Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Microscopic Examination
2.3. Urine Culture
2.4. Identification of Bacterial Isolates
2.5. Antimicrobial Susceptibility
2.6. Statistical Analysis
3. Results
3.1. Characterization of Patients with Bacterial UTI
3.2. Bacteria Implicated in UTI
3.3. Antimicrobial Resistance Patterns of the Main Bacteria Implicated in UTI
3.4. Calculated Bacterial Resistance to Recommended Antimicrobials
3.5. Multidrug-Resistant Bacteria Implicated in UTI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McLellan, L.K.; Hunstad, D.A. Urinary Tract Infection: Pathogenesis and Outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes: Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.E. Clinical Presentations and Epidemiology of Urinary Tract Infections. Microbiol. Spectr. 2016, 4, 4–5. [Google Scholar] [CrossRef]
- Smelov, V.; Naber, K.; Bjerklund Johansen, T.E. Improved Classification of Urinary Tract Infection: Future Considerations. Eur. Urol. Suppl. 2016, 15, 71–80. [Google Scholar] [CrossRef]
- Karam, M.R.A.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef]
- Ny, S.; Edquist, P.; Dumpis, U.; Gröndahl-Yli-Hannuksela, K.; Hermes, J.; Kling, A.-M.; Klingeberg, A.; Kozlov, R.; Källman, O.; Lis, D.O.; et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2019, 17, 25–34. [Google Scholar] [CrossRef]
- Lee, D.S.; Lee, S.-J.; Choe, H.-S. Community-Acquired Urinary Tract Infection by Escherichia coli in the Era of Antibiotic Resistance. BioMed Res. Int. 2018, 2018, 7656752. [Google Scholar] [CrossRef]
- Kolman, K.B. Cystitis and Pyelonephritis: Diagnosis, Treatment, and Prevention. Prim. Care Clin. Off. Pract. 2019, 46, 191–202. [Google Scholar] [CrossRef]
- Wei Tan, C.; Chlebicki, M.P. Urinary tract infections in adults. Singap. Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagenlehner, F.; Mezei, T.; Pilatz, A.; et al. EUA Guidelines on Urological Infections; EAU Guidelines Office: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Linhares, I.; Raposo, T.; Rodrigues, A.; Almeida, A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: A ten-year surveillance study (2000–2009). BMC Infect. Dis. 2013, 13, 19. [Google Scholar] [CrossRef] [PubMed]
