Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles—Effects of Menthol and Lactic Acid on Its Potentialization
Abstract
1. Introduction
2. Material and Methods
2.1. Bacterial Strains
2.2. Elaboration of Alginate Nanoparticles (Alg NPs)
2.3. Preparation of Alg NPs Loaded with Dermaseptin (DRS-B2) and Small Molecules (Menthol or Lactic Acid)
2.4. Preparations of Different DRS-B2 Formulations for HPLC Analyses
2.5. Determination of Minimal Inhibitory Concentrations
2.6. Hemolysis
2.7. Cytotoxicity Assay
2.8. In Vitro Stability of the Alg NPs-Based Formulations in Conditions Mimicking the Gastrointestinal Tract
2.9. Statistical Analysis
3. Results
3.1. Quantification of DRS-B2 Adsorbed on Alg NPs by HPLC
3.2. Determination of the Antibacterial Activity of Alg NPs + DRS-B2 + Small Molecules Formulations
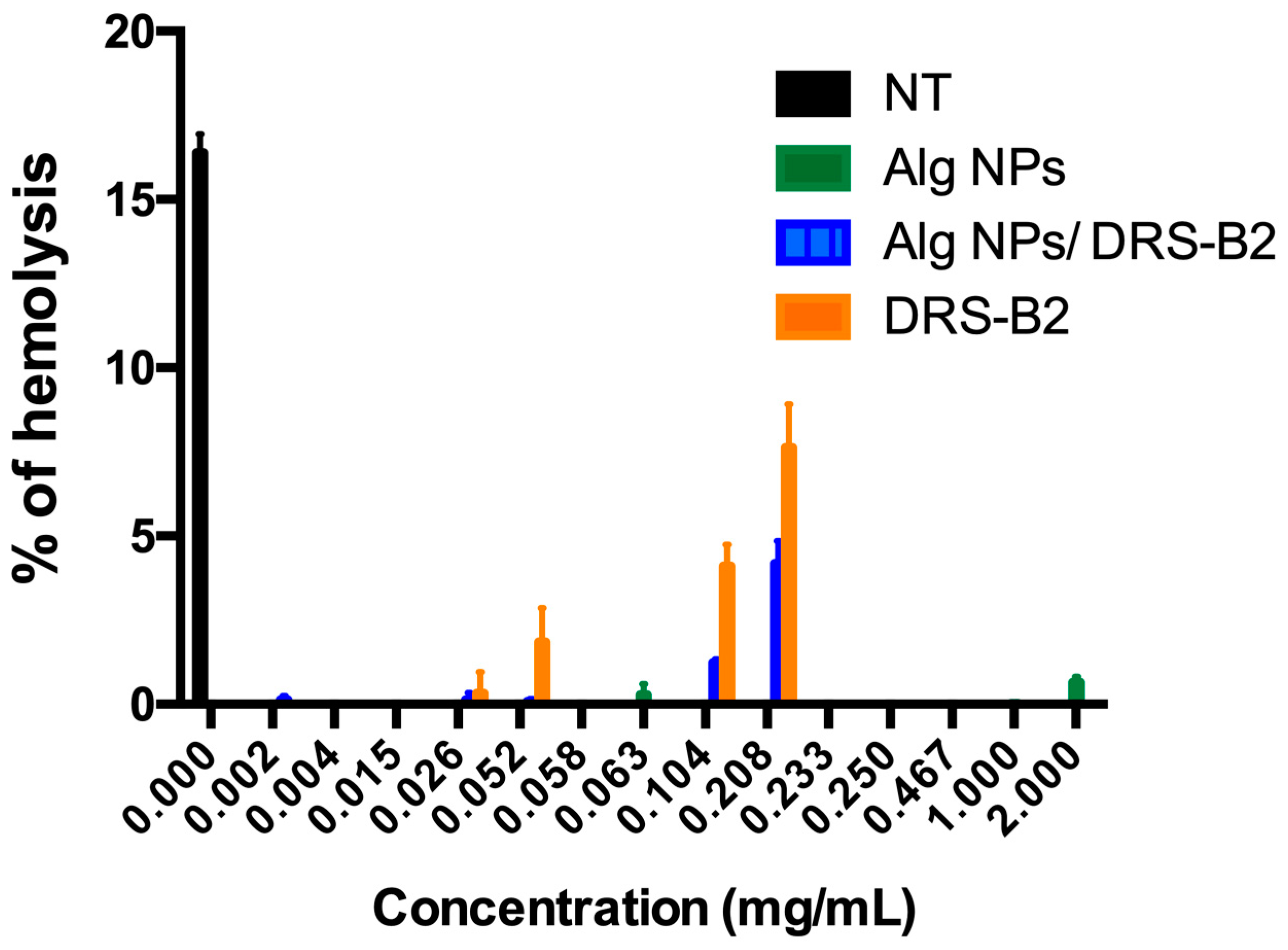
3.3. Hemolytic Activity of DRS-B2, Alg NPs, and Alg NPs + DRS-B2
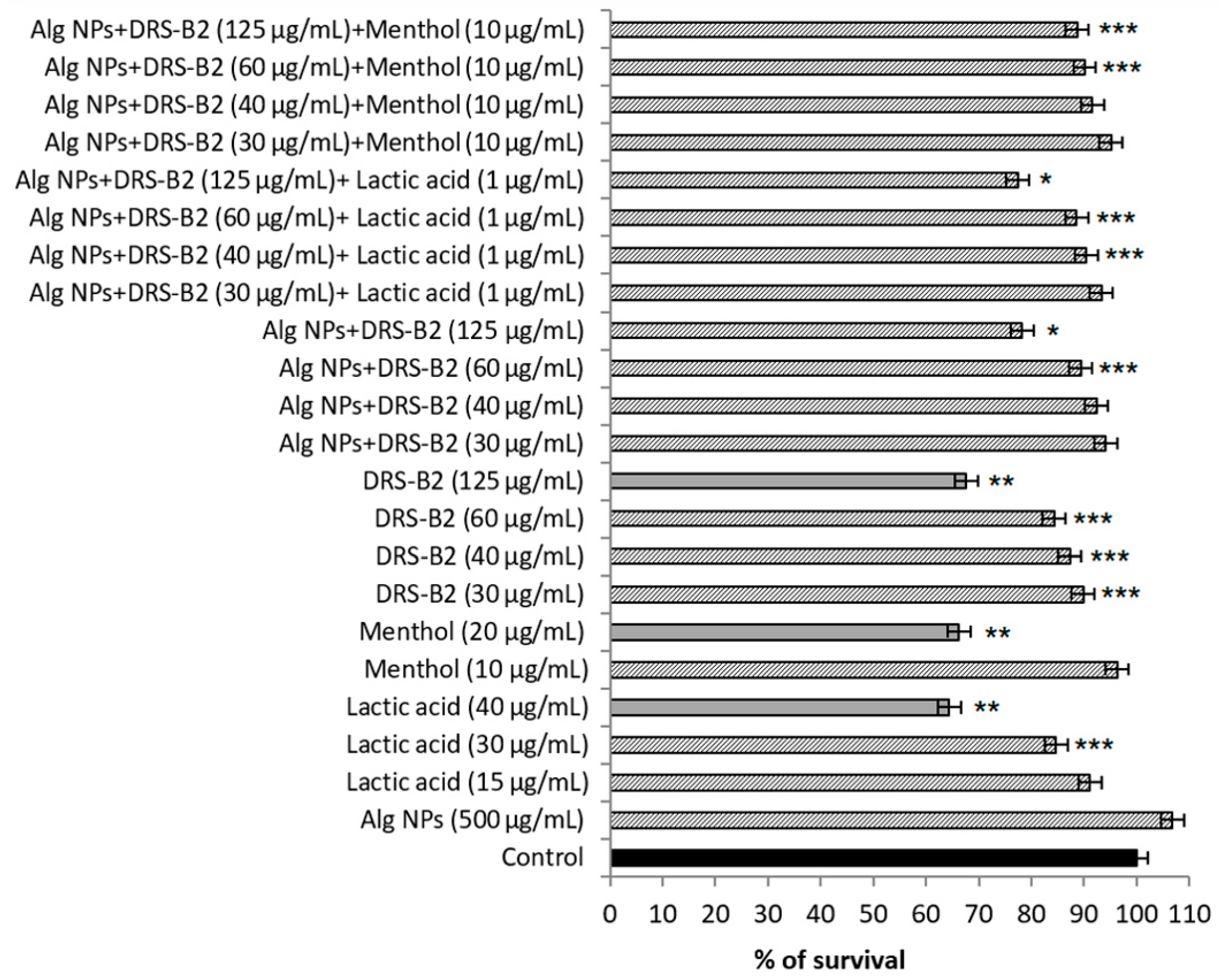
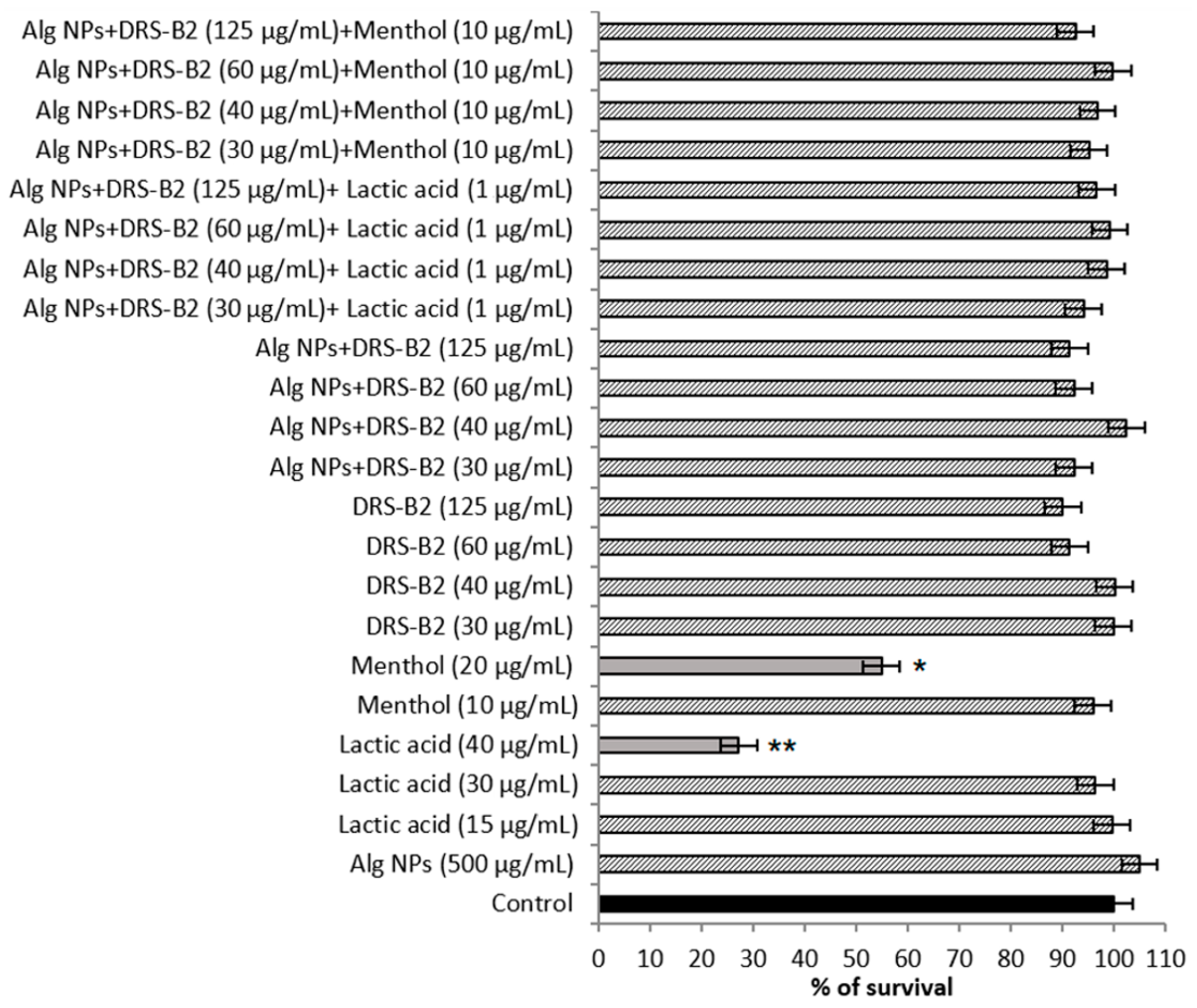
3.4. Assessment of Cytotoxicity on IPEC-1 and HT29 Cell Lines
3.5. Stability of DRS-B2 + Alginate Nanoparticles after Enzymatic Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amiche, M.; Ladram, A.; Nicolas, P. A consistent nomenclature of antimicrobial peptides isolated from frogs of the subfamily Phyllomedusinae. Peptides 2008, 29, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.J.H.; Dekker, D.; Amiche, M. Dermaseptins, Multifunctional Antimicrobial Peptides: A Review of Their Pharmacology, Effectivity, Mechanism of Action, and Possible Future Directions. Front. Pharmacol. 