Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation
Abstract
:1. Introduction
2. Experiment
2.1. Chemicals and Reagents
2.2. Hydrothermal Syntesis of BiVO4
2.3. Characterization
2.4. Photodegradation Study
2.4.1. Photocatalytic Degradation of the Antibiotics
2.4.2. Study of the Photocatalytic Degradation Mechanism
2.4.3. Cycling Ability of the Photocatalyst
3. Results and Discussion
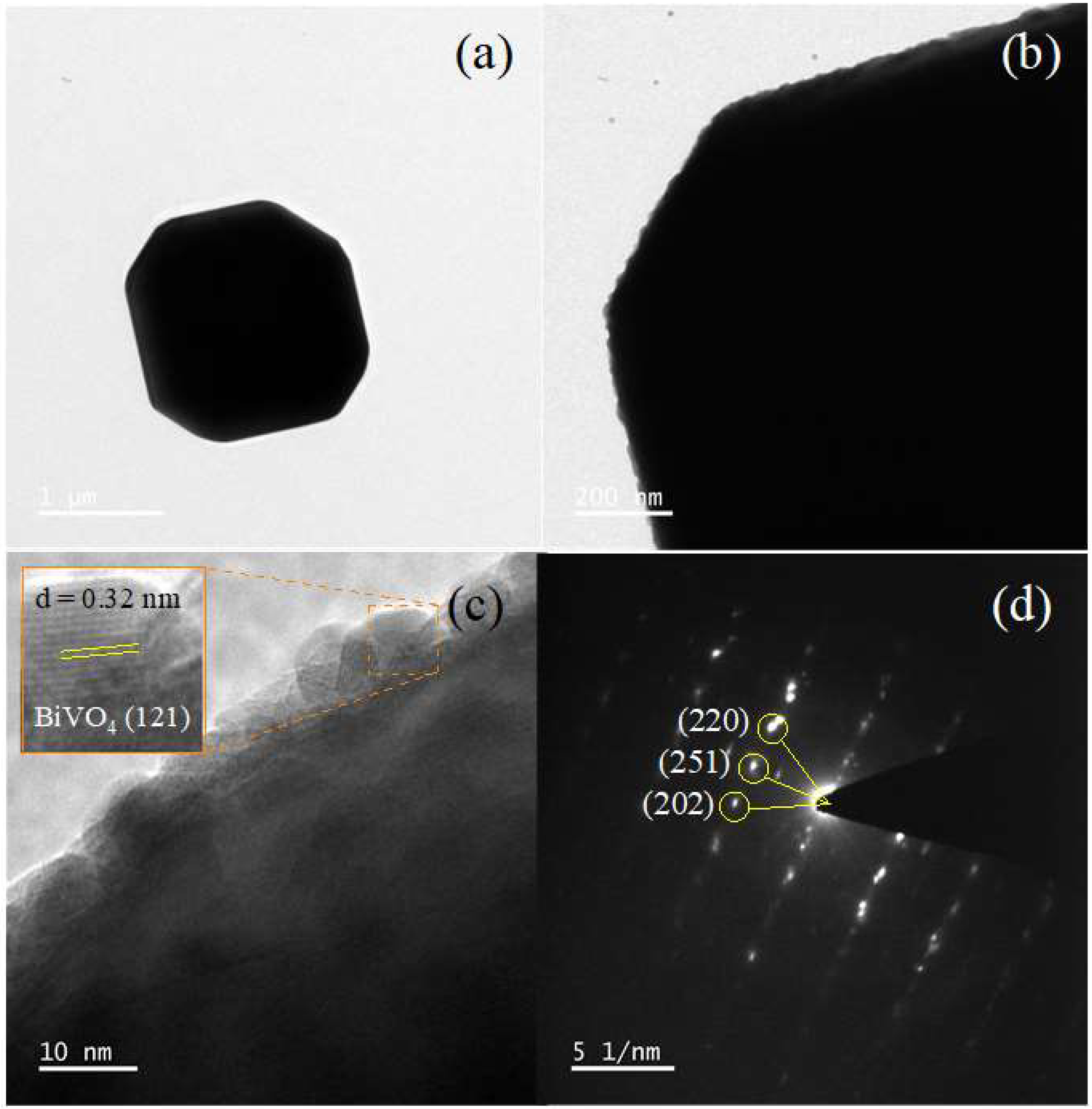
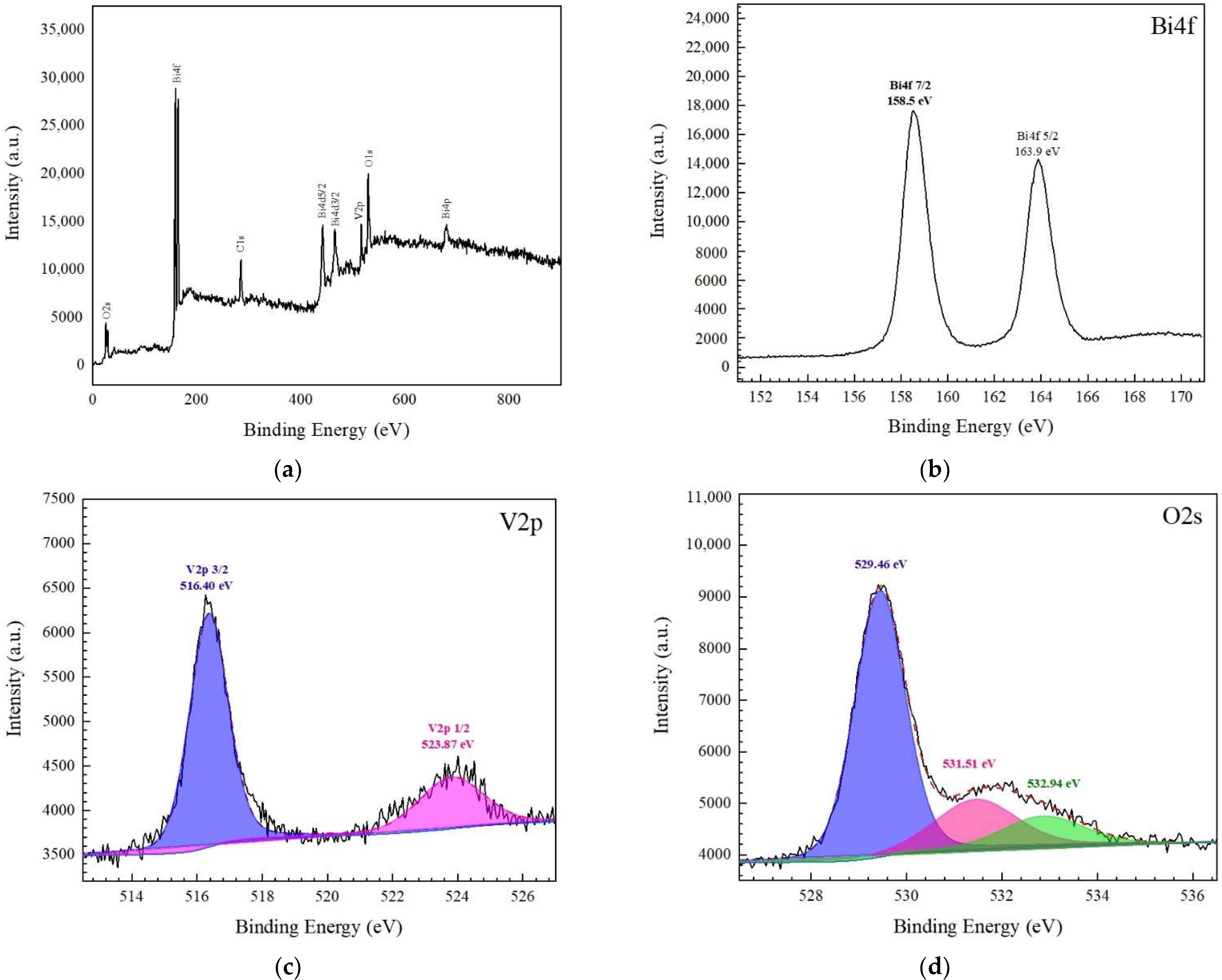
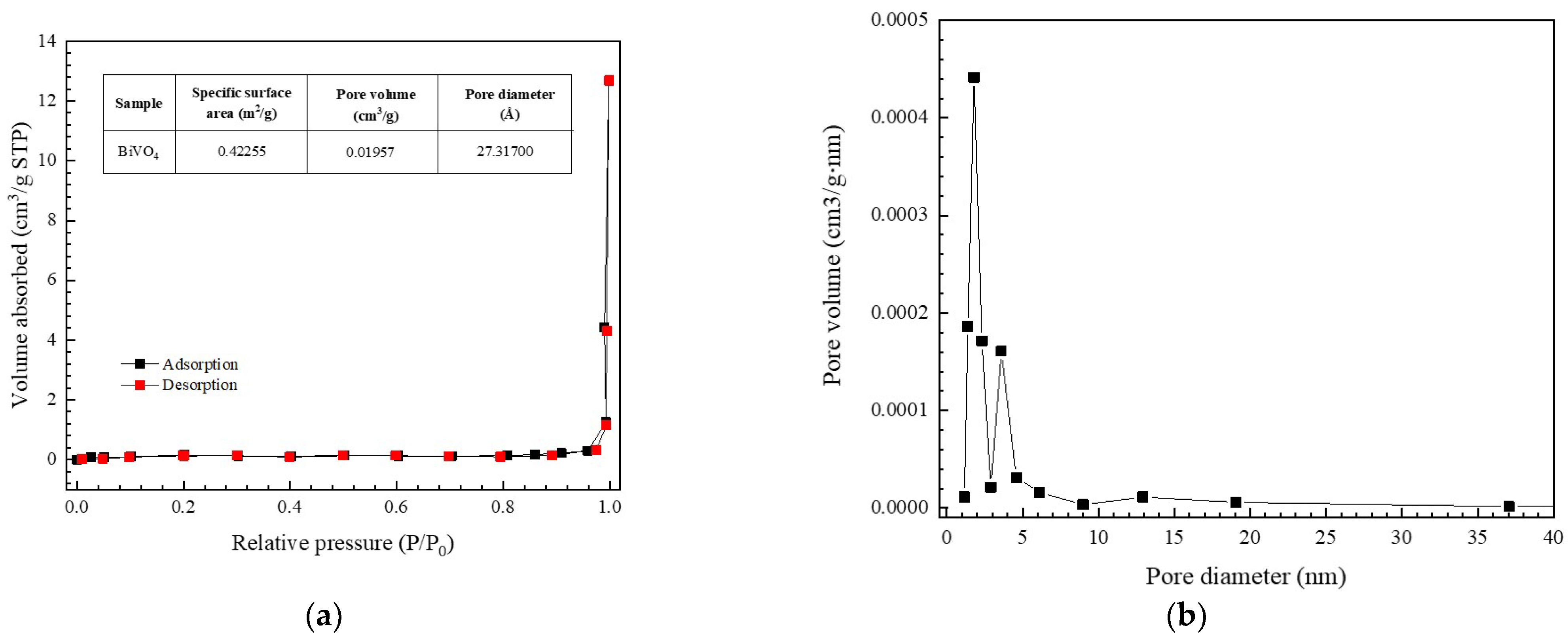
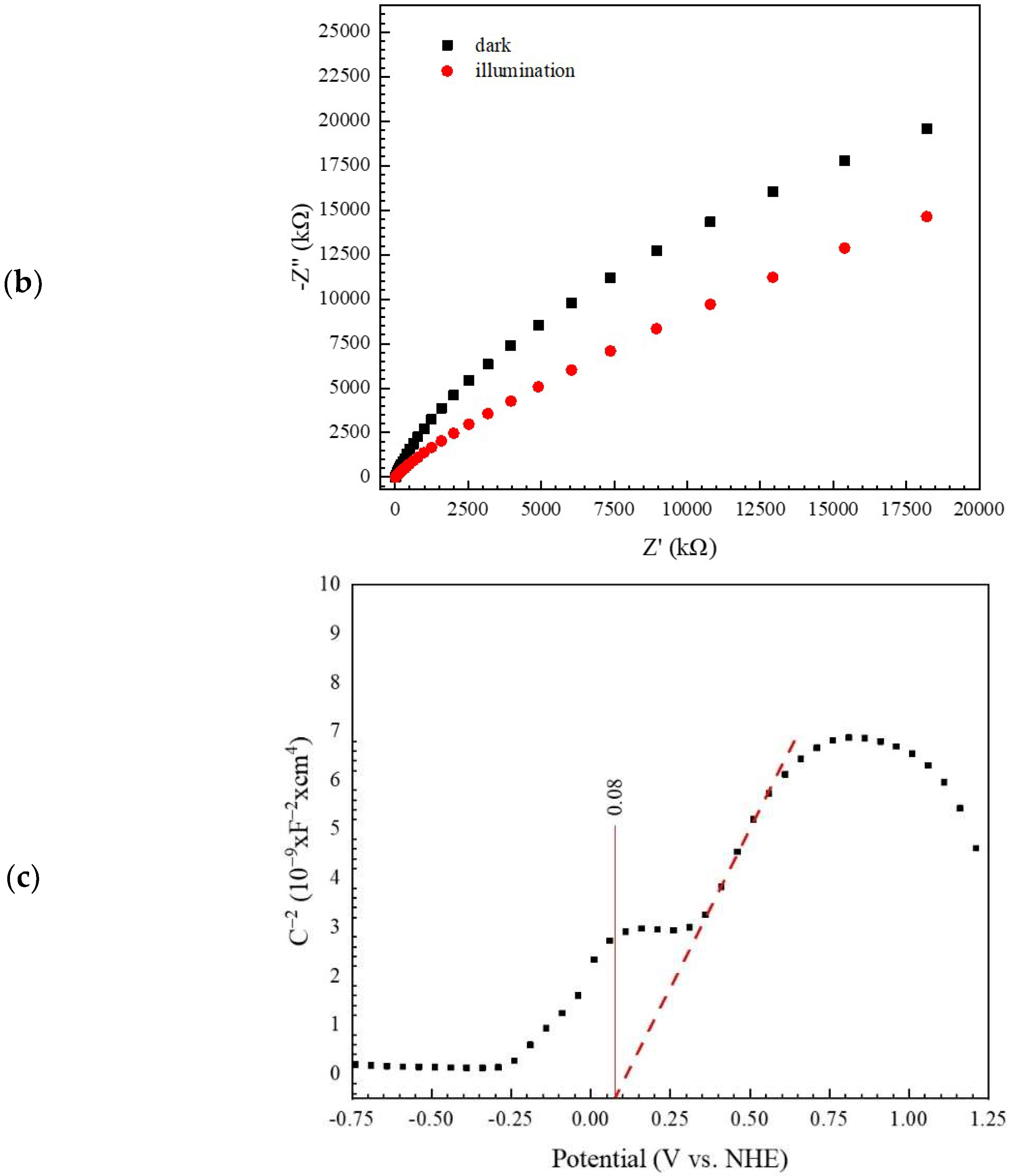
3.1. Characterization of the BiVO4 Catalyst
3.2. Photocatalytic Degradation of the Antibiotics
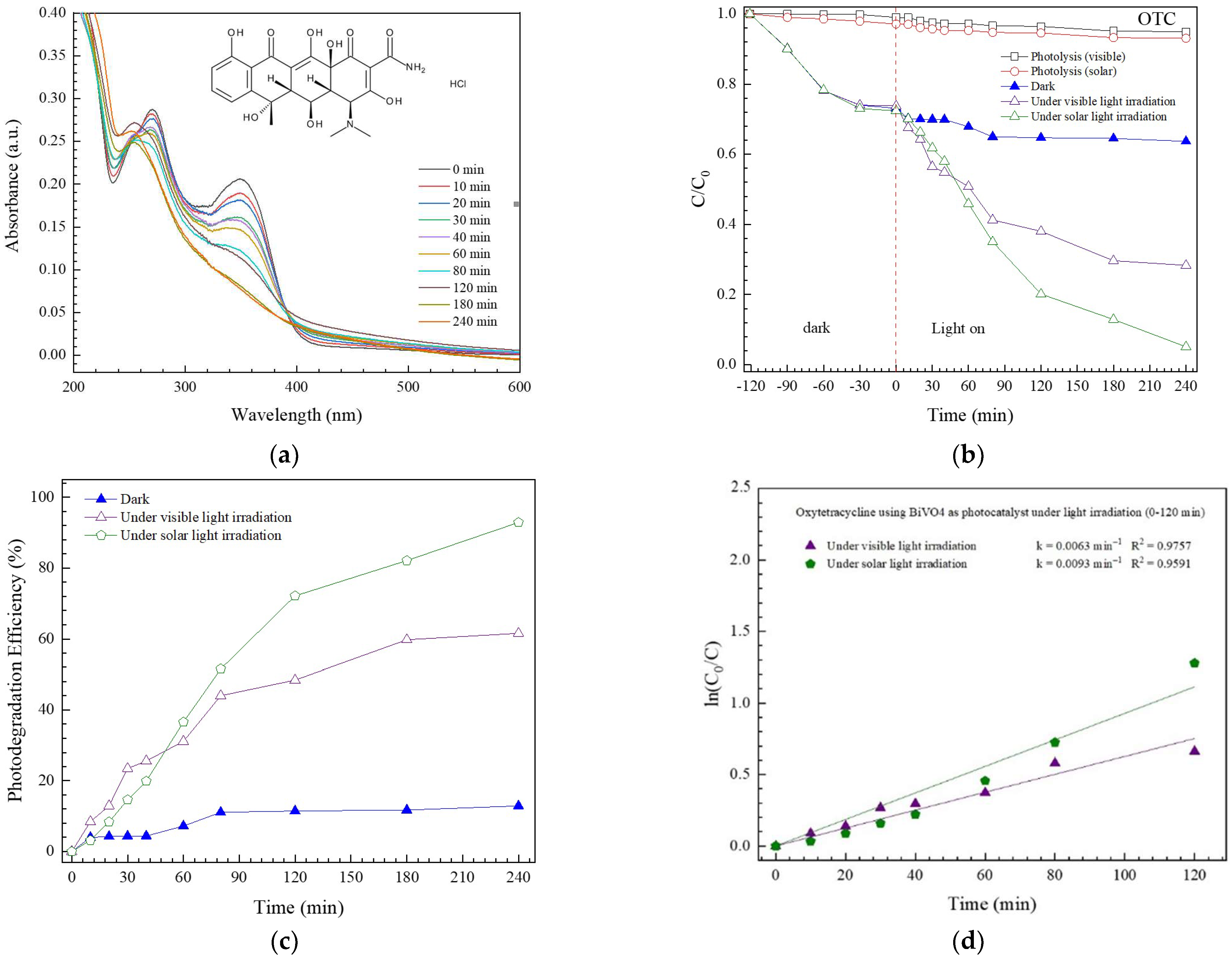
3.2.1. Photodegradation of OTC Antibiotic
3.2.2. Photodegradation of TC Antibiotic
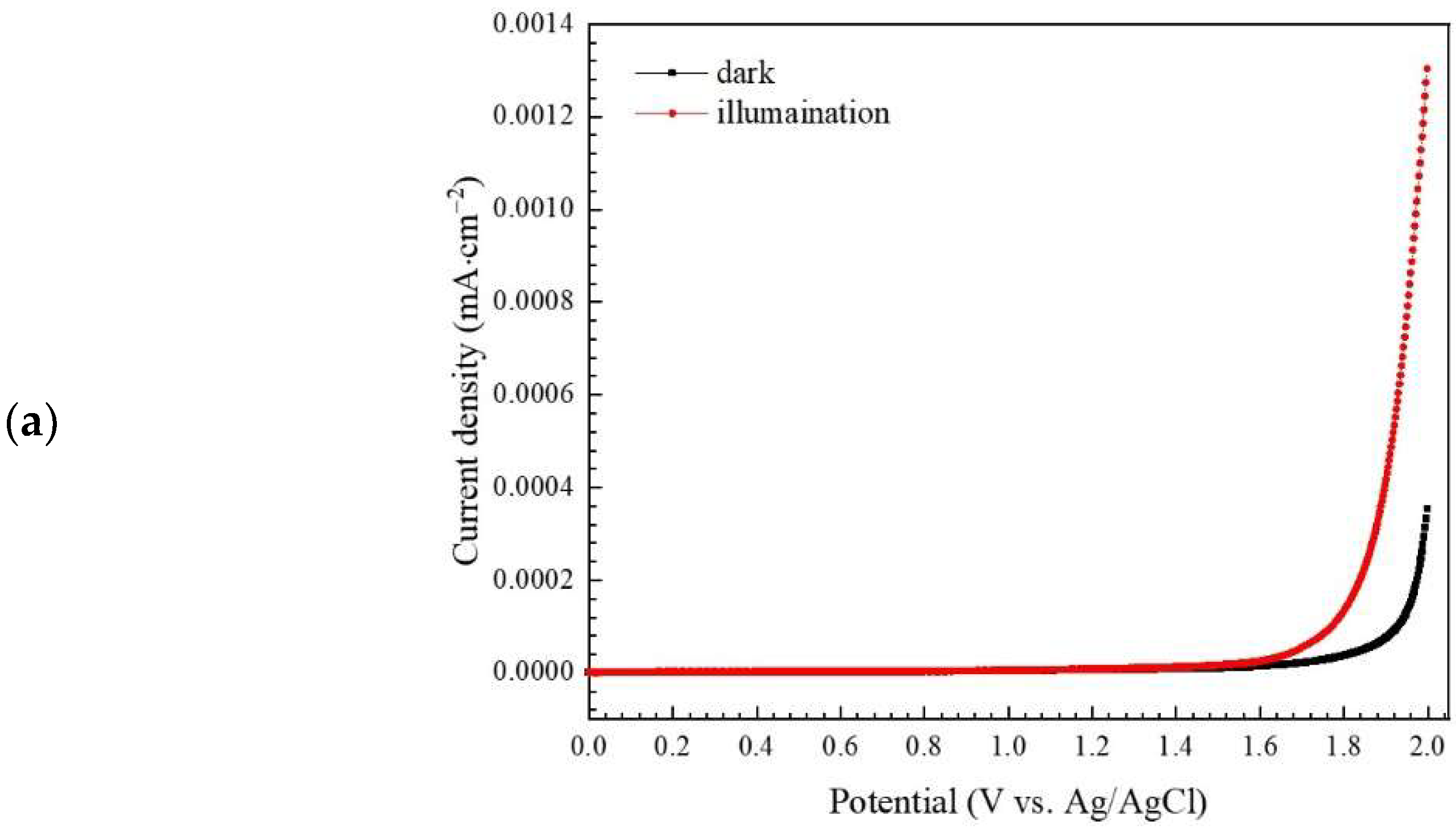
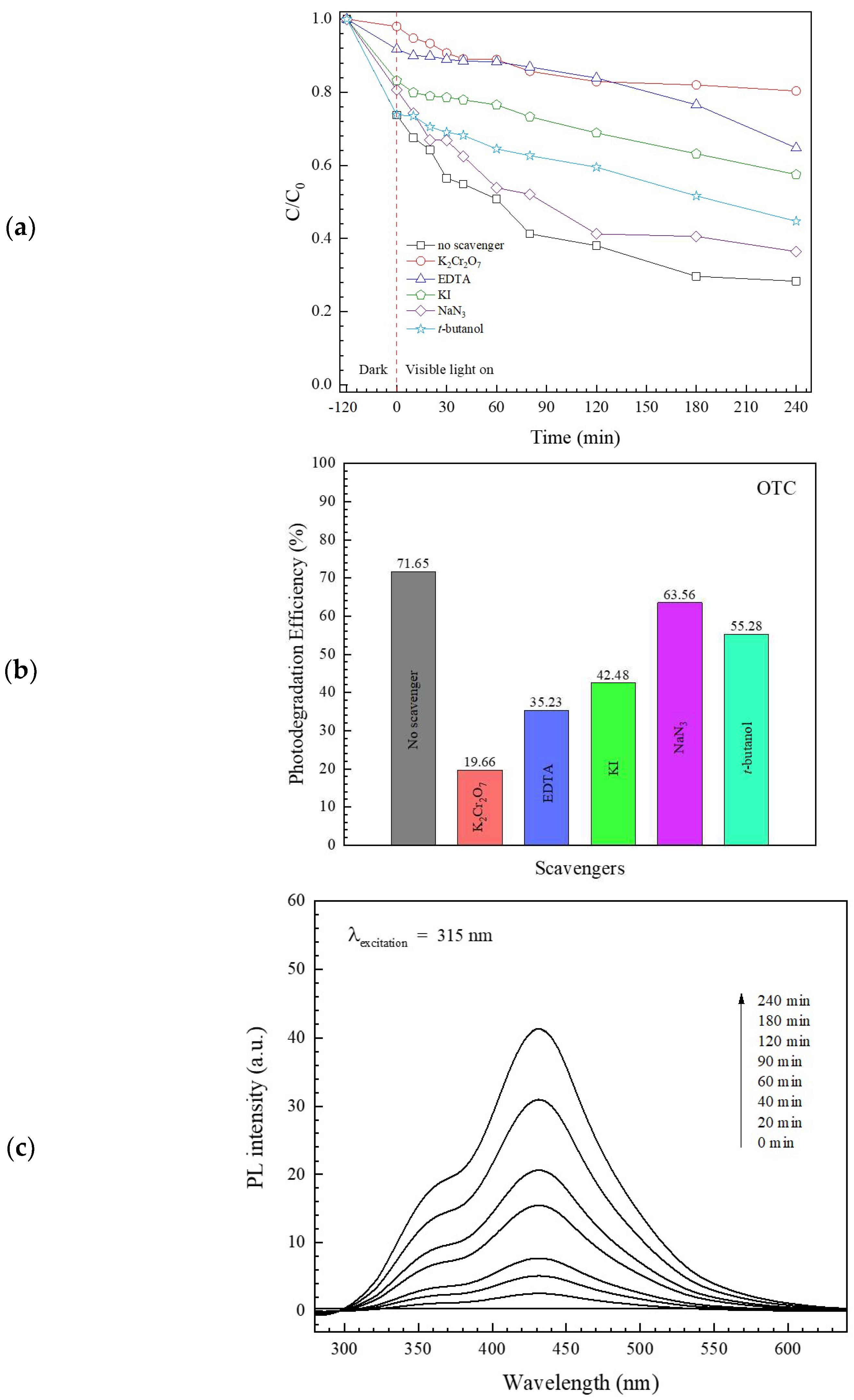
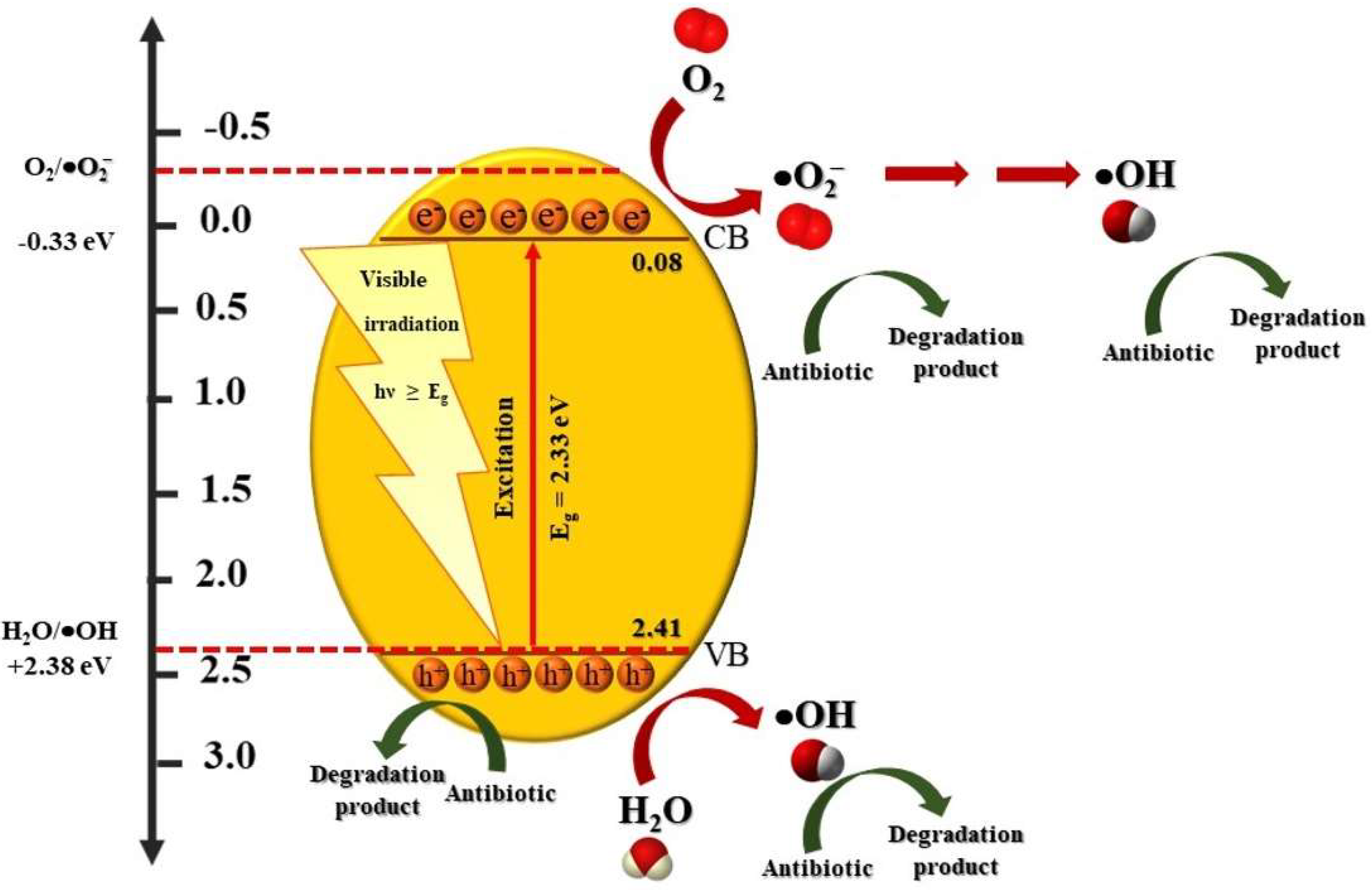
3.2.3. Study of Photocatalytic Degradation Mechanism
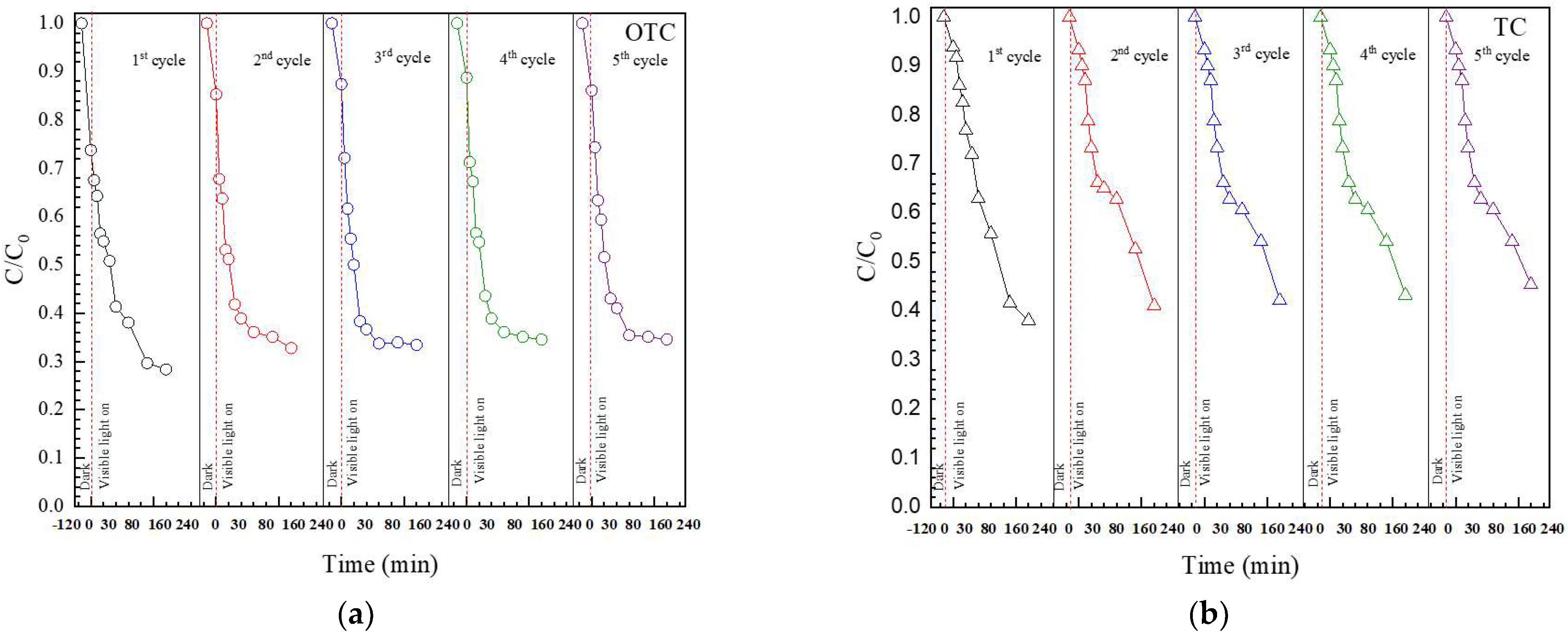
3.2.4. Cycling Ability of the Catalyst
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Q.; Xin, J.; Mao, L.; Cai, X.; Ding, B.; Zhang, L.; Zhang, J.; Zheng, S.; Yang, Y. In situ growth of BiVO4/HoVO4 heterojunction with OO bond connection for enhanced photodegradation activity. Mater. Lett. 2021, 284, 128952–128956. [Google Scholar] [CrossRef]
- Ma, C.; Seo, W.C.; Lee, J.; Kim, Y.; Jung, H.; Yang, W. Chemosphere construction of quantum dots self-decorated BiVO4/reduced graphene hydrogel composite photocatalyst with improved photocatalytic performance for antibiotics degradation. Chemosphere 2021, 275, 130052. [Google Scholar] [CrossRef] [PubMed]
