- Datta, S.; Niwa, H.; Itoh, K. Prevalence of 11 pathogenic genes of Campylobacter jejuni by PCR in strains isolated from humans, poultry meat and broiler and bovine faeces. J. Med. Microbiol. 2003, 52, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Meng, J.; Zhao, S.; Singh, R.; Song, W. Adherence to and invasion of human intestinal epithelial cells by Campylobacter jejuni and Campylobacter coli isolates from retail meat products. J. Food Prot. 2006, 69, 768–774. [Google Scholar] [CrossRef]
- Breakpoints tables for interpretation of MICs and zone diameters, Version 12.0; The European Committee on Antimicrobial Susceptibility Testing: 2022. Available online: http://eucast.org (accessed on 1 January 2022).
- CLSI. Methods for Antimicrobial Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria Isolated From Animals, 1st ed.; CLSI supplement VET06; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Ribot, E.M.; Fitzgerald, C.; Kubota, K.; Swaminathan, B.; Barrett, T.J. Rapid Pulsed-Field Gel Electrophoresis Protocol for Subtyping of Campylobacter jejuni. J. Clin. Microbiol. 2001, 39, 1889–1894. [Google Scholar] [CrossRef]
- IBM SPSS Statistics for Windows, version 28; IBM Corp: Armonk, NY, USA, 2022.
- Kohn, M.A.; Senyak, J. Sample Size Calculators. UCSF CTSI. 20 December 2021. Available online: https://www.sample-size.net/ (accessed on 26 May 2022).
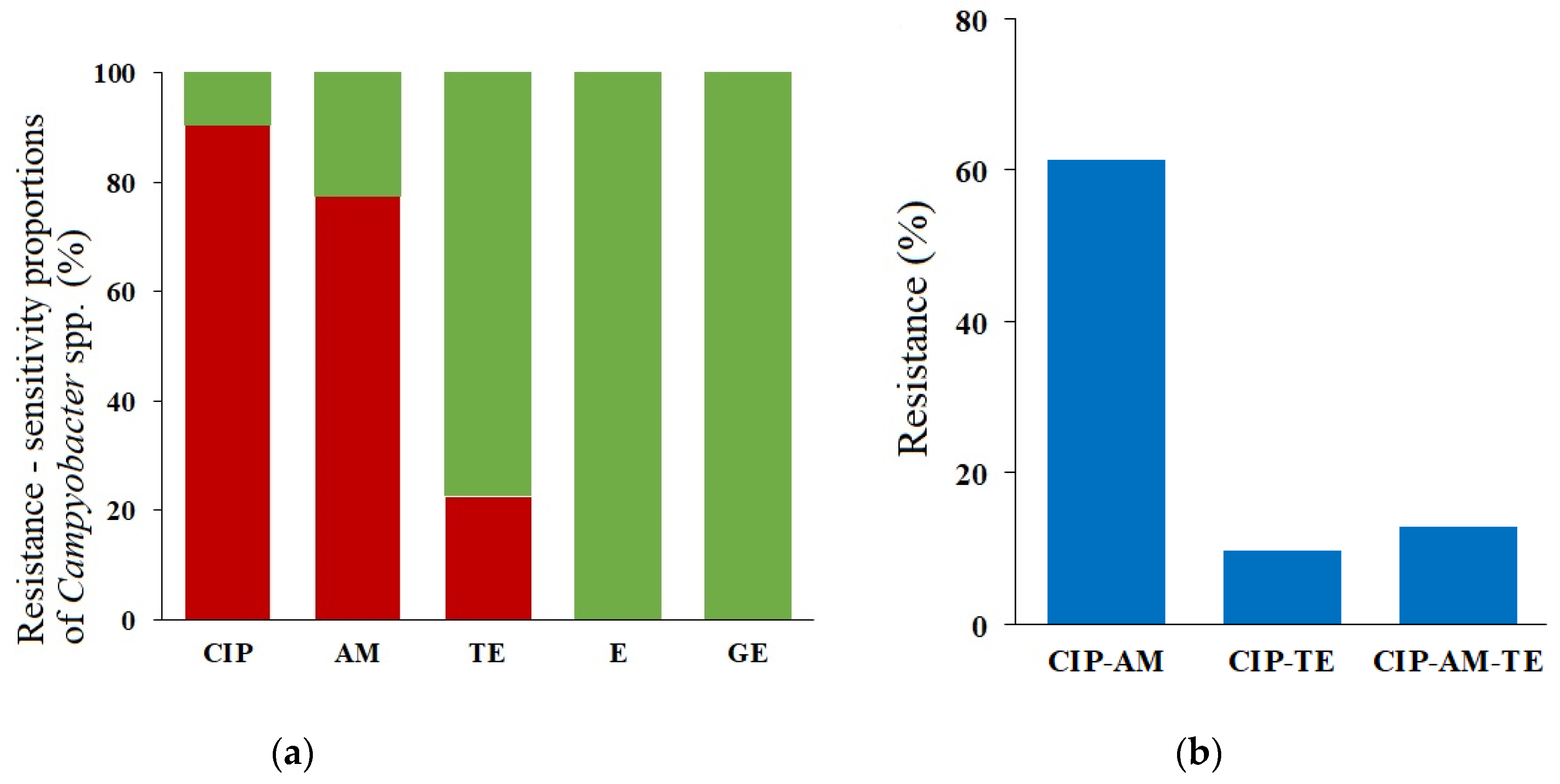
| Resistance Profile | Number of Isolates | Frequency [%] | Species Included [%] |
|---|---|---|---|
| CIPR AMR TES | 19 | 61.3 | C. jejuni (47.4), C. lari (15.8), C. upsaliensis (36.8) |
| CIPR AMR TER | 4 | 12.9 | C. jejuni (100) |
| CIPR AMS TER | 3 | 9.6 | C. jejuni (100) |
| CIPR AMS TES | 2 | 6.5 | C. jejuni (100) |
| CIPS AMS TES | 2 | 6.5 | C. jejuni (100) |
| CIPS AMR TES | 1 | 3.2 | C. jejuni (100) |
| Total | 31 |
| Target Species | Primer | Amplicon Size [bp] | Primer Sequence 5′-3′ | Tm * [°C] | Ref. |