- The European Committe on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 9.0. 2019. Available online: http://www.eucast.org, (accessed on 1 May 2022).
- Costa, T.; Linhares, I.; Ferreira, R.; Neves, J.; Almeida, A. Frequency and Antibiotic Resistance of Bacteria Implicated in Community Urinary Tract Infections in North Aveiro Between 2011 and 2014. Microb. Drug Resist. 2018, 24, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Curto, C.; Rosendo, I.; Santiago, L. Perfil de Sensibilidade aos Antibióticos na Infeção Urinária em Ambulatório no Distrito de Coimbra: Um Estudo Transversal. Acta Med. Port. 2019, 32, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Passadouro, R.; Fonseca, R.; Figueiredo, F.; Lopes, A.; Fernandes, C. Avaliação do Perfil de Sensibilidade aos Antibióticos na Infeção Urinária da Comunidade. Acta Med. Port. 2014, 27, 737–742. [Google Scholar] [CrossRef]
- Amna, M.A.; Chazan, B.; Raz, R.; Edelstein, H.; Colodner, R. Risk factors for non-Escherichia coli community-acquired bacteriuria. Infection 2013, 41, 473–477. [Google Scholar] [CrossRef]
- Shackley, D.C.; Whytock, C.; Parry, G.; Clarke, L.; Vincent, C.; Harrison, A.; John, A.; Provost, L.; Power, M. Variation in the prevalence of urinary catheters: A profile of National Health Service patients in England. BMJ Open 2017, 7, e013842. [Google Scholar] [CrossRef]
- Prieto, J.; Wilson, J.; Bak, A.; Denton, A.; Flores, A.; Lusardi, G.; Reid, M.; Shepherd, L.; Whittome, N.; Loveday, H. A prevalence survey of patients with indwelling urinary catheters on district nursing caseloads in the United Kingdom: The Community Urinary Catheter Management (CCaMa) Study. J. Infect. Prev. 2020, 21, 129–135. [Google Scholar] [CrossRef]
- Cole, S.J.; Records, A.R.; Orr, M.W.; Linden, S.B.; Lee, V.T. Catheter-Associated Urinary Tract Infection by Pseudomonas aeruginosa Is Mediated by Exopolysaccharide-Independent Biofilms. Infect. Immun. 2014, 82, 2048–2058. [Google Scholar] [CrossRef]