2019, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, P.; El Amri, C. The dermaseptin superfamily: A gene-based combinatorial library of antimicrobial peptides. Biochim. Biophys. Acta BBA-Biomembr. 2009, 1788, 1537–1550. [Google Scholar] [CrossRef]
- Mor, A.; Van Huong, N.; Delfour, A.; Migliore-Samour, D.; Nicolas, P. Isolation, amino acid sequence and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 1991, 30, 8824–8830. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Hani, K.; Nicolas, P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J. Biol. Chem. 1994, 269, 31635–31641. [Google Scholar] [CrossRef]
- Amiche, M.; Ducancel, F.; Lajeunesse, E.; Boulain, J.; Menez, A.; Nicolas, P. Molecular Cloning of a cDNA Encoding the Precursor of Adenoregulin from Frog Skin: Relationships with the Vertebrate Defensive Peptides, Dermaseptins. Biochem. Biophys. Res. Commun. 1993, 191, 983–990. [Google Scholar] [CrossRef]
- Amiche, M.; Ducancel, F.; Mor, A.; Boulain, J.; Menez, A.; Nicolas, P. Precursors of vertebrate peptide antibiotics dermaseptin b and adenoregulin have extensive sequence identities with precursors of opioid peptides dermorphin, dermenkephalin, and deltorphins. J. Biol. Chem. 1994, 269, 17847–17852. [Google Scholar] [CrossRef]
- Daly, J.W.; Caceres, J.; Moni, R.W.; Gusovsky, F.; Moos, M.; Seamon, K.B.; Milton, K.; Myers, C.W. Frog secretions and hunting magic in the upper Amazon: Identification of a peptide that interacts with an adenosine receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 10960–10963. [Google Scholar] [CrossRef]
