Co-Administration of Lactulose Crystals with Amoxicillin Followed by Prolonged Lactulose Treatment Promotes Recovery of the Human Gut Microbiome In Vitro
Abstract
:1. Introduction
2. Results
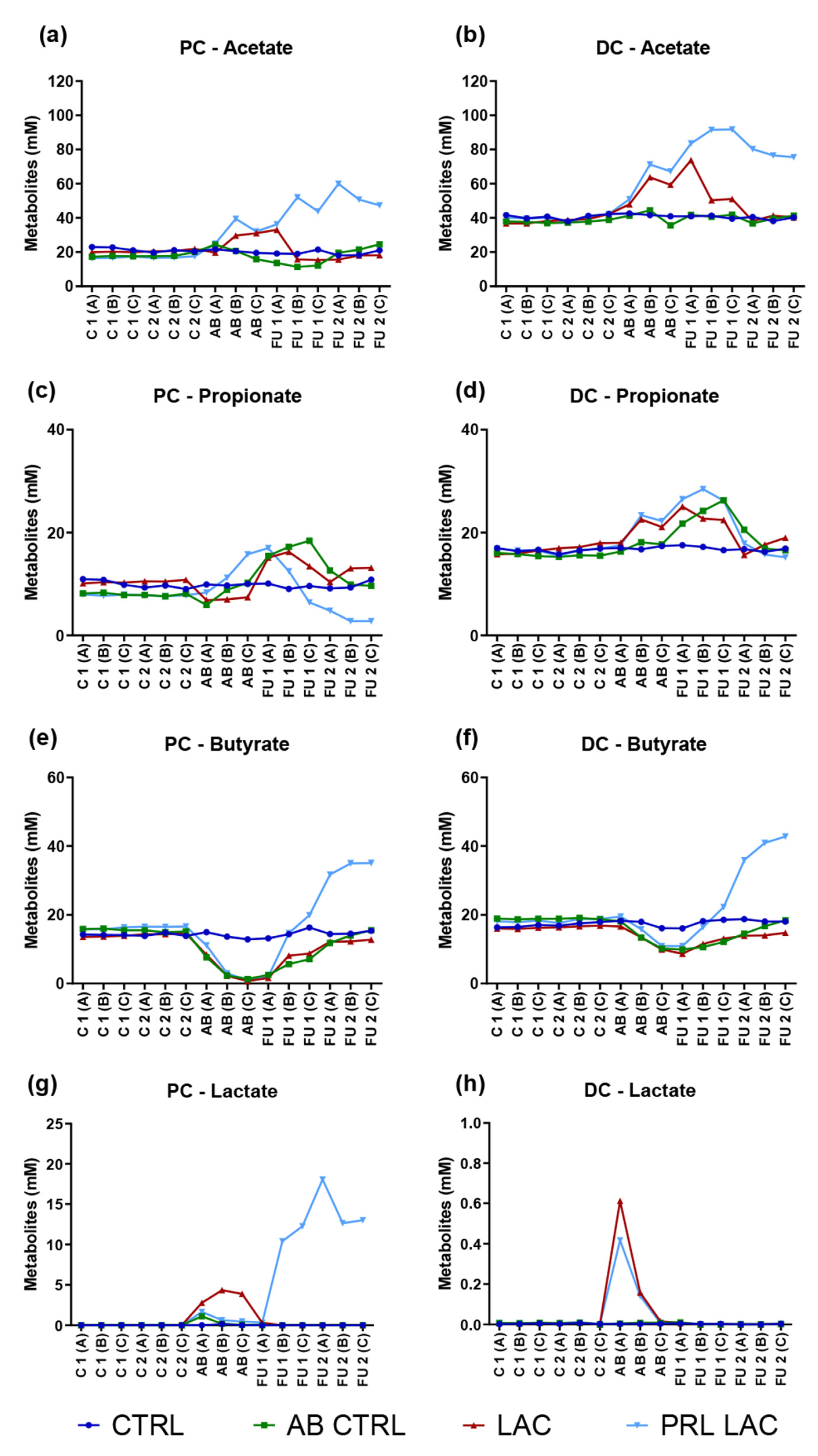
2.1. Analysis of the Microbial Metabolic Activity
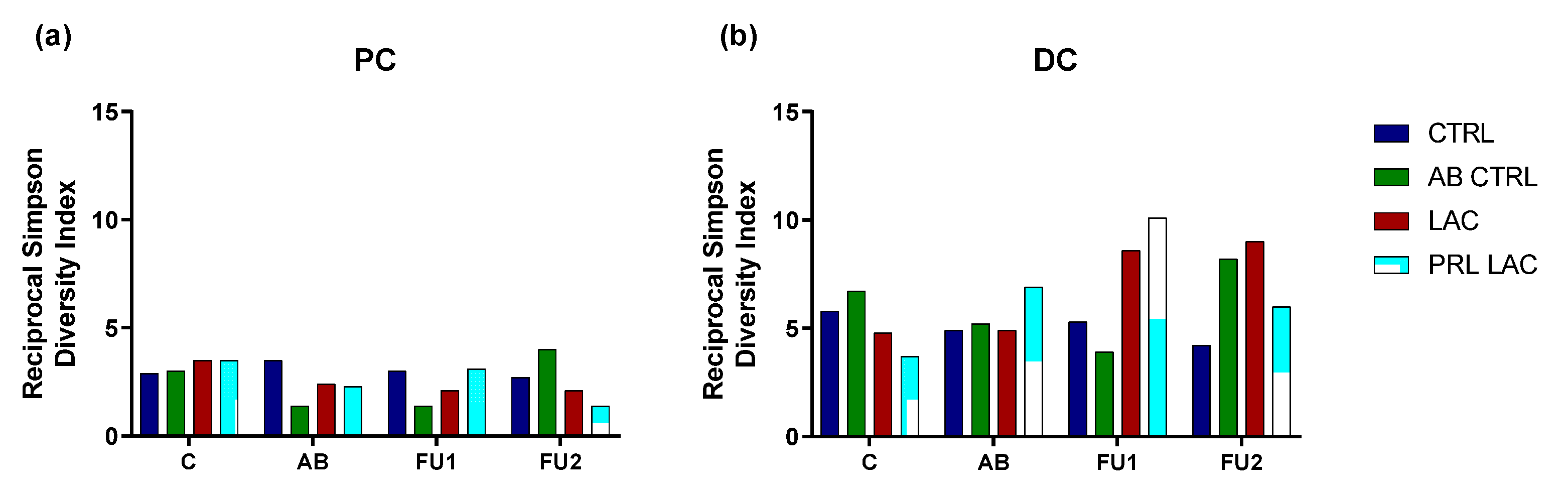
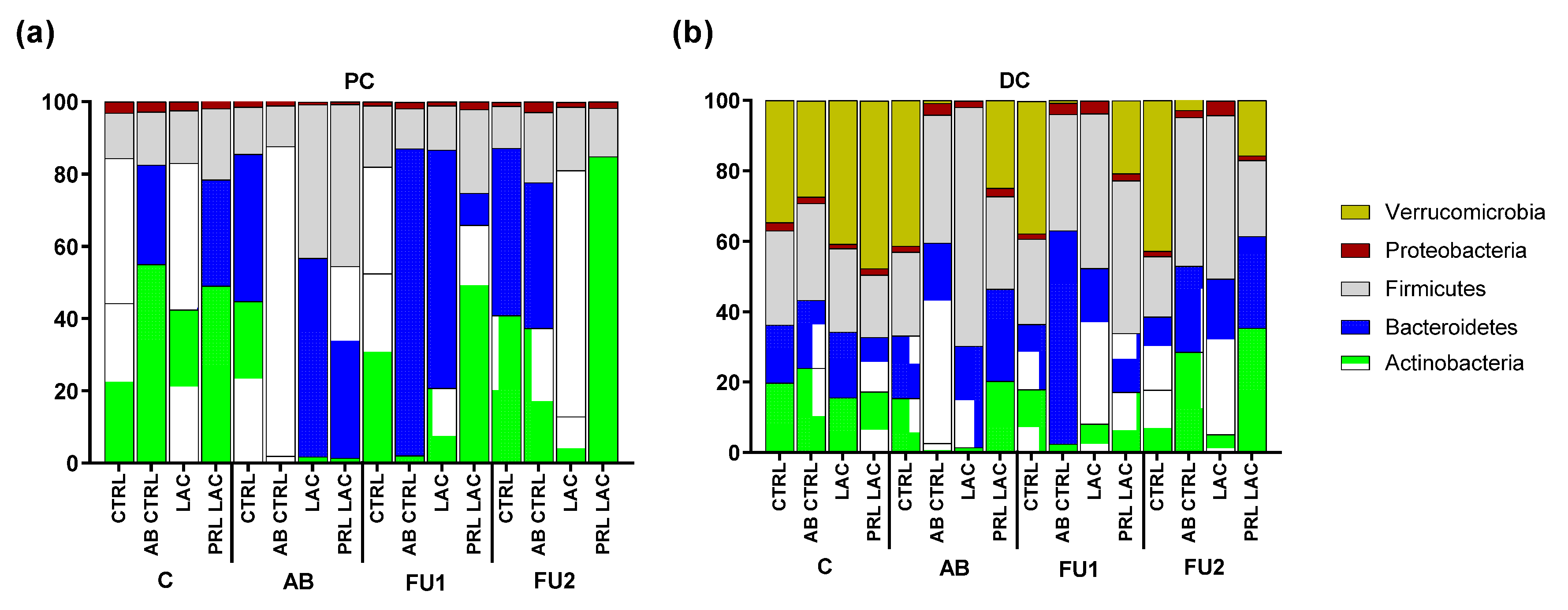
2.2. Analysis of Microbial Community Composition
3. Discussion
4. Materials and Methods
4.1. Chemicals and Test Product
4.2. Simulator of the Human Intestinal Microbial Ecosystem (SHIME®)
4.3. Microbial Metabolic Activity
4.4. Microbial Community Analysis
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brötz-Oesterhelt, H.; Brunner, N.A. How many modes of action should an antibiotic have? Curr. Opin. Pharmacol. 2008, 8, 564–573. [Google Scholar] [CrossRef] [PubMed]
- McFarland, L.V. Antibiotic-associated diarrhea: Epidemiology, trends and treatment. Futur. Microbiol. 2008, 3, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulevic, J.; Drakoularakou, A.; Yaqoob, P.; Tzortzis, G.; Gibson, G.R. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am. J. Clin. Nutr. 2008, 88, 1438–1446. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. S2), S1–S63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; McCartney, A.L.; Rastall, R.A. Prebiotics and resistance to gastrointestinal infections. Br. J. Nutr. 2007, 93, S31–S34. [Google Scholar] [CrossRef] [PubMed]
- Ladirat, S.E.; Schoterman, M.H.C.; Rahaoui, H.; Mars, M.; Schuren, F.H.J.; Gruppen, H.; Nauta, A.; Schols, H.A. Exploring the effects of galacto-oligosaccharides on the gut microbiota of healthy adults receiving amoxicillin treatment. Br. J. Nutr. 2014, 112, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Ladirat, S.E.; Schuren, F.H.J.; Schoterman, M.H.C.; Nauta, A.; Gruppen, H.; Schols, H.A. Impact of galacto-oligosaccharides on the gut microbiota composition and metabolic activity upon antibiotic treatment during in vitro fermentation. FEMS Microbiol. Ecol. 2014, 87, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldi, S.; Vasileiadis, S.; Lohner, S.; Uggeri, F.; Puglisi, E.; Molinari, P.; Donner, E.; Sieland, C.; Decsi, T.; Sailer, M.; et al. Prebiotic supplementation over a cold season and during antibiotic treatment specifically modulates the gut microbiota composition of 3-6 year-old children. Benef. Microbes 2019, 10, 253–263. [Google Scholar] [CrossRef]