- Lara-Isla, A.; Medina-Polo, J.; Alonso-Isa, M.; Benítez-Sala, R.; Sopeña-Sutil, R.; Justo-Quintas, J.; Gil-Moradillo, J.; González-Padilla, D.A.; García-Rojo, E.; Passas-Martínez, J.B.; et al. Urinary Infections in Patients with Catheters in the Upper Urinary Tract: Microbiological Study. Urol. Int. 2017, 98, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Tien, B.Y.Q.; Goh, H.M.S.; Chong, K.K.L.; Bhaduri-Tagore, S.; Holec, S.; Dress, R.; Ginhoux, F.; Ingersoll, M.A.; Williams, R.B.H.; Kline, K.A. Enterococcus faecalis Promotes Innate Immune Suppression and Polymicrobial Catheter-Associated Urinary Tract Infection. Infect. Immun. 2017, 85, e00378-17. [Google Scholar] [CrossRef] [PubMed]
- Gaston, J.R.; Andersen, M.J.; Johnson, A.O.; Bair, K.L.; Sullivan, C.M.; Guterman, L.B.; White, A.N.; Brauer, A.L.; Learman, B.S.; Flores-Mireles, A.L.; et al. Enterococcus faecalis Polymicrobial Interactions Facilitate Biofilm Formation, Antibiotic Recalcitrance, and Persistent Colonization of the Catheterized Urinary Tract. Pathogens 2020, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Grigoryan, L.; Trautner, B. Urinary Tract Infection. Ann. Intern. Med. 2017, 167, 49–64. [Google Scholar] [CrossRef]
- Rowe, T.A.; Juthani-Mehta, M. Diagnosis and Management of Urinary Tract Infection in Older Adults. Infect. Dis. Clin. N. Am. 2014, 28, 75–89. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Zanichelli, V.; Huttner, A.; Harbarth, S.; Kronenberg, A.; Huttner, B. Antimicrobial resistance trends in Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis urinary isolates from Switzerland: Retrospective analysis of data from a national surveillance network over an 8-year period (2009–2016). Swiss Med. Wkly. 2019, 149, 25–33. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Babiker, A.; Master, R.N.; Luu, T.; Mathur, A.; Bordon, J. Antibiotic Resistance among Urinary Isolates from Female Outpatients in the United States in 2003 and 2012. Antimicrob. Agents Chemother. 