- Galanth, C.; Abbassi, F.; Lequin, O.; Ayala-Sanmartin, J.; Ladram, A.; Nicolas, P.; Amiche, M. Mechanism of Antibacterial Action of Dermaseptin B2: Interplay between Helix−Hinge−Helix Structure and Membrane Curvature Strain. Biochemistry 2009, 48, 313–327. [Google Scholar] [CrossRef]
- Charpentier, S.; Amiche, M.; Mester, J.; Vouille, V.; Le Caer, J.-P.; Nicolas, P.; Delfour, A. Structure, Synthesis, and Molecular Cloning of Dermaseptins B, a Family of Skin Peptide Antibiotics. J. Biol. Chem. 1998, 273, 14690–14697. [Google Scholar] [CrossRef]
- Galanth, C. Etude Du Mécanisme d’action d’un Peptide Cationique Antibactérien, La Dermaseptine B2. Ph.D. Thesis, Université Pierre et Marie-Curie, Paris, France, 2009. [Google Scholar]
- Ghosh, J.K.; Shaool, D.; Guillaud, P.; Cicéron, L.; Mazier, D.; Kustanovich, I.; Shai, Y.; Mor, A. Selective Cytotoxicity of Dermaseptin S3 toward IntraerythrocyticPlasmodium falciparum and the Underlying Molecular Basis. J. Biol. Chem. 1997, 272, 31609–31616. [Google Scholar] [CrossRef] [PubMed]
- Krugliak, M.; Feder, R.; Zolotarev, V.Y.; Gaidukov, L.; Dagan, A.; Ginsburg, H.; Mor, A. Antimalarial Activities of Dermaseptin S4 Derivatives. Antimicrob. Agents Chemother. 2000, 44, 2442–2451. [Google Scholar] [CrossRef] [PubMed]
- Dagan, A.; Efron, L.; Gaidukov, L.; Mor, A.; Ginsburg, H. In Vitro Antiplasmodium Effects of Dermaseptin S4 Derivatives. Antimicrob. Agents Chemother. 