|---|---|---|---|---|---|
| C. jejuni | mapAF | 604 | ATGTTTAAAAAATTTTTG | 55 | [51] |
| mapAR | AAGTTCAGAGATTAAACTAG | ||||
| C. upsaliensis | CHCU146F | 879 | GGGACAACACTTAGAAATGAG | 55 | [52] |
| CU1024R | CACTTCCGTATCTCTACAGA | ||||
| C. helveticus | CHCU146F | 1226 | GGGACAACACTTAGAAATGAG | 52 | [52] |
| CH1371R | CCGTGACATGGCTGATTCAC | ||||
| C. lari | lpxAF | 233 | TRCCAAATGTTAAAATAGGCGA | 50 | [53] |
| lpxAR | CAATCATGDGCDATATGASAATAHGCCAT | ||||
| C. coli | Mu3 | 364 | AGGCAAGGGAGCCTTTAATC | 61 | [51] |
| Mu4 | TATCCCTATCTACAATTCGC |
| Target Gene | Primer | Amplicon Size [bp] | Primer Sequence 5′- 3′ | Tm * [°C] | Ref. |
|---|---|---|---|---|---|
| flaA | flaA-F | 1728 | GGATTTCGTATTAACACAAATGGTGC | 45 | [54] |
| flaA-R | CTGTAGTAATCTTAAAACATTTTG | ||||
| flaB | fB1 | 260 | AAGGATTTAAAATGGGTTTTAGAATAAACACC | 54 | [55] |
| fA2 | GCTCATCCATAGCTTTATCTGC | ||||
| cdtA | cdtA-F | 370 | CCTTGTGATGCAAGCAATC | 46 | [56] |
| cdtA-R | ACACTCCATTTGCTTTCTG | ||||
| cdtB | cdtB-F | 620 | CAGAAAGCAAATGGAGTGTT | 47 | [57] |
| cdtB-R | AGCTAAAAGCGGTGGAGTAT | ||||
| cdtC | cdtC-F | 182 | TTGGCATTATAGAAAATACAGTT | 46 | [57] |
| cdtC-R | CGATGAGTTAAAACAAAAAGATA | ||||
| ciaB | ciaB-F | 527 | TGCGAGATTTTTCGAGAATG | 47 | [58] |
| ciaB-R | TGCCCGCCTTAGAACTTACA | ||||
| pldA | pldA-F | 385 | AAGAGTGAGGCGAAATTCCA | 49 | [58] |
| pldA-R | GCAAGATGGCAGGATTATCA | ||||
| flpA | flpAF | 1017 | GCTTTTGAATGGGAGTCTTTATAT | 49 | This study |
| flpAR | ATCAATAGCAATAACTTCATAACTATA | ||||
| cadF | cadF_F | 580 | TTTGAGTGCTATTAAAGGTATTG | 47 | This study |
| cadF_R | TCTTTCTGAAAGCTTTTGATTATA | ||||
| cadF (C. lari) | cadF_LF | 589 | GCGCACGACCTTCTTTAGT | 50 | This study |
| cadF_LR | GCTTATGAAAATAAAAGCGGTATG | ||||
| cadF (C. upsaliensis) | cadF_UF | 510 | CTCTCTTGGTTCTTCAGGACA | 52 | This study |
| cadF_UR | GATAATCGCTATGCACCAGGGA | ||||
| tetO | tetO_F | 559 | GGCGTTTTGTTTA | 49 | [37] |
| tetO_R | ATGGACAACCCGACAGAAGC | ||||