2016, 60, 2680–2683. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.W.; Trautner, B.W.; Jump, R. Urinary Tract Infection and Asymptomatic Bacteriuria in Older Adults. Infect. Dis. Clin. N. Am. 2017, 31, 673–688. [Google Scholar] [CrossRef]
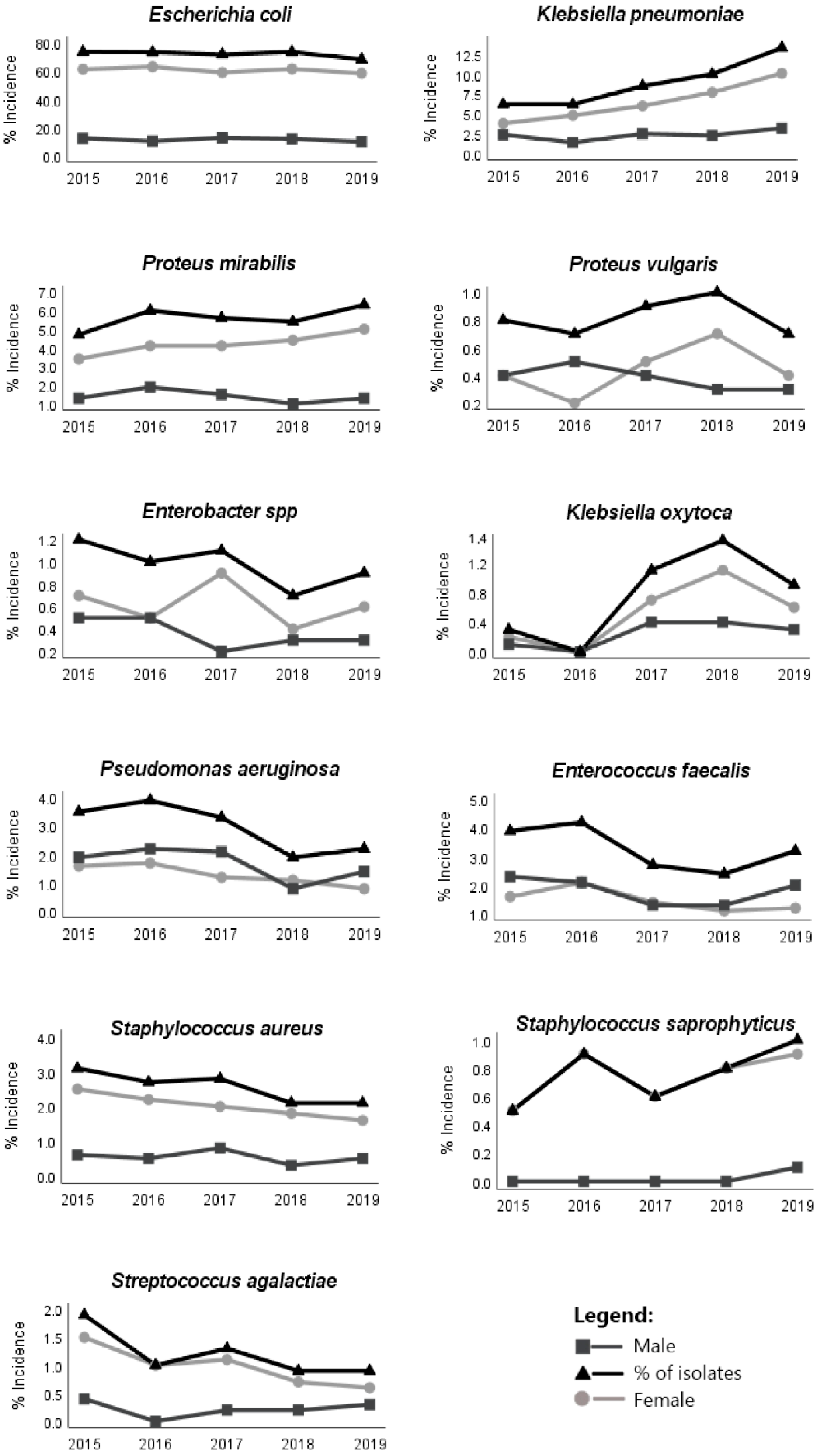
| Children | Adolescents | Young Adults | Adults | Elderly | Isolates in the 5 Years (%) 1 (N = 15,439) | Females (%) 1 (N = 11,959) | Males (%) 1 (N = 3066) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–12 Years | 13–18 Years | 19–34 Years | 35–64 Years | >65 Years | ||||||||||||||
| Total 1 | F 2 | M 2 | Total 1 | F 2 | M 2 | Total 1 | F 2 | M 2 | Total 1 | F 2 | M 2 | Total 1 | F 2 | M 2 | ||||
| Bacteria | N = 282 | n = 232 | n = 50 | N = 157 | n = 147 | n = 10 | N = 1277 | n = 1206 | n = 70 | N = 4753 | n = 4044 | n = 709 | N = 8556 | n = 6330 | n = 2226 | |||
| E. coli | 74.8 | 67.4 3 | 7.4 | 63.1 | 59.3 3 | 3.8 | 75.3 | 71.1 3 | 4.2 | 77.1 4 | 67.2 3 | 9.9 | 68.7 | 55.2 3 | 13.5 | 70.1 | 76.2 3 | 55.6 |
| K.pneumoniae | 1.8 | 1.1 | 0.7 | 6.4 | 5.8 3 | 0.6 | 4.5 | 4.1 3 | 0.4 | 6.2 | 4.9 3 | 1.3 | 11.7 4 | 8.3 3 | 3.4 | 8.9 | 8.4 | 11.7 3 |
| P. mirabilis | 16.3 4 | 8.9 | 7.4 | 10.2 | 9.6 3 | 0.6 | 5.3 | 5.2 3 | 0.1 | 5.2 | 4.4 3 | 0.8 | 5.5 | 3.0 3 | 2.5 | 5.5 | 5.4 | 6.7 3 |
| P. vulgaris | 1.1 | 0.4 | 0.7 | 0.6 | 0.6 | 0.0 | 0.1 | 0.1 | 0.0 | 0.4 | 0.2 | 0.2 | 1.2 4 | 0.7 | 0.5 | 0.8 | 0.6 | 1.9 |
| Enterobacter spp. | 0.0 | 0.0 | 0.0 | 1.3 4 | 1.3 | 0.0 | 0.4 | 0.4 | 0.0 | 0.8 | 0.6 3 | 0.2 | 1.2 | 0.6 | 0.6 | 0.9 | 0.8 | 1.8 3 |
| K. oxytoca | 0.4 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.3 | 0.2 | 0.1 | 0.5 | 0.4 3 | 0.1 | 1.1 4 | 0.7 3 | 0.4 | 0.8 | 0.7 | 1.3 3 |
| P.aeruginosa | 2.1 | 1.8 | 0.4 | 0.0 | 0.0 | 0.0 | 0.2 | 0.1 | 0.1 | 1.3 | 0.3 3 | 1.3 | 4.2 4 | 1.9 | 2.3 | 2.8 | 1.6 | 8.1 3 |
| E. faecalis | 1.1 | 0.7 | 0.4 | 0.6 | 0.6 | 0.0 | 2.5 | 2.1 3 | 0.4 | 2.1 | 1.2 | 0.9 | 4.1 4 | 1.5 3 | 2.6 | 3.2 | 1.8 | 8.8 3 |