- Lewis, S.; Burmeister, S.; Cohen, S.; Brazier, J.; Awasthi, A. Failure of dietary oligofructose to prevent antibiotic-associated diarrhoea. Aliment. Pharmacol. Ther. 2005, 21, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Preidis, G.A.; Versalovic, J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: Gastroenterology enters the metagenomics era. Gastroenterology 2009, 136, 2015–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bass, P.; Dennis, S. The laxative effects of lactulose in normal and constipated subjects. J. Clin. Gastroenterol. 1981, 3 (Suppl. S1), 23–28. [Google Scholar] [CrossRef] [PubMed]
- Weber, F.L., Jr. Lactulose and combination therapy of hepatic encephalopathy: The role of the intestinal microflora. Dig. Dis. 1996, 14 (Suppl. S1), 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bouhnik, Y.; Attar, A.; Joly, F.A.; Riottot, M.; Dyard, F.; Flourie, B. Lactulose ingestion increases faecal bifidobacterial counts: A randomised double-blind study in healthy humans. Eur. J. Clin. Nutr. 2004, 58, 462–466. [Google Scholar] [CrossRef]
- Mizota, T.; Mori, T.; Yaeshima, T.; Yanagida, T.; Iwatsuki, K.; Ishibashi, N.; Tamura, Y.; Fukuwatari, Y. Effects of low dosages of lactulose on the intestinal function of healthy adults. Milchwissenschaft 2002, 57, 312–315. [Google Scholar]
- Bothe, M.K.; Maathuis, A.J.H.; Bellmann, S.; van der Vossen, J.; Berressem, D.; Koehler, A.; Schwejda-Guettes, S.; Gaigg, B.; Kuchinka-Koch, A.; Stover, J.F. Dose-Dependent Prebiotic Effect of Lactulose in a Computer-Controlled In Vitro Model of the Human Large Intestine. Nutrients 2017, 9, 767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, M.; Jonkers, D.M.; Troost, F.J.; Roeselers, G.; Venema, K. In vitro characterization of the impact of different substrates on metabolite production, energy extraction and composition of gut microbiota from lean and obese subjects. PLoS ONE 2014, 9, e113864. [Google Scholar] [CrossRef] [Green Version]
- Bothe, M.; Maathuis, A.; Lange, K.; Koenen, M.; van der Vossen, J. Lactulose Crystals and Liquid Both Show A Dose-Dependent Prebiotic Effect in a Computer-Controlled In Vitro Model of the Human Proximal Colon. J. Food Technol. Food Chem. 2018, 1, 1–7. [Google Scholar]
- Duysburgh, C.; Van Den Abbeele, P.; Bothe, M.; Stover, J.; Kuchinka-Koch, A.; Schwejda-Guettes, S.; Marzorati, M. Lactulose Crystals Beneficially Affect Community Composition Along Entire Human Colon in Vitro, Resulting in Donor-Dependent Prebiotic Effects at Metabolic Level. Int. J. Food. Sci. Nutr. 2019, 8, 10. [Google Scholar] [CrossRef]