2002, 46, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.R.S.A.; Brand, G.D.; Silva, L.P.; Kückelhaus, S.A.S.; Bento, W.R.C.; Araújo, A.L.T.; Martins, G.R.; Lazzari, A.M.; Bloch, C. Dermaseptins from Phyllomedusa oreades and Phyllomedusa distincta: Secondary structure, antimicrobial activity, and mammalian cell toxicity. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 336–343. [Google Scholar] [CrossRef]
- Morton, C.O.; dos Santos, S.C.; Coote, P. An Amphibian-Derived, Cationic, Alpha-Helical Antimicrobial Peptide Kills Yeast by Caspase-Independent but AIF-Dependent Programmed Cell Death. Mol. Microbiol. 2007, 65, 494–507. [Google Scholar] [CrossRef]
- Soltaninejad, H.; Zare-Zardini, H.; Ordooei, M.; Ghelmani, Y.; Ghadiri-Anari, A.; Mojahedi, S.; Hamidieh, A.A. Antimicrobial Peptides from Amphibian Innate Immune System as Potent Antidiabetic Agents: A Literature Review and Bioinformatics Analysis. J. Diabetes Res. 2021, 2021, 2894722. [Google Scholar] [CrossRef]
- Chaves, R.X.; Quelemes, P.V.; Leite, L.M.; Aquino, D.S.; Amorim, L.V.; Rodrigues, K.A.; Campelo, Y.D.; Veras, L.M.; Bemquerer, M.P.; Ramos-Jesus, J.; et al. Antileishmanial and Immunomodulatory Effects of Dermaseptin-01, A Promising Peptide Against Leishmania amazonensis. Curr. Bioact. Compd. 2017, 13, 305–311. [Google Scholar] [CrossRef]
- Tønnesen, H.H.; Karlsen, J. Alginate in Drug Delivery Systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Bidhendi, S.; Zolfagharian, H.; Dounighi, N.; Saraei, F.; Khaki, P.; Inanlou, F. Design and Evaluate Alginate Nanoparticles As a Protein Delivery System; Archives of Razi Institute: Karaj, Iran, 2013; pp. 139–146. [Google Scholar]