- Senasu, T.; Youngme, S.; Hemavibool, K.; Nanan, S. Sunlight-driven photodegradation of oxytetracycline antibiotic by BiVO4 photocatalyst. J. Solid State Chem. 2021, 297, 122088. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, Y.; Kong, J.; Yuan, J.; Sun, C.; Xian, Q.; Yang, S.; He, H. High photocatalytic degradation efficiency of oxytetracycline hydrochloride over Ag/AgCl/BiVO4 plasmonic photocatalyst. Solid State Sci. 2019, 96, 105946. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Zhai, C. Construction of a novel p-n heterojunction CdS QDs/LaMnO3 composite for photodegradation of oxytetracycline. Mater. Sci. Semicond. Process. 2022, 144, 106568. [Google Scholar] [CrossRef]
- Ye, S.; Zhou, X.; Xu, Y.; Lai, W.; Yan, K.; Huang, L.; Ling, J.; Zheng, L. Photocatalytic performance of multi-walled carbon nanotube/BiVO4 synthesized by electro-spinning process and its degradation mechanisms on oxytetracycline. Chem. Eng. J. 2019, 373, 880–890. [Google Scholar] [CrossRef]
- Huyen, N.T.K.; Pham, T.-D.; Cam, N.T.D.; Van Quan, P.; Van Noi, N.; Hanh, N.T.; Tung, M.H.T.; Dao, V.-D. Fabrication of titanium doped BiVO4 as a novel visible light driven photocatalyst for degradation of residual tetracycline pollutant. Ceram. Int. 2021, 47, 34253–34259. [Google Scholar] [CrossRef]
- Kang, J.; Tang, Y.; Wang, M.; Jin, C.; Liu, J.; Li, S.; Li, Z.; Zhu, J. The enhanced peroxymonosulfate-assisted photocatalytic degradation of tetracycline under visible light by g-C3N4/Na-BiVO4 heterojunction catalyst and its mechanism. J. Environ. Chem. Eng. 2021, 9, 105524. