Candida Carriers among Individuals with Tongue Piercing—A Real-Time PCR Study
Abstract
:1. Introduction
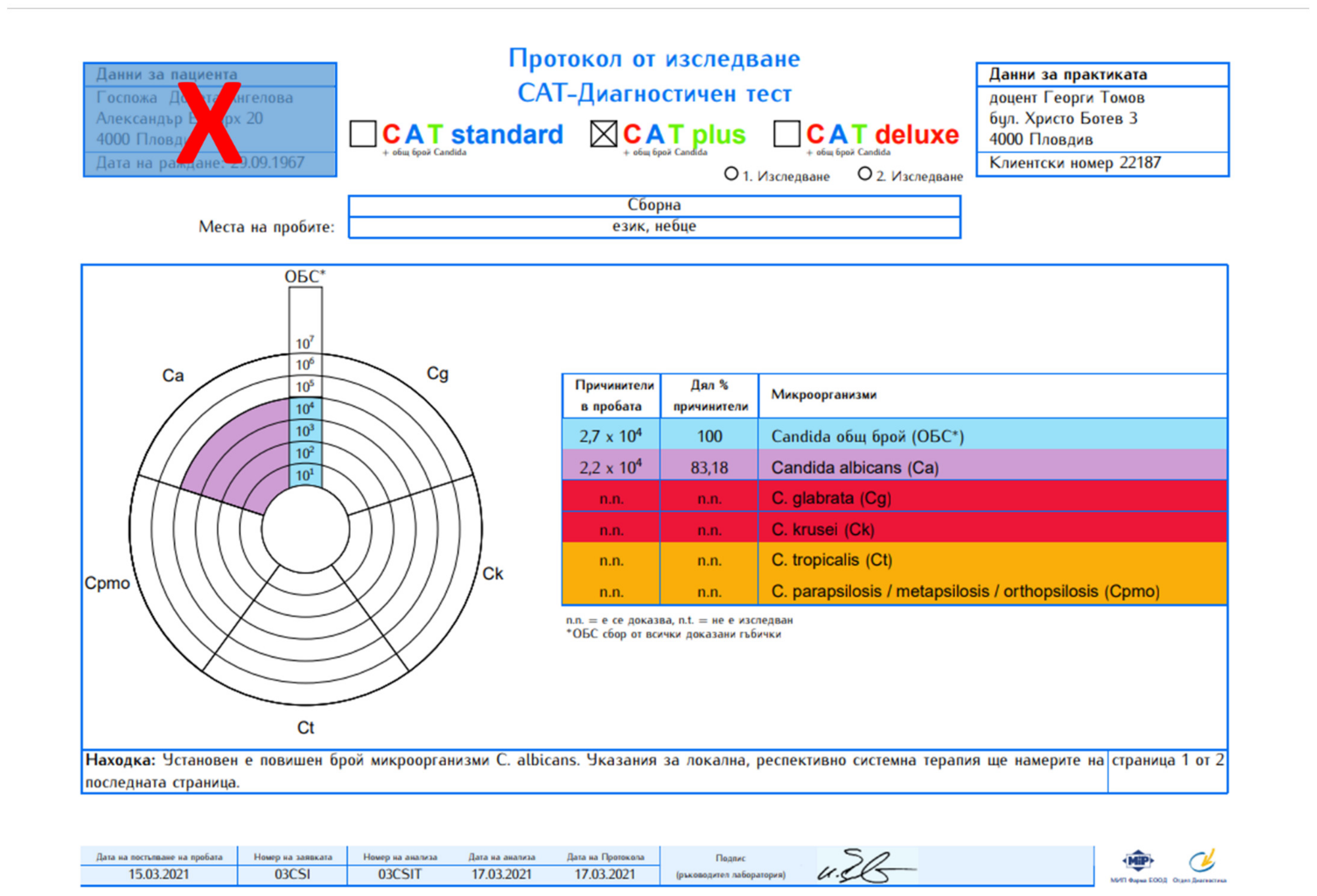
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
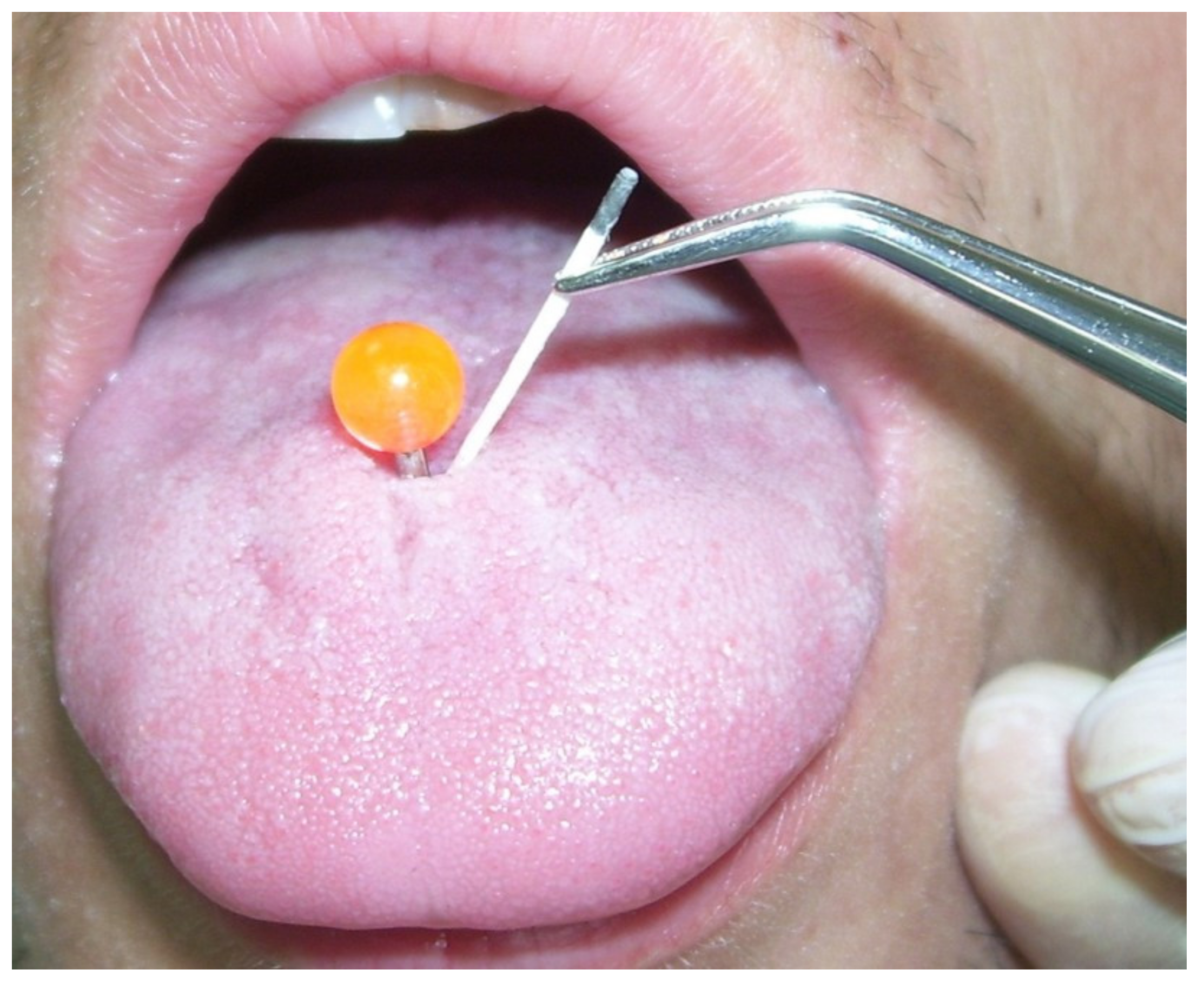
4.2. Laboratory Methods
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cannon, R.D. Oral Fungal Infections: Past, Present, and Future. Front. Oral Health 2022, 3, 838639. [Google Scholar] [CrossRef]
- Lalla, R.V.; Patton, L.L.; Dongari-Bagtzoglou, A. Oral candidiasis: Pathogenesis, clinical presentation, diagnosis and treatment strategies. J. Calif. Dent. Assoc. 2013, 41, 263–268. [Google Scholar]
- Cannon, R.D.; Holmes, A.R.; Mason, A.B.; Monk, B.C. Oral Candida: Clearance, colonization, or candidiasis? J. Dent. Res. 1995, 74, 1152–1161. [Google Scholar] [CrossRef]
- Sandin, R.L.; Rogers, A.L.; Patterson, R.J.; Beneke, E.S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect. Immun. 1982, 35, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Samaranayake, L.P.; MacFarlane, T.W. An in-vitro study of the adherence of Candida albicans to acrylic surfaces. Arch. Oral Biol. 1980, 25, 603–609. [Google Scholar] [CrossRef]
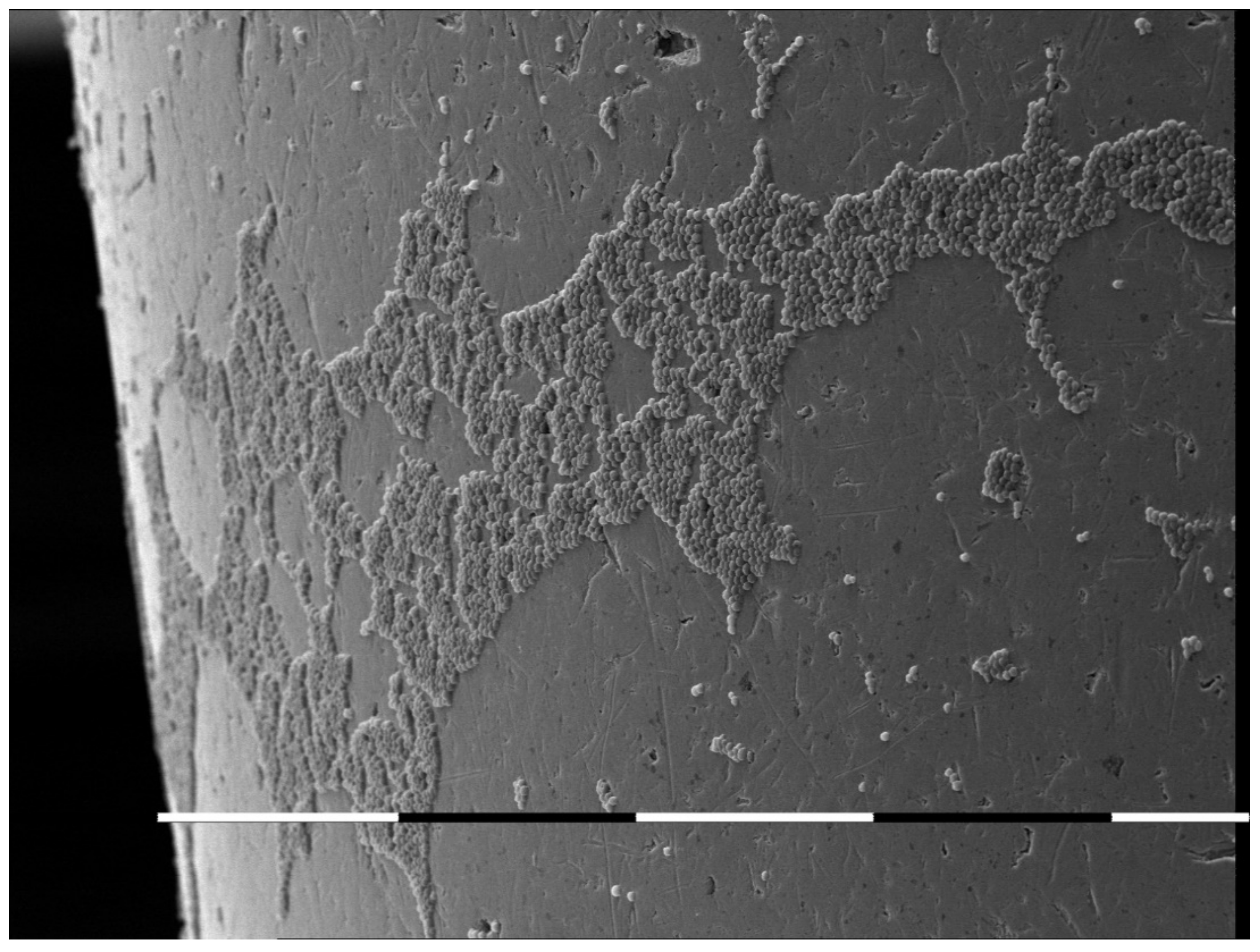