- Gombotz, W.R.; Wee, S.F. Protein release from alginate matrices. Adv. Drug Deliv. Rev. 1998, 31, 267–285. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a useful natural polymer for microencapsulation and therapeutic applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe Vera Hydrogel Films for Biomedical Applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Zgheib, H.; Belguesmia, Y.; Boukherroub, R.; Drider, D. Alginate Nanoparticles Enhance Anti-Clostridium perfringens Activity of the Leaderless Two-Peptide Enterocin DD14 and Affect Expression of Some Virulence Factors. Probiotics Antimicrob. Proteins 2021, 13, 1213–1227. [Google Scholar] [CrossRef] [PubMed]
- Belguesmia, Y.; Hazime, N.; Kempf, I.; Boukherroub, R.; Drider, D. New Bacteriocins from Lacticaseibacillus paracasei CNCM I-5369 Adsorbed on Alginate Nanoparticles Are Very Active against Escherichia coli. Int. J. Mol. Sci. 2020, 21, 8654. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of Antibacterial Action of Three Monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Dos Santos, C.; Hamadat, S.; Le Saux, K.; Newton, C.; Mazouni, M.; Zargarian, L.; Miro-Padovani, M.; Zadigue, P.; Delbé, J.; Hamma-Kourbali, Y.; et al. Studies of the antitumor mechanism of action of dermaseptin B2, a multifunctional cationic antimicrobial peptide, reveal a partial implication of cell surface glycosaminoglycans. PLoS ONE 2017, 12, e0182926. [Google Scholar] [CrossRef]
- CLSI M100Ed30|Performance Standards for Antimicrobial Susceptibility Testing, 30th Edition. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 30 April 2020).