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, J.; Chen, Y.; Zhou, Y.; Ding, L.; Liang, H.; Li, X. Degradation of tetracycline hydrochloride by coupling of photocatalysis and peroxymonosulfate oxidation processes using CuO-BiVO4 heterogeneous catalyst. Process Saf. Environ. Prot. 2021, 145, 364–377. [Google Scholar] [CrossRef]
- Xu, J.; Bian, Z.; Xin, X.; Chen, A.; Wang, H. Size dependence of nanosheet BiVO4 with oxygen vacancies and exposed {001} facets on the photodegradation of oxytetracycline. Chem. Eng. J. 2018, 337, 684–696. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Yenjai, C.; Nanan, S. Utilization of formononetin and pinocembrin from stem extract of Dalbergia parviflora as capping agents for preparation of ZnO photocatalysts for degradation of RR141 azo dye and ofloxacin antibiotic. Catal. Today 2022, 384–386, 279–293. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Komchoo, N.; Senasu, T.; Piriyanon, J.; Youngme, S.; Hemavibool, K.; Nanan, S. Silver decorated ZnO photocatalyst for effective removal of reactive red azo dye and ofloxacin antibiotic under solar light irradiation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127034. [Google Scholar] [CrossRef]
- Sansenya, T.; Masri, N.; Chankhanittha, T.; Senasu, T.; Piriyanon, J.; Mukdasai, S.; Nanan, S. Hydrothermal synthesis of ZnO photocatalyst for detoxification of anionic azo dyes and antibiotic. J. Phys. Chem. Solids 2022, 160, 110353. [Google Scholar] [CrossRef]
- Nur, A.S.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Belousov, A.S.; Suleimanov, E.V. Application of metal–organic frameworks as an alternative to metal oxide-based photocatalysts for the production of industrially important organic chemicals. Green Chem. 2021, 23, 6172–6204. [Google Scholar] [CrossRef]