- Stamenov, N.; Tomov, G.; Denkova, Z.; Dobrev, I. In Vitro Study on the Adhesion and Colonization of Candida albicans on Metal and Acrylic Piercings. Acta Med. Bulg. 2016, 43, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.B. Diagnosis and treatment of oropharyngeal candidiasis. Oral Maxillofac. Surg. Clin. N. Am. 2003, 15, 91–102. [Google Scholar] [CrossRef]
- Zadik, Y.; Burnstein, S.; Derazne, E.; Sandler, V.; Ianculovici, C.; Halperin, T. Colonization of Candida: Prevalence among tongue-pierced and non-pierced immunocompetent adults. Oral Dis. 2010, 16, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Ledan-Muntean, S.; Tudor, B. Colonization of Candida After the Placement of a Tongue Piercing, a Case Report. Acta Med. Transilv. 2020, 25, 63–65. [Google Scholar] [CrossRef]
- Ventolini, G.; Tsai, P.; Moore, L.D. C. dubliniensis in an immunocompetent patient with metal lingual frenulum piercing. Med. Mycol. Case Rep. 2016, 14, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G. The oral microbiome in health and disease. Pharmacol. Res. 2013, 69, 137–143. [Google Scholar] [CrossRef]
- Stirn, A. Body piercing: Medical consequences and psychological motivations. Lancet 2003, 361, 1205–1215. [Google Scholar] [CrossRef]
- Levin, L.; Zadik, Y. Oral piercing: Complications and side effects. Am. J. Dent. 2007, 20, 340–344. [Google Scholar]