- Nikolaou, E.; Kamilari, E.; Savkov, D.; Sergeev, A.; Zakharova, I.; Vogazianos, P.; Tomazou, M.; Antoniades, A.; Shammas, C. Intestinal microbiome analysis demonstrates azithromycin post-treatment effects improve when combined with lactulose. World J. Pediatr. 2019, 16, 168–176. [Google Scholar] [CrossRef]
- Possemiers, S.; Verthe, K.; Uyttendaele, S.; Verstraete, W. PCR-DGGE-based quantification of stability of the microbial community in a simulator of the human intestinal microbial ecosystem. FEMS Microbiol. Ecol. 2004, 49, 495–507. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gerard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M.; et al. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef] [Green Version]
- Tsitko, I.; Wiik-Miettinen, F.; Mattila, O.; Rosa-Sibakov, N.; Seppänen-Laakso, T.; Maukonen, J.; Nordlund, E.; Saarela, M. A Small In Vitro Fermentation Model for Screening the Gut Microbiota Effects of Different Fiber Preparations. Int. J. Mol. Sci. 2019, 20, 1925. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.; El khader, I.; Casellas, F.; López Vivancos, J.; García Cors, M.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-term effect of antibiotics on human gut microbiota. PLoS ONE 2014, 9, e95476. [Google Scholar] [CrossRef] [PubMed]
- Barc, M.C.; Bourlioux, F.; Rigottier-Gois, L.; Charrin-Sarnel, C.; Janoir, C.; Boureau, H.; Dore, J.; Collignon, A. Effect of amoxicillin-clavulanic acid on human fecal flora in a gnotobiotic mouse model assessed with fluorescence hybridization using group-specific 16S rRNA probes in combination with flow cytometry. Antimicrob. Agents Chemother. 2004, 48, 1365–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef] [Green Version]
- Moens, F.; Rivière, A.; Selak, M.; De Vuyst, L. Inulin-type fructan degradation capacity of interesting butyrate-producing colon bacteria and cross-feeding interactions of Faecalibacterium prausnitzii DSM 17677(T) with bifidobacteria. Arch. Public Health 2014, 72, O6. [Google Scholar] [CrossRef] [Green Version]
- Carter, M.E.; Chengappa, M.M. Enterobacteria. In Diagnostic Procedure in Veterinary Bacteriology and Mycology, 5th ed.; Carter, G.R., Cole, J.R., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 107–128. [Google Scholar] [CrossRef]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the human intestinal microbial flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [Green