- Couty, M.; Dusaud, M.; Miro-Padovani, M.; Zhang, L.; Zadigue, P.; Zargarian, L.; Lequin, O.; de la Taille, A.; Delbe, J.; Hamma-Kourbali, Y.; et al. Antitumor Activity and Mechanism of Action of Hormonotoxin, an LHRH Analog Conjugated to Dermaseptin-B2, a Multifunctional Antimicrobial Peptide. Int. J. Mol. Sci. 2021, 22, 11303. [Google Scholar] [CrossRef]
- Belguesmia, Y.; Madi, A.; Sperandio, D.; Merieau, A.; Feuilloley, M.; Prevost, H.; Drider, D.; Connil, N. Growing insights into the safety of bacteriocins: The case of enterocin S37. Res. Microbiol. 2011, 162, 159–163. [Google Scholar] [CrossRef]
- Zhong, R.Z.; Xia, J.Q.; Sun, H.; Qin, G.X. Effects of different sources of protein on the growth performance, blood chemistry and polypeptide profiles in the gastrointestinal tract digesta of newly weaned piglets. J. Anim. Physiol. Anim. Nutr. 2017, 101, e312–e322. [Google Scholar] [CrossRef]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.M.B.; Dias, S.C.; Franco, O.L. Antimicrobial Peptides and Nanotechnology, Recent Advances and Challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef]
- Drider, D.; Boukherroub, R.; Le Devendec, L.; Belguesmia, Y.; Hazime, N.; Mourand, G.; Paboeuf, F.; Kempf, I. Impact of colistin and colistin-loaded on alginate nanoparticles on pigs infected with a colistin-resistant enterotoxigenic Escherichia coli strain. Veter. Microbiol. 2022, 266, 109359. [Google Scholar] [CrossRef] [PubMed]
- Hofman, D.L.; Van Buul, V.J.; Brouns, F.J.P.H. Nutrition, Health, and Regulatory Aspects of Digestible Maltodextrins. Crit. Rev. Food Sci. Nutr. 2016, 56, 2091–2100. [Google Scholar] [CrossRef] [PubMed]
- Youta, C.L.D. Utilisation de Nanoparticules pour Délivrer des Protéines dans les Épithéliums Respiratoires: Caractérisation des Mécanismes Impliqués. Ph.D. Thesis, Université du Droit et de la Santé, Lille, France, 2012. [Google Scholar]
- Nicolas, J.; Couvreur, P. Les nanoparticules polymères pour la délivrance de principes actifs anticancéreux. Med. Sci. 2017, 33, 11–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murata, Y.; Jinno, D.; Liu, D.; Isobe, T.; Kofuji, K.; Kawashima, S. The Drug Release Profile from Calcium-induced Alginate Gel Beads Coated with an Alginate Hydrolysate. Molecules 2007, 12, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chi, Y.-Q.; Yu, C.-H.; Xie, Y.; Xia, M.-Y.; Zhang, C.-L.; Han, X.; Peng, Q. Drug-free and non-crosslinked chitosan scaffolds with efficient antibacterial activity against both Gram-negative and Gram-positive bacteria. Carbohydr. Polym. 2020, 241, 116386. [Google Scholar] [CrossRef]
- Rojo, L.; Barcenilla, J.M.; Vázquez, B.; González, R.; Román, J.S. Intrinsically Antibacterial Materials Based on Polymeric Derivatives of Eugenol for Biomedical Applications. Biomacromolecules 2008, 9, 2530–2535. [Google Scholar] [CrossRef]