| S. aureus | 1.8 | 1.8 | 0.0 | 14.0 4 | 12.7 3 | 1.3 | 7.0 | 6.8 3 | 0.2 | 3.5 | 3.2 3 | 0.3 | 1.2 | 0.4 3 | 0.8 | 2.5 | 2.5 | 2.8 3 |
| S.saprophyticus | 0.7 | 0.7 | 0.0 | 3.2 4 | 3.2 | 0.0 | 3.1 | 3.0 3 | 0.1 | 1.3 | 1.3 3 | 0.0 | 0.1 | 0.1 | 0.0 | 0.8 | 1.0 3 | 0.2 |
| S. agalactiae | 0.0 | 0.0 | 0.0 | 0.6 | 0.6 | 0.0 | 1.3 | 1.2 3 | 0.1 | 1.6 4 | 1.4 3 | 0.2 | 1.0 | 0.7 3 | 0.3 | 1.1 | 1.2 3 | 1.1 |
| Total (%) | 1.9 | 1.6 | 0.3 | 1.0 | 0.9 | 0.1 | 8.5 | 8.0 | 0.5 | 31.6 | 26.9 | 4.7 | 56.9 | 42.1 | 14.8 | - | 79.6 | 20.4 |
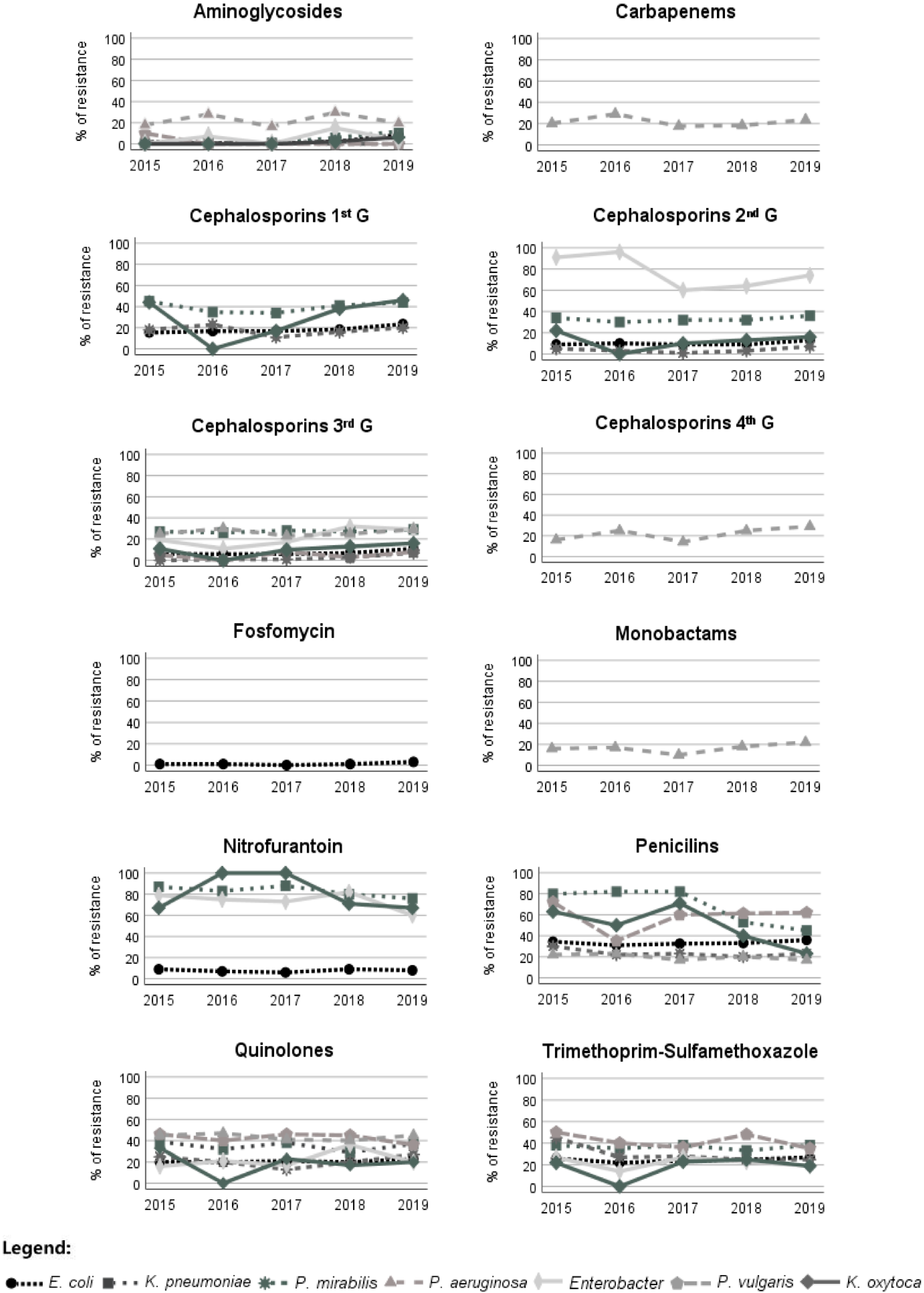
| Antimicrobial Group | Antimicrobials | E. coli | K. pneumoniae | P. mirabilis | P. vulgaris | Enterobacter spp. | K. oxytoca | P. aeruginosa | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | F | M | N | % | F | M | N | % | F | M | N | % | F | M | N | % | F | M | N | % | F | M | N | % | F | M | |||
| Aminoglycosides | Amikacin | 7246 | 1.6 | 1.5 * | 2.4 | 952 | 3.9 | 3.3 | 5.2 | 573 | 0.7 | 0.6 | 0.0 | 100 | 2.0 | 0.0 | 3.3 | 139 | 4.3 | 2.3 | 7.5 | 104 | 1.9 | 1.4 | 3.1 | 424 | 17.3 | 13.6 | 19.9 | |
| Gentamicin | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 425 | 31.1 | 27.9 | 33.3 | ||
| Tobramycin | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 416 | 21.2 | 15.5 * | 25.2 | ||
| β-lactam | Carbapenems | Imipenem | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 430 | 15.3 | 17.4 * | 26.0 |
| Cephalosporins (first G) | Cefazolin | 9474 | 14.9 | 13.1 * | 24.7 | 1105 | 39.3 | 31.8 * | 58.6 | 737 | 15.2 | 14.0 | 18.8 | 111 | 100.0 | 100.0 | 100.0 | 125 | 100.0 | 100.0 | 100.0 | 102 | 32.4 | 28.2 | 41.9 | - | - | - | - | |
| Cephalosporins (second G) | Cefuroxime | 10,814 | 10.0 | 8.5 * | 17.9 | 1368 | 33.4 | 26.7 * | 52.2 | 848 | 4.0 | 3.3 | 6.3 | 124 | 100.0 | 100.0 | 100.0 | 144 | 77.8 | 75.6 | 81.5 | 120 | 16.7 | 15.0 | 20.0 | - | - | - | - | |
| Cephalosporins (third G) | Cefotaxime | 10,796 | 7.2 | 6.1 * | 13.2 | 1360 | 28.8 | 22.8 * | 45.7 | 844 | 1.5 | 1.6 | 1.5 | 123 | 4.9 | 4.5 | 5.6 | 142 | 21.1 | 13.5 * | 34.0 | 120 | 12.5 | 10.0 | 17.5 | - | - | - | - | |