Version]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Molly, K.; Vande Woestyne, M.; Verstraete, W. Development of a 5-step multi-chamber reactor as a simulation of the human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 1993, 39, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Alric, M.; Blanquet-Diot, S.; Bornhorst, G.; Cueva, C.; Deglaire, A.; Denis, S.; Ferrua, M.; Havenaar, R.; Lelieveld, J.; et al. Can dynamic in vitro digestion systems mimic the physiological reality? Crit. Rev. Food Sci. Nutr. 2019, 59, 1546–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Velde, F.; de Winter, B.C.M.; Koch, B.C.P.; van Gelder, T.; Mouton, J.W.; COMBACTE-NET consortium. Non-linear absorption pharmacokinetics of amoxicillin: Consequences for dosing regimens and clinical breakpoints. J. Antimicrob. Chemother. 2016, 71, 2909–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legen, I.; Kračun, M.; Salobir, M.; Kerč, J. The evaluation of some pharmaceutically acceptable excipients as permeation enhancers for amoxicillin. Int. J. Pharm. 2006, 308, 84–89. [Google Scholar] [CrossRef] [PubMed]
- EMEA. Committee for Veterinary Medicinal Products-Clavulanic Acid; Unit, V.M.E., Ed.; The European Agency for the Evaluation of Medicinal Products Veterinary Medicines and Information Technology: London, UK, 2001; pp. 1–6. [Google Scholar]
- De Weirdt, R.; Possemiers, S.; Vermeulen, G.; Moerdijk-Poortvliet, T.C.W.; Boschker, H.T.S.; Verstraete, W.; Van de Wiele, T. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol. Ecol. 2010, 74, 601–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duysburgh, C.; Van den Abbeele, P.; Krishnan, K.; Bayne, T.F.; Marzorati, M. A synbiotic concept containing spore-forming Bacillus strains and a prebiotic fiber blend consistently enhanced metabolic activity by modulation of the gut microbiome in vitro. Int. J. Pharm. X 2019, 1, 100021. [Google Scholar] [CrossRef]
- Boon, N.; Top, E.M.; Verstraete, W.; Siciliano, S.D. Bioaugmentation as a tool to protect the structure and function of an activated-sludge microbial community against a 3-chloroaniline shock load. Appl. Environ. Microbiol. 2003, 69, 1511–1520. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L. Assessing and improving methods used in operational taxonomic unit-based approaches for 16S rRNA gene sequence analysis. Appl. Environ. Microbiol. 2011, 77, 3219–3226. [Google Scholar] [CrossRef] [Green Version]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