- Patel, T.; Ishiuji, Y.; Yosipovitch, G. Menthol: A refreshing look at this ancient compound. J. Am. Acad. Dermatol. 2007, 57, 873–878. [Google Scholar] [CrossRef]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and antiplasmid activities of essential oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef]
- de Billerbeck, V.-G. Huiles essentielles et bactéries résistantes aux antibiotiques. Phytothérapie 2007, 5, 249–253. [Google Scholar] [CrossRef]
- Turcheniuk, V.; Raks, V.; Issa, R.; Cooper, I.R.; Cragg, P.J.; Jijie, R.; Dumitrascu, N.; Mikhalovska, L.I.; Barras, A.; Zaitsev, V.; et al. Antimicrobial activity of menthol modified nanodiamond particles. Diam. Relat. Mater. 2015, 57, 2–8. [Google Scholar] [CrossRef]
- Vigan, M. Essential oils: Renewal of interest and toxicity. Eur. J. Dermatol. 2010, 20, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential Oils as a Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef] [PubMed]
- Génin, G. L’acide lactique et ses applications. Le Lait 1960, 40, 27–37. [Google Scholar] [CrossRef]
- Pohanka, M. D-Lactic Acid as a Metabolite: Toxicology, Diagnosis, and Detection. BioMed Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef]
- Lidon, F.; Silva, M.M. An Overview on Applications and Side Effects of Antioxidant Food Additives. Emir. J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Polyak, B.; Geresh, A.S.; Marks, R.S. Synthesis and Characterization of a Biotin-Alginate Conjugate and Its Application in a Biosensor Construction. Biomacromolecules 2004, 5, 389–396. [Google Scholar] [CrossRef]
- Nossol, C.; Barta-Böszörményi, A.; Kahlert, S.; Zuschratter, W.; Faber-Zuschratter, H.; Reinhardt, N.; Ponsuksili, S.; Wimmers, K.; Diesing, A.-K.; Rothkötter, H.-J. Comparing Two Intestinal Porcine Epithelial Cell Lines (IPECs): Morphological Differentiation, Function and Metabolism. PLoS ONE 2015, 10, e0132323. [Google Scholar] [CrossRef]
- Martínez-Maqueda, D.; Miralles, B.; Recio, I. HT29 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; ISBN 978-3-319-15791-7. [Google Scholar]
| Formulations (on Basis of DRS-B2) | (DRS-B2) µg/mL | (Alg NPs) µg/mL | (Lactic Acid) µg/mL | (Menthol) µg/mL |
|---|---|---|---|---|
| F1 | 125 | - | - | - |
| F2 | 125 | 500 | - | - |
| F3 | 125 | - | 1 | - |
| F4 | 125 | - | - | 10 |
| F5 | 125 | 500 | - | 10 |
| F6 | 125 | 500 | 1 | - |
| F7 | 60 | - | - | - |
| F8 | 60 | 500 | - | - |
| F9 | 30 | - | - | - |
| F10 | 30 | 500 | - | - |
| F11 | 40 | 500 | - | - |
| F12 | 40 | 500 | - | - |
| F13 | 40 | 500 | - | 10 |
| F14 | 40 | 500 | 1 | - |
| F15 | 40 | 500 | - | 10 |
| F16 | 40 | 500 | 1 | - |
| F17 | 30 | 500 | - | 10 |
| F18 | 30 | 500 | 1 | - |
| F19 | 30 | 500 | - | 10 |
| F20 | 30 | 1 | - |
| (DRS-B2) (µg/mL) before Dialysis | Peak Area before Dialysis | Peak Are after Dialysis (Y) | (DRS-B2) (µg/mL) after Dialysis (X) * | ** Percentage of Initial DRS-B2 Ad Sorbed on Alg NPs |