- Subhiksha, V.; Kokilavani, S.; Khan, S.S. Recent advances in degradation of organic pollutant in aqueous solutions using bismuth based photocatalysts: A review. Chemosphere 2022, 290, 133228. [Google Scholar] [CrossRef]
- Belousov, A.S.; Suleimanov, E.V.; Fukina, D.G. Pyrochlore oxides as visible light-responsive photocatalysts. New J. Chem. 2021, 45, 22531–22558. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Teng, F.; Gu, W.; Yang, Z.; Zhang, A.; Zhao, F.; Shah, A.H. Enhanced visible light photocatalytic activity of CeO2@Zn0.5Cd0.5S by facile Ce(IV)/Ce(III) cycle. Arab. J. Chem. 2020, 13, 4198–4209. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Teng, F. Fe2O3-promoted interface charge separation and visible-light activity of Fe2O3@Zn0.3Cd0.7S. Mater. Chem. Phys. 2020, 246, 122811–122819. [Google Scholar] [CrossRef]
- Shah, A.H.; Gu, W.; Abideen, Z.U.; Teng, F. Removal of chromium from aqueous solution by porous Bi2MoO6@BiOCl nanostructure. J. Solid State Chem. 2020, 292, 121719–121729. [Google Scholar] [CrossRef]
- Gu, W.; Teng, F.; Chu, Y.; Zhang, A.; Abideen, Z.U. An interesting charge accumulation process of Bi12O15Cl6. J. Electroanal. Chem. 2019, 846, 113169–113173. [Google Scholar] [CrossRef]
- Gu, W.; Xu, J.; Teng, F.; Abideen, Z.U. Investigation on the Different Photocatalytic Properties of Bismuths Oxychlorides: Bi12O15Cl6 versus Bi3O4Cl versus BiOCl. ChemistrySelect 2018, 3, 10721–10726. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Teng, F. Enhanced photochemical activity and stability of ZnS by a simple alkaline treatment approach. CrystEngComm 2018, 20, 7866–7879. [Google Scholar] [CrossRef]