- Singh, A.; Verma, R.; Murari, A.; Agrawal, A. Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 2014, 18 (Suppl. S1), S81–S85. [Google Scholar] [CrossRef]
- Makhoul, I.R.; Sujov, P.; Ardekian, L.; Kassis, I.; Smolkin, T.; Abu-Elnaa’j, I.; Tamir, A.; Laufer, D. Factors influencing oral colonization in premature infants. Isr. Med. Assoc. J. 2002, 4, 98–102. [Google Scholar]
- Kinkela Devcic, M.; Simonic-Kocijan, S.; Prpic, J.; Paskovic, I.; Cabov, T.; Kovac, Z.; Glazar, I. Oral Candidal Colonization in Patients with Different Prosthetic Appliances. J. Fungi 2021, 7, 662. [Google Scholar] [CrossRef]
- Rodríguez, L.; Rosa, A.; Nastri, L.; Nastri, N.; Jewtuchowicz, V.M. Candida parapsilosis Sensu Stricto: A Pathobiont in Conditions of Oral Dysbiosis? Arch. Clin. Microbiol. 2019, 10, 96. [Google Scholar] [CrossRef]
- Richardson, J.; Moyes, D. Adaptive immune responses to Candida albicans infection. Virulence 2015, 6, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Gow, N.; van de Veerdonk, F.; Brown, A. Candida albicans morphogenesis and host defence: Discriminating invasion from colonization. Nat. Rev. Microbiol. 2012, 10, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Jewtuchowicz, V.; Mujica, M.; Brusca, M. Phenotypic and genotypic identification of Candida dubliniensis from subgingival sites in immunocompetent subjects in Argentina. Oral Microbiol. Immunol. 2008, 23, 505–509. [Google Scholar] [CrossRef]