| Ceftazidime | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 431 | 26.9 | 24.3 | 28.9 | ||
| Cephalosporins (fourth G) | Cefepime | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 431 | 20.6 | 19.5 | 21.5 | |
| Monobactams | Aztreonam | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 428 | 16.4 | 17.5 | 25.5 | |
| Penicillins | Amoxicillin | 10,816 | 46.3 | 44.1 * | 57.7 | 1366 | 100.0 | 100.0 | 100.0 | 847 | 36.6 | 34.3 * | 43.7 | 124 | 100.0 | 100.0 | 100.0 | 144 | 100.0 | 100.0 | 100.0 | 120 | 100.0 | 100.0 | 100.0 | - | - | - | - | |
| AMX-CLA | 10,807 | 20.3 | 18.8 * | 28.5 | 1365 | 69.4 | 65.7 * | 79.7 | 846 | 9.9 | 7.8 * | 16.6 | 124 | 58.9 | 56.1 | 62.1 | 144 | 100.0 | 100.0 | 100.0 | 120 | 55.0 | 51.3 | 62.5 | - | - | - | - | ||
| PIP-TAZ | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 432 | 20.1 | 17.3 | 22.3 | ||
| Quinolones | Ciprofloxacin | 10,489 | 20.5 | 18.0 * | 33.7 | 1351 | 34.6 | 27.8 * | 53.7 | 788 | 21.6 | 18.6 * | 31.0 | 120 | 42.5 | 42.2 | 42.9 | 142 | 21.1 | 11.4 * | 37.0 | 119 | 20.2 | 16.3 | 28.2 | 430 | 44.0 | 39.9 | 47.0 | |
| Miscellaneous agents | Nitrofurantoin | 10,804 | 7.0 | 6.4 * | 10.2 | 1273 | 81.7 | 79.8 * | 86.8 | 843 | 100.0 | 100.0 | 100.0 | 124 | 100.0 | 100.0 | 100.0 | 138 | 73.9 | 72.1 | 76.9 | 113 | 77.9 | 73.7 | 86.5 | - | - | - | - | |
| Fosfomycin | 9829 | 1.4 | 1.4 | 1.4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | ||
| SXT | 10,811 | 24.8 | 23.3 * | 33.1 | 1365 | 36.5 | 30.4 * | 53.3 | 846 | 28.8 | 28.2 | 30.7 | 124 | 41.9 | 47.0 | 36.2 | 144 | 22.2 | 15.6 * | 33.3 | 120 | 22.5 | 18.8 | 30.0 | - | - | - | - | ||
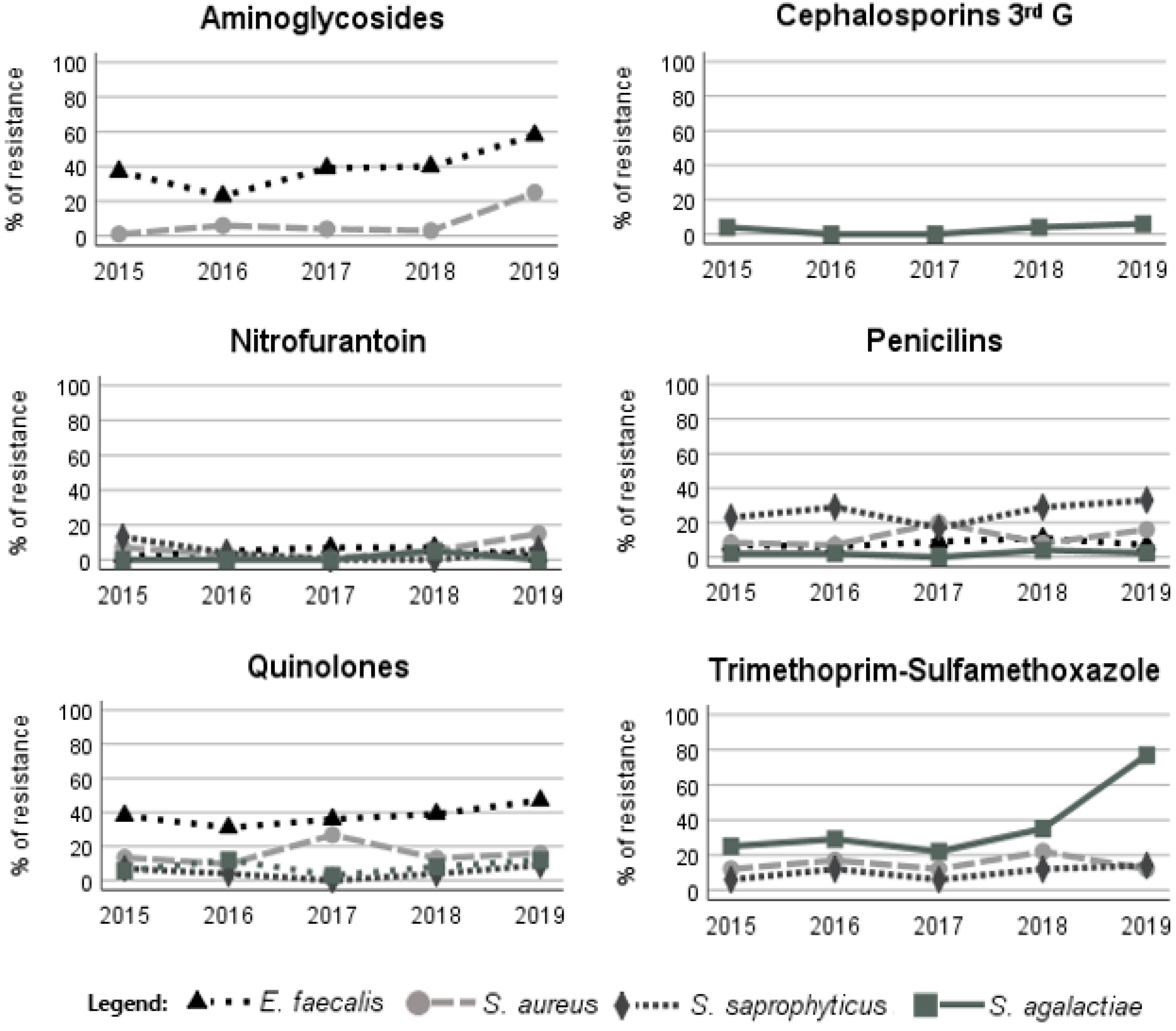
| Antimicrobial Group | Antimicrobials | E. faecalis | S. aureus | S. saprophyticus | S. agalactiae | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | F | M | N | % | F | M | N | % | F | M | N | % | F | M | |||
| Aminoglycosides | Gentamicin | 473 | 37.8 | 33.0 | 41.8 | 270 | 4.1 | 2.0 * | 10.4 | - | - | - | - | - | - | - | - | |