|---|---|---|---|---|
| 199 | 4,091,385 | 3,954,214 | 198.4 | 40 |
| 225 | 4,521,891 | 3,998,514 | 200.6 | 40 |
| 250 | 5,149,931 | 4,031,252 | 202.3 | 40.4 |
| MIC (µg/mL) | |||
|---|---|---|---|
| Formulations | (DRS-B2) | E. coli 184 | E. coli ATCC8739 |
| (DRS-B2) µg/mL | - | 7.5 | 3.75 |
| Alg NPs (500 µg/mL) | 125 | 3.91 | 1.95 |
| + | 40 | 2.5 | 1.25 |
| (DRS-B2) µg/mL | 30 | 3.75 | 1.87 |
| MIC (µg/mL) | |||
|---|---|---|---|
| Formulations | (DRS-B2) | E. coli 184 | E. coli ATCC8739 |
| Alg NPs (500 µg/mL) | 125 | 1.95 | 0.97 |
| + | |||
| (DRS-B2) µg/mL | 40 | 1.25 | 0.62 |
| + | |||
| Menthol (10 µg/mL) | 30 | 1.87 | 0.94 |
| MIC (µg/mL) | |||
|---|---|---|---|
| Formulations | (DRS-B2) | E. coli 184 | E. coli ATCC8739 |
| Alg NPs (500 µg/mL) | 125 | 1.95 | 0.97 |
| + | |||
| (DRS-B2) µg/mL | 40 | 1.25 | 0.62 |
| + | |||
| Lactic acid (1 µg/mL) | 30 | 1.87 | 0.94 |
| MICs of DRS-B2 (µg/mL) | ||||
|---|---|---|---|---|
| Enzymes Mix | Without Treatment | Treatment with pH Variation Only * (Incubation 30 min at pH 3 and Then 2 h at pH 6) | Treatment with Pepsin * (Incubation 30 min at pH 3 with Pepsin and Then 2 h at pH 6) | Treatment with Digestive Enzymes * (Incubation 30 min at pH 3 with Pepsin and Then 2h at pH 6 with Trypsin + Chymotrypsin) |
| DRS-B2 (40 µg/mL) | 7.5 | 10 | ≥10 | ≥10 |
| Alg NPs (500 µg/mL) | 2.5 | 2.5 | 5 | 10 |
| + | ||||
| DRS-B2 (40 µg/mL) | ||||
| Alg NPs (500 µg/mL) | 1.25 | 1.25 | 2.5 | 2.5 |
| + | ||||
| DRS-B2 (40 µg/mL) | ||||
| + | ||||
| Lactic acid (1 µg/mL) | ||||
| Alg NPs (500 µg/mL) | 1.25 | 1.25 | 2.5 | 2.5 |
| + | ||||
| DRS-B2 (40 µg/mL) | ||||
| + | ||||
| Menthol (10 µg/mL) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazime, N.; Belguesmia, Y.; Barras, A.; Amiche, M.; Boukherroub, R.; Drider, D. Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles—Effects of Menthol and Lactic Acid on Its Potentialization. Antibiotics 2022, 11, 787. https://doi.org/10.3390/antibiotics11060787
Hazime N, Belguesmia Y, Barras A, Amiche M, Boukherroub R, Drider D. Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles—Effects of Menthol and Lactic Acid on Its Potentialization. Antibiotics. 2022; 11(6):787. https://doi.org/10.3390/antibiotics11060787
Chicago/Turabian StyleHazime, Noura, Yanath Belguesmia, Alexandre Barras, Mohamed Amiche, Rabah Boukherroub, and Djamel Drider. 2022. "Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles—Effects of Menthol and Lactic Acid on Its Potentialization" Antibiotics 11, no. 6: 787. https://doi.org/10.3390/antibiotics11060787
APA StyleHazime, N., Belguesmia, Y., Barras, A., Amiche, M., Boukherroub, R., & Drider, D. (2022). Enhanced Antibacterial Activity of Dermaseptin through Its Immobilization on Alginate Nanoparticles—Effects of Menthol and Lactic Acid on Its Potentialization. Antibiotics, 11(6), 787. https://doi.org/10.3390/antibiotics11060787