- Chankhanittha, T.; Somaudon, V.; Watcharakitti, J.; Piyavarakorn, V.; Nanan, S. Performance of solvothermally grown Bi2MoO6 photocatalyst toward degradation of organic azo dyes and fluoroquinolone antibiotics. Mater. Lett. 2020, 258, 126764–126769. [Google Scholar] [CrossRef]
- Rathi, V.; Panneerselvam, A.; Sathiyapriya, R. A novel hydrothermal induced BiVO4/g-C3N4 heterojunctions visible-light photocatalyst for effective elimination of aqueous organic pollutants. Vacuum 2020, 180, 109458–109467. [Google Scholar] [CrossRef]
- Senasu, T.; Narenuch, T.; Wannakam, K.; Chankhanittha, T.; Nanan, S. Solvothermally grown BiOCl catalyst for photodegradation of cationic dye and fluoroquinolone-based antibiotics. J. Mater. Sci. Mater. Electron. 2020, 31, 9685–9694. [Google Scholar] [CrossRef]
- Peleyeju, G.M.; Umukoro, E.H.; Babalola, J.O.; Arotiba, O.A. Solar-Light-Responsive Titanium-Sheet-Based Carbon Nanoparticles/B-BiVO4/WO3 Photoanode for the Photoelectrocatalytic Degradation of Orange II Dye Water Pollutant. ACS Omega 2020, 5, 4743–4750. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-H.; Jiang, Y.-S.; Lin, H.-Y. Easy Synthesis of BiVO4 for Photocatalytic Overall Water Splitting. ACS Omega 2020, 5, 8927–8933. [Google Scholar] [CrossRef] [Green Version]
- Baral, B.; Parida, K. {040/110} Facet Isotype Heterojunctions with Monoclinic Scheelite BiVO4. Inorg. Chem. 2020, 59, 10328–10342. [Google Scholar] [CrossRef]