| β-lactam | Cephalosporins (third G) | Cefotaxime | - | - | - | - | - | - | - | - | - | - | - | - | 176 | 2.8 | 2.8 | 2.9 |
| Penicillins | Ampicillin | 465 | 7.5 | 8.1 | 7.1 | - | - | - | - | - | - | - | - | 175 | 1.1 | 0.7 | 3.0 | |
| Amoxicillin | 436 | 8.3 | 8.2 | 8.3 | - | - | - | - | 103 | 48.5 | 47.4 | 66.7 | 166 | 2.4 | 2.3 | 3.0 | ||
| AMX-CLA | - | - | - | - | 379 | 11.6 | 6.4 * | 29.8 | 120 | 6.7 | 6.1 | 16.7 | - | - | - | - | ||
| Quinolones | Ciprofloxacin | 485 | 46.4 | 43.1 | 49.1 | 363 | 15.7 | 10.3 * | 33.7 | 114 | 5.3 | 4.7 | 14.3 | - | - | - | - | |
| Levofloxacin | 472 | 38.3 | 29.0 * | 45.8 | - | - | - | - | - | - | - | - | 173 | 7.5 | 7.1 | 9.4 | ||
| Miscellaneous agents | Nitrofurantoin | 470 | 3.2 | 3.3 | 3.1 | 374 | 5.9 | 2.7 * | 17.7 | 120 | 4.2 | 3.5 | 14.3 | 146 | 0.7 | 0.9 | 0.0 | |
| SXT | - | - | - | - | 383 | 14.6 | 9.7 * | 31.8 | 120 | 10.8 | 10.5 | 16.7 | 134 | 39.6 | 38.0 | 46.2 | ||
| Resistance to First-Line Therapy | |||||
|---|---|---|---|---|---|
| Bacteria | Incidence | FOM (%) | FOM (%) 1 | NIT (%) | NIT (%) 1 |
| E. coli | 70.1 | 1.4 | 1.0 | 7.0 | 4.9 |
| K. pneumoniae | 8.9 | - | - | 81.7 | 7.3 |
| P. mirabilis | 5.5 | - | - | 100.0 | 5.4 |
| E. faecalis | 3.2 | - | - | 3.2 | 0.1 |
| S. aureus | 2.5 | - | - | 5.9 | 0.1 |
| S. agalactiae | 1.1 | - | - | 0.7 | 0.0 |
| Enterobacter spp. | 0.9 | - | - | 73.9 | 0.7 |
| P. vulgaris | 0.8 | - | - | 100.0 | 0.8 |
| S. saprophyticus | 0.8 | - | - | 4.2 | 0.0 |
| K. oxytoca | 0.8 | - | - | 77.9 | 0.6 |
| Average (%) | - | - | 1.0 | - | 19.9 |
| Resistance to Alternative Therapy | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacteria | Incidence | CEP 1st (%) | CEP 1st (%) 1 | CEP 2nd (%) | CEP 2nd (%) 1 | CEP 3rd (%) | CEP 3rd (%) 1 | QUI (%) | QUI (%) 1 | AMX-CLA (%) | AMX-CLA (%) 1 | SXT (%) | SXT (%) 1 |
| E. coli | 70.1 | 14.9 | 10.4 | 10.0 | 7.0 | 7.2 | 5.0 | 20.5 | 14.4 | 20.3 | 14.2 | 24.8 | 17.4 |
| K. pneumoniae | 8.9 | 39.3 | 3.5 | 33.4 | 3.3 | 28.8 | 2.6 | 34.6 | 3.1 | 69.4 | 6.2 | 36.5 | 3.2 |