| C | AB | FU1 | FU2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acetate (mM) | PC | CTRL | 21.3 | (±1.3) | 20.6 | (±1.0) | 19.8 | (±1.4) | 19.1 | (±1.6) |
| AB CTRL | 18.0 | (±1.0) | 20.3 | (±4.3) | 12.4 | (±1.2) | 21.8 | (±2.5) | ||
| LAC | 20.5 | (±0.7) | 26.8 | (±6.2) | 21.4 | (±10.1) | 17.3 | (±1.4) | ||
| PRL LAC | 16.9 | (±0.4) | 32.2 | (±7.3) | 44.0 | (±7.9) | 52.7 | (±6.5) | ||
| DC | CTRL | 40.6 | (±1.5) | 41.7 | (±0.9) | 40.6 | (±0.9) | 39.6 | (±1.3) | |
| AB CTRL | 37.8 | (±0.7) | 40.5 | (±4.5) | 41.4 | (±0.7) | 39.2 | (±2.3) | ||
| LAC | 38.7 | (±2.0) | 57.0 | (±8.1) | 58.4 | (±13.3) | 39.9 | (±1.6) | ||
| PRL LAC | 40.0 | (±1.5) | 63.1 | (±10.7) | 89.0 | (±4.7) | 77.4 | (±2.4) | ||
| Propionate (mM) | PC | CTRL | 9.9 | (±0.8) | 9.9 | (±0.2) | 9.6 | (±0.5) | 9.8 | (±0.9) |
| AB CTRL | 8.0 | (±0.3) | 8.3 | (±2.2) | 17.0 | (±1.5) | 10.7 | (±1.7) | ||
| LAC | 10.4 | (±0.2) | 7.1 | (±0.3) | 14.9 | (±1.4) | 12.2 | (±1.6) | ||
| PRL LAC | 7.8 | (±0.1) | 11.8 | (±3.7) | 12.0 | (±5.3) | 3.5 | (±1.2) | ||
| DC | CTRL | 16.5 | (±0.4) | 17.1 | (±0.3) | 17.1 | (±0.5) | 16.6 | (±0.3) | |
| AB CTRL | 15.6 | (±0.3) | 17.4 | (±0.9) | 24.1 | (±2.3) | 18.0 | (±2.2) | ||
| LAC | 16.7 | (±0.8) | 20.6 | (±2.3) | 23.4 | (±1.4) | 17.4 | (±1.7) | ||
| PRL LAC | 16.5 | (±0.5) | 21.1 | (±3.1) | 27.0 | (±1.2) | 16.3 | (±1.4) | ||
| Butyrate (mM) | PC | CTRL | 14.2 | (±0.3) | 13.8 | (±1.0) | 14.6 | (±1.6) | 14.8 | (±0.5) |
| AB CTRL | 15.5 | (±0.4) | 3.8 | (±3.4) | 5.1 | (±2.4) | 13.8 | (±1.8) | ||
| LAC | 14.1 | (±0.4) | 3.8 | (±4.1) | 6.1 | (±4.0) | 12.4 | (±0.3) | ||
| PRL LAC | 16.3 | (±0.4) | 5.0 | (±5.4) | 12.2 | (±9.2) | 34.0 | (±1.9) | ||
| DC | CTRL | 17.0 | (±0.6) | 17.5 | (±1.2) | 17.6 | (±1.3) | 18.3 | (±0.4) | |
| AB CTRL | 18.9 | (±0.1) | 13.9 | (±4.0) | 10.9 | (±1.1) | 16.6 | (±1.9) | ||
| LAC | 16.4 | (±0.4) | 13.3 | (±3.3) | 11.1 | (±2.2) | 14.3 | (±0.5) | ||
| PRL LAC | 18.3 | (±0.5) | 15.4 | (±4.3) | 16.5 | (±5.7) | 39.9 | (±3.5) | ||
| Lactate (mM) | PC | CTRL | 0.01 | (±0.01) | 0.05 | (±0.09) | 0.01 | (±0.00) | 0.00 | (±0.00) |
| AB CTRL | 0.01 | (±0.00) | 0.46 | (±0.57) | 0.01 | (±0.00) | 0.00 | (±0.00) | ||
| LAC | 0.01 | (±0.00) | 3.67 | (±0.81) | 0.11 | (±0.17) | 0.00 | (±0.00) | ||
| PRL LAC | 0.01 | (±0.00) | 0.92 | (±0.64) | 7.67 | (±6.43) | 14.58 | (±3.04) | ||
| DC | CTRL | 0.004 | (±0.000) | 0.004 | (±0.000) | 0.003 | (±0.000) | 0.001 | (±0.000) | |
| AB CTRL | 0.006 | (±0.000) | 0.008 | (±0.000) | 0.003 | (±0.010) | 0.001 | (±0.000) | ||