- Mudavakkat, V.; Atuchin, V.; Kruchinin, V.; Kayani, A.; Ramana, C. Structure, morphology and optical properties of nanocrystalline yttrium oxide (Y2O3) thin films. Opt. Mater. 2012, 34, 893–900. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Jiang, X.; Lin, Z.; Atuchin, V.V. Comparative investigations of the crystal structure and photoluminescence property of eulytite-type Ba3Eu(PO4)3and Sr3Eu(PO4)3. Dalton Trans. 2015, 44, 7679–7686. [Google Scholar] [CrossRef] [PubMed]
- Atuchin, V.; Chimitova, O.; Adichtchev, S.; Bazarov, J.; Gavrilova, T.; Molokeev, M.; Surovtsev, N.; Bazarova, Z. Synthesis, structural and vibrational properties of microcrystalline β-RbSm(MoO4)2. Mater. Lett. 2013, 106, 26–29. [Google Scholar] [CrossRef]
- Wang, G.-L.; Shan, L.-W.; Wu, Z.; Dong, L.-M. Enhanced photocatalytic properties of molybdenum-doped BiVO4 prepared by sol–gel method. Rare Met. 2017, 36, 129–133. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Cui, W.; Sui, H. Synthesis and characterization of a core–shell BiVO4@g-C3N4 photo-catalyst with enhanced photocatalytic activity under visible light irradiation. RSC Adv. 2017, 7, 8167–8177. [Google Scholar] [CrossRef] [Green Version]
- Yanga, R.; Zhua, Z.; Hua, C.; Zhongb, S.; Zhangb, L.; Liuad, B.; Wangc, W. One-step preparation (3D/2D/2D) BiVO4/FeVO4@rGO heterojunction composite photocatalyst for the removal of tetracycline and hexavalent chromium ions in water. Chem. Eng. J. 2020, 390, 124522. [Google Scholar] [CrossRef]