| gyrA | gyrA_F | 290 | ATTATAGGTCGTGCTTTGCCT | 50 | This study |
| gyrA_R | GCTTCAGTATAACGCATCGCA |
| Antimicrobial Agent | Antimicrobial Class | Concentration Range Tested [mg/L] | Breakpoints for MIC Testing | ||
|---|---|---|---|---|---|
| S ≤ | I | R ≥ | |||
| CIP b * | Fluoroquinolones | 0.002–32 | 0.001 | - | 0.5 |
| TE a | Tetracyclines | 0.016–256 | 4 | 8 | 16 |
| AM b | β-lactams | 0.016–256 | 2 | - | 8 |
| E a | Macrolides | 0.016–256 | 8 | 16 | 32 |
| GE b | Aminoglycosides | 0.016–256 | 0.5 | - | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murawska, M.; Sypecka, M.; Bartosik, J.; Kwiecień, E.; Rzewuska, M.; Sałamaszyńska-Guz, A. Should We Consider Them as a Threat? Antimicrobial Resistance, Virulence Potential and Genetic Diversity of Campylobacter spp. Isolated from Varsovian Dogs. Antibiotics 2022, 11, 964. https://doi.org/10.3390/antibiotics11070964
Murawska M, Sypecka M, Bartosik J, Kwiecień E, Rzewuska M, Sałamaszyńska-Guz A. Should We Consider Them as a Threat? Antimicrobial Resistance, Virulence Potential and Genetic Diversity of Campylobacter spp. Isolated from Varsovian Dogs. Antibiotics. 2022; 11(7):964. https://doi.org/10.3390/antibiotics11070964
Chicago/Turabian StyleMurawska, Małgorzata, Monika Sypecka, Justyna Bartosik, Ewelina Kwiecień, Magdalena Rzewuska, and Agnieszka Sałamaszyńska-Guz. 2022. "Should We Consider Them as a Threat? Antimicrobial Resistance, Virulence Potential and Genetic Diversity of Campylobacter spp. Isolated from Varsovian Dogs" Antibiotics 11, no. 7: 964. https://doi.org/10.3390/antibiotics11070964
APA StyleMurawska, M., Sypecka, M., Bartosik, J., Kwiecień, E., Rzewuska, M., & Sałamaszyńska-Guz, A. (2022). Should We Consider Them as a Threat? Antimicrobial Resistance, Virulence Potential and Genetic Diversity of Campylobacter spp. Isolated from Varsovian Dogs. Antibiotics, 11(7), 964. https://doi.org/10.3390/antibiotics11070964