| LAC | 0.006 | (±0.000) | 0.262 | (±0.310) | 0.003 | (±0.000) | 0.001 | (±0.000) | ||
| PRL LAC | 0.007 | (±0.000) | 0.189 | (±0.210) | 0.003 | (±0.010) | 0.002 | (±0.000) | ||
| C | AB | FU1 | FU2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCFA (mM) | PC | CTRL | 2.82 | (±0.11) | 2.66 | (±0.24) | 2.61 | (±0.10) | 2.59 | (±0.19) |
| AB CTRL | 2.80 | (±0.05) | 0.92 | (±0.58) | 2.22 | (±0.57) | 2.64 | (±0.09) | ||
| LAC | 2.76 | (±0.04) | 0.87 | (±1.15) | 1.80 | (±1.26) | 2.66 | (±0.13) | ||
| PRL LAC | 2.66 | (±0.05) | 0.85 | (±1.00) | 1.74 | (±0.80) | 1.79 | (±0.07) | ||
| DC | CTRL | 2.95 | (±0.05) | 2.92 | (±0.28) | 2.86 | (±0.21) | 2.92 | (±0.03) | |
| AB CTRL | 3.01 | (±0.09) | 2.62 | (±0.40) | 2.12 | (±0.26) | 2.88 | (±0.13) | ||
| LAC | 2.88 | (±0.11) | 1.70 | (±1.01) | 1.66 | (±1.13) | 2.73 | (±0.15) | ||
| PRL LAC | 2.93 | (±0.10) | 1.62 | (±1.10) | 1.30 | (±0.26) | 2.15 | (±0.02) | ||
| Ammonium (mg/L) | PC | CTRL | 232 | (±7) | 225 | (±29) | 231 | (±22) | 220 | (±25) |
| AB CTRL | 210 | (±12) | 143 | (±38) | 203 | (±35) | 238 | (±1) | ||
| LAC | 232 | (±10) | 139 | (±50) | 182 | (±44) | 243 | (±2) | ||
| PRL LAC | 203 | (±10) | 138 | (±36) | 150 | (±25) | 98 | (±16) | ||
| DC | CTRL | 351 | (±13) | 345 | (±14) | 365 | (±22) | 353 | (±29) | |
| AB CTRL | 359 | (±17) | 345 | (±20) | 334 | (±65) | 362 | (±6) | ||
| LAC | 339 | (±17) | 236 | (±102) | 274 | (±74) | 309 | (±53) | ||
| PRL LAC | 348 | (±11) | 240 | (±93) | 223 | (±27) | 233 | (±27) | ||
| Phylum | Family | PC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | AB | FU1 | FU2 | ||||||||||||||
| CTRL | AB CTRL | LAC | PRL LAC | CTRL | AB CTRL | LAC | PRL LAC | CTRL | AB CTRL | LAC | PRL LAC | CTRL | AB CTRL | LAC | PRL LAC | ||
| Actinobacteria | Bifidobacteriaceae | 44.2 | 54.4 | 42.3 | 48.6 | 44.5 | 0.6 | 1.3 | 0.5 | 52.4 | 1.0 | 20.4 | 64.8 | 40.8 | 37.0 | 12.8 | 84.7 |
| Microbacteriaceae | 0.0 | 0.4 | 0.1 | 0.4 | 0.1 | 1.2 | 0.5 | 0.8 | 0.0 | 1.1 | 0.2 | 1.0 | 0.0 | 0.2 | 0.1 | 0.1 | |
| Bacteroidetes | Bacteroidaceae | 40.1 | 27.5 | 40.6 | 29.4 | 40.8 | 85.6 | 54.9 | 53.0 | 29.5 | 84.9 | 65.9 | 8.9 | 46.3 | 40.3 | 68.1 | 0.0 |
| Firmicutes | Acidaminococcaceae | 1.2 | 2.6 | 1.2 | 1.7 | 2.0 | 0.4 | 0.0 | 2.5 | 1.5 | 3.6 | 1.9 | 0.8 | 1.5 | 1.9 | 2.7 | 0.2 |
| Enterococcaceae | 0.2 | 0.5 | 0.3 | 0.0 | 0.5 | 1.5 | 0.5 | 0.0 | 0.7 | 1.0 | 0.7 | 0.1 | 0.2 | 0.5 | 0.7 | 0.5 | |