- Ma, J.; Jin, D.; Li, Y.; Xiao, D.; Jiao, G.; Liu, Q.; Guo, Y.; Xiao, L.; Chen, X.; Li, X.; et al. Photocatalytic conversion of biomass-based monosaccharides to lactic acid by ultrathin porous oxygen doped carbon nitride. Appl. Catal. B Environ. 2021, 283, 119520–119533. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Liu, C.; Wang, D.; Yan, G.; Liu, B.; Che, G. Enhanced visible-light-induced photocatalytic degradation of tetracycline using BiOI/MIL-125(Ti) composite photocatalyst. J. Alloy. Compd. 2021, 854, 157166–157176. [Google Scholar] [CrossRef]
- Ma, F.; Yang, Q.; Wang, Z.; Liu, Y.; Xin, J.; Zhang, J.; Hao, Y.; Li, L. Enhanced visible-light photocatalytic activity and photostability of Ag3PO4/Bi2WO6 heterostructures toward organic pollutant degradation and plasmonic Z-scheme mechanism. RSC Adv. 2018, 8, 15853–15862. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Zhang, B.; Fan, L.; Liu, H.; Valvo, M.; Edström, K.; Cuartero, M.; de Marco, R.; Crespo, G.A.; Sun, L. Efficient BiVO4 Photoanodes by Postsynthetic Treatment: Remarkable Improvements in Photoelectrochemical Performance from Facile Borate Modification. Angew. Chem. Int. Ed. 2019, 58, 19027–19033. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Bai, Y.; Si, W.; Mao, W.; Gao, Y.; Liu, S. Heterogeneous photo-Fenton system of novel ternary Bi2WO6/BiFeO3/g-C3N4 heterojunctions for highly efficient degrading persistent organic pollutants in wastewater. J. Photochem. Photobiol. A Chem. 2021, 404, 112856–112867. [Google Scholar] [CrossRef]
- Ni, S.; Zhou, T.; Zhang, H.; Cao, Y.; Yang, P. BiOI/BiVO4 Two-Dimensional Heteronanostructures for Visible-Light Photocatalytic Degradation of Rhodamine B. ACS Appl. Nano Mater. 2018, 1, 5128–5141. [Google Scholar] [CrossRef]