| P. mirabilis | 5.5 | 15.2 | 0.8 | 4.0 | 0.2 | 1.5 | 0.0 | 21.6 | 1.2 | 9.9 | 0.5 | 28.8 | 1.6 |
| E. faecalis | 3.2 | - | - | - | - | - | - | 42.4 | 1.4 | - | - | - | - |
| P. aeruginosa | 2.8 | - | - | - | - | - | - | 44.0 | 1.2 | - | - | - | - |
| S. aureus | 2.5 | - | - | - | - | - | - | 15.7 | 0.4 | 11.6 | 0.3 | 14.6 | 0.4 |
| S. agalactiae | 1.1 | - | - | - | - | 2.8 | 0.0 | 7.5 | 0.1 | - | - | 39.6 | 0.4 |
| Enterobacter spp. | 0.9 | 100.0 | 0.9 | 77.8 | 0.7 | 21.1 | 0.2 | 21.1 | 0.2 | 100.0 | 0.9 | 22.2 | 0.2 |
| P. vulgaris | 0.8 | 100.0 | 0.8 | 100.0 | 0.8 | 4.9 | 0.0 | 42.5 | 0.3 | 58.9 | 0.5 | 41.9 | 0.3 |
| S. saprophyticus | 0.8 | - | - | - | - | - | - | 5.3 | 0.0 | 6.7 | 0.1 | 10.8 | 0.1 |
| K. oxytoca | 0.8 | 32.4 | 0.3 | 16.7 | 0.1 | 12.5 | 0.1 | 20.2 | 0.2 | 55.0 | 0.4 | 22.5 | 0.2 |
| Average (%) | - | - | 16.7 | - | 12.1 | - | 7.9 | - | 22.5 | - | 23.1 | - | 23.8 |
| Bacteria in UTI | % of MDR Isolates |
|---|---|
| E. coli | 23.3 |
| K. pneumoniae | 40.4 |
| P. mirabilis | 10.0 |
| P. vulgaris | 19.4 |
| Enterobacter spp. | 29.9 |
| K. oxytoca | 24.2 |
| P. aeruginosa | 34.7 |
| S. aureus | 18.3 |
| S. saprophyticus | 5.8 |
| S. agalactiae | 6.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.; Costa, E.; Freitas, A.; Almeida, A. Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics 2022, 11, 768. https://doi.org/10.3390/antibiotics11060768
Silva A, Costa E, Freitas A, Almeida A. Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics. 2022; 11(6):768. https://doi.org/10.3390/antibiotics11060768
Chicago/Turabian StyleSilva, Andreia, Elisabeth Costa, Américo Freitas, and Adelaide Almeida. 2022. "Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections" Antibiotics 11, no. 6: 768. https://doi.org/10.3390/antibiotics11060768
APA StyleSilva, A., Costa, E., Freitas, A., & Almeida, A. (2022). Revisiting the Frequency and Antimicrobial Resistance Patterns of Bacteria Implicated in Community Urinary Tract Infections. Antibiotics, 11(6), 768. https://doi.org/10.3390/antibiotics11060768