| Lachnospiraceae | 9.0 | 3.6 | 11.8 | 10.7 | 8.2 | 6.5 | 31.2 | 40.2 | 12.1 | 5.8 | 7.9 | 9.9 | 9.1 | 11.3 | 12.4 | 9.0 | |
| Lactobacillaceae | 1.3 | 3.6 | 0.3 | 2.6 | 0.3 | 2.8 | 10.7 | 2.1 | 0.4 | 0.3 | 1.1 | 2.9 | 0.1 | 0.1 | 0.3 | 0.1 | |
| Ruminococcaceae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Veillonellaceae | 0.8 | 4.3 | 1.1 | 4.7 | 1.9 | 0.1 | 0.0 | 0.0 | 2.2 | 0.4 | 0.7 | 9.1 | 0.8 | 5.7 | 1.3 | 3.4 | |
| Proteobacteria | Burkholderiaceae | 0.4 | 0.6 | 0.6 | 0.6 | 0.4 | 0.0 | 0.1 | 0.1 | 0.5 | 0.2 | 0.4 | 0.7 | 0.3 | 1.0 | 0.2 | 1.2 |
| Desulfovibrionaceae | 2.5 | 1.6 | 1.1 | 0.3 | 0.8 | 0.1 | 0.1 | 0.0 | 0.5 | 0.8 | 0.2 | 0.0 | 0.4 | 0.9 | 0.8 | 0.0 | |
| Enterobacteriaceae | 0.1 | 0.3 | 0.6 | 0.3 | 0.1 | 0.0 | 0.3 | 0.1 | 0.0 | 0.5 | 0.2 | 0.2 | 0.0 | 0.2 | 0.2 | 0.1 | |
| Pseudomonadaceae | 0.1 | 0.3 | 0.0 | 0.7 | 0.3 | 0.9 | 0.3 | 0.5 | 0.1 | 0.2 | 0.2 | 1.1 | 0.4 | 0.7 | 0.2 | 0.4 | |
| Xanthomonadaceae | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | |
| Phylum | Family | DC | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | AB | FU1 | FU2 | ||||||||||||||
| CTRL | AB CTRL | LAC | PRL LAC | CTRL | AB CTRL | LAC | PRL LAC | CTRL | AB CTRL | LAC | PRL LAC | CTRL | AB CTRL | LAC | PRL LAC | ||
| Actinobacteria | Bifidobacteriaceae | 19.6 | 23.8 | 15.5 | 17.2 | 15.3 | 2.3 | 1.4 | 20.0 | 17.9 | 2.2 | 7.6 | 16.9 | 17.7 | 28.4 | 5.0 | 34.9 |
| Coriobacteriaceae | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | |
| Microbacteriaceae | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.3 | 0.1 | 0.1 | 0.0 | 0.2 | 0.5 | 0.2 | 0.0 | 0.1 | 0.1 | 0.3 | |
| Bacteroidetes | Bacteroidaceae | 10.9 | 9.3 | 11.4 | 7.8 | 13.0 | 50.4 | 23.2 | 22.3 | 14.1 | 59.5 | 41.9 | 9.7 | 18.7 | 19.8 | 35.8 | 18.5 |
| Prevotellaceae | 1.0 | 2.4 | 2.0 | 1.8 | 1.1 | 4.0 | 0.3 | 2.1 | 2.0 | 0.1 | 0.0 | 0.2 | 0.7 | 0.6 | 5.1 | 1.5 | |
| Rikenellaceae | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 1.5 | 0.0 | 0.1 | 0.0 | 2.1 | |
| Tannerellaceae | 4.5 | 7.6 | 5.2 | 6.0 | 3.5 | 2.3 | 5.3 | 1.6 | 2.4 | 1.0 | 2.2 | 5.2 | 1.3 | 3.9 | 3.3 | 4.0 | |
| Firmicutes | Acidaminococcaceae | 0.7 | 0.6 | 1.0 | 0.5 | 0.9 | 0.6 | 1.3 | 0.4 | 1.1 | 1.1 | 2.3 | 0.9 | 1.3 | 1.2 | 1.9 | 0.3 |
| Enterococcaceae | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | |
| Lachnospiraceae | 22.9 | 23.5 | 21.3 | 16.1 | 20.4 | 25.1 | 59.9 | 24.5 | 21.0 | 23.6 | 32.5 | 40.2 | 14.1 | 34.8 | 42.9 | 20.1 | |
| Lactobacillaceae | 0.2 | 0.1 | 0.0 | 0.1 | 0.1 | 0.7 | 2.7 | 0.5 | 0.1 | 0.1 | 0.8 | 0.4 | 0.0 | 0.1 | 0.1 | 0.0 | |