- Alrabiah, M.; Alshagroud, R.; Alsahhaf, A. Presence of Candida species in the subgingival oral biofilm of patients with peri-implantitis. Clin. Implant Dent. Relat. Res. 2019, 21, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.T.; Knorst, J.K.; Ortiz, F.R.; Ardenghi, T.M. Scope and challenges of machine learning-based diagnosis and prognosis in clinical dentistry: A literature review. J. Clin. Transl. Res. 2021, 7, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Hao, Y.; Di Gianfilippo, R.; Sugai, J.; Li, J.; Gong, W.; Kornman, K.S.; Wang, H.; Kamada, N.; Xie, Y.; et al. Machine learning-assisted immune profiling stratifies peri-implantitis patients with unique microbial colonization and clinical outcomes. Theranostics 2021, 11, 6703–6716. [Google Scholar] [CrossRef] [PubMed]
| Factor | n | Colonizationn% | Univariate Analysis | Logistic Regression Analysis | ||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |||
| Tongue piercing | ||||||||
| No | 31 | 3 (9.67%) | 1.000 | 0.441–2.743 | 1.000 | 0.519–3.423 | ||
| Yes | 31 | 4 (12.9%) | 1.200 | 1.000 | 1.333 | 0.550 | ||
| Gender | ||||||||
| M | 10 | 3 (30%) | 1.000 | 0.949–6.749 | 1.300 | 1.172–9.472 | ||
| F | 52 | 4 (7.69%) | 2.578 | 0.069 | 3.332 | 0.024 * | ||