| Ruminococcaceae | 2.9 | 3.2 | 1.2 | 0.8 | 2.0 | 9.3 | 4.0 | 0.7 | 1.8 | 7.5 | 7.2 | 1.5 | 1.6 | 5.8 | 1.0 | 0.2 | |
| Veillonellaceae | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 | 0.5 | 0.0 | 0.1 | 0.1 | 0.6 | 1.1 | 0.3 | 0.1 | 0.2 | 0.4 | 0.9 | |
| Proteobacteria | Burkholderiaceae | 0.2 | 0.3 | 0.1 | 0.2 | 0.2 | 1.1 | 0.6 | 1.0 | 0.1 | 0.4 | 0.5 | 0.7 | 0.2 | 0.4 | 0.3 | 0.3 |
| Desulfovibrionaceae | 1.8 | 1.3 | 1.1 | 1.3 | 1.2 | 1.3 | 0.7 | 0.5 | 1.0 | 0.7 | 2.5 | 0.8 | 0.7 | 0.8 | 1.3 | 0.4 | |
| Enterobacteriaceae | 0.0 | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| Pseudomonadaceae | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.2 | 0.1 | 0.1 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.7 | 0.2 | 0.1 | |
| uncultured | 0.2 | 0.2 | 0.1 | 0.2 | 0.1 | 0.6 | 0.3 | 0.9 | 0.1 | 1.8 | 0.3 | 0.3 | 0.4 | 0.0 | 2.3 | 0.5 | |
| Verrucomicrobia | Akkermansiaceae | 34.7 | 27.3 | 40.8 | 47.7 | 41.4 | 0.8 | 0.0 | 24.9 | 37.7 | 0.7 | 0.0 | 20.8 | 42.8 | 2.9 | 0.0 | 15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duysburgh, C.; Van den Abbeele, P.; Franckenstein, D.; Westphal, M.; Kuchinka-Koch, A.; Marzorati, M. Co-Administration of Lactulose Crystals with Amoxicillin Followed by Prolonged Lactulose Treatment Promotes Recovery of the Human Gut Microbiome In Vitro. Antibiotics 2022, 11, 962. https://doi.org/10.3390/antibiotics11070962
Duysburgh C, Van den Abbeele P, Franckenstein D, Westphal M, Kuchinka-Koch A, Marzorati M. Co-Administration of Lactulose Crystals with Amoxicillin Followed by Prolonged Lactulose Treatment Promotes Recovery of the Human Gut Microbiome In Vitro. Antibiotics. 2022; 11(7):962. https://doi.org/10.3390/antibiotics11070962
Chicago/Turabian StyleDuysburgh, Cindy, Pieter Van den Abbeele, Dennis Franckenstein, Martin Westphal, Angelika Kuchinka-Koch, and Massimo Marzorati. 2022. "Co-Administration of Lactulose Crystals with Amoxicillin Followed by Prolonged Lactulose Treatment Promotes Recovery of the Human Gut Microbiome In Vitro" Antibiotics 11, no. 7: 962. https://doi.org/10.3390/antibiotics11070962
APA StyleDuysburgh, C., Van den Abbeele, P., Franckenstein, D., Westphal, M., Kuchinka-Koch, A., & Marzorati, M. (2022). Co-Administration of Lactulose Crystals with Amoxicillin Followed by Prolonged Lactulose Treatment Promotes Recovery of the Human Gut Microbiome In Vitro. Antibiotics, 11(7), 962. https://doi.org/10.3390/antibiotics11070962