| Smoking | 0.434–2.758 0.533–2.851 | 0.522–3.421 1.169–9.475 | ||||||
| 0 | 26 | 1 (3.84%) | 1.200 | 0.068 | 1.000 | 0.550 | ||
| 1–10 | 20 | 2 (10%) | 1.100 | 1.332 | ||||
| >10 | 16 | 4 (25%) | 2.100 | 0.683 | 3.334 | 0.021 * | ||
| Tongue brushing | ||||||||
| Yes | 43 | 2 (4.65%) | 1.100 | 0.952–6.754 | 1.333 | 1.175–9.469 | ||
| No | 19 | 5 (26.31%) | 2.536 | 0.069 | 3.331 | 0.028 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomov, G.; Stamenov, N.; Neychev, D.; Atliev, K. Candida Carriers among Individuals with Tongue Piercing—A Real-Time PCR Study. Antibiotics 2022, 11, 742. https://doi.org/10.3390/antibiotics11060742
Tomov G, Stamenov N, Neychev D, Atliev K. Candida Carriers among Individuals with Tongue Piercing—A Real-Time PCR Study. Antibiotics. 2022; 11(6):742. https://doi.org/10.3390/antibiotics11060742
Chicago/Turabian StyleTomov, Georgi, Nikola Stamenov, Deyan Neychev, and Kiril Atliev. 2022. "Candida Carriers among Individuals with Tongue Piercing—A Real-Time PCR Study" Antibiotics 11, no. 6: 742. https://doi.org/10.3390/antibiotics11060742
APA StyleTomov, G., Stamenov, N., Neychev, D., & Atliev, K. (2022). Candida Carriers among Individuals with Tongue Piercing—A Real-Time PCR Study. Antibiotics, 11(6), 742. https://doi.org/10.3390/antibiotics11060742