- Ouyang, K.; Yang, C.; Xu, B.; Wang, H.; Xie, S. Synthesis of novel ternary Ag/BiVO4/GO photocatalyst for degradation of oxytetracycline hydrochloride under visible light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126978. [Google Scholar] [CrossRef]
- Chen, F.; Wu, C.; Wang, J.; François-Xavier, C.P.; Wintgens, T. Highly efficient Z-scheme structured visible-light photocatalyst constructed by selective doping of Ag@AgBr and Co3O4 separately on {010} and {110} facets of BiVO4: Pre-separation channel and hole-sink effects. Appl. Catal. B Environ. 2019, 250, 31–41. [Google Scholar] [CrossRef]
- Wang, W.; Han, Q.; Zhu, Z.; Zhang, L.; Zhong, S.; Liu, B. Enhanced photocatalytic degradation performance of organic contaminants by heterojunction photocatalyst BiVO4/TiO2/RGO and its compatibility on four different tetracycline antibiotics. Adv. Powder Technol. 2019, 30, 1882–1896. [Google Scholar] [CrossRef]
- Li, Y.; Sun, X.; Tang, Y.; Ng, Y.H.; Li, L.; Jiang, F.; Wang, J.; Chen, W.; Li, L. Understanding photoelectrocatalytic degradation of tetracycline over three-dimensional coral-like ZnO/BiVO4 nanocomposite. Mater. Chem. Phys. 2021, 271, 124871. [Google Scholar] [CrossRef]
- Xu, G.; Du, M.; Li, T.; Guan, Y.; Guo, C. Facile synthesis of magnetically retrievable Fe3O4/BiVO4/CdS heterojunction composite for enhanced photocatalytic degradation of tetracycline under visible light. Sep. Purif. Technol. 2021, 275, 119157. [Google Scholar] [CrossRef]
- Ma, C.; Din, S.T.U.; Seo, W.C.; Lee, J.; Kim, Y.; Jung, H.; Yang, W. BiVO4 ternary photocatalyst co-modified with N-doped graphene nanodots and Ag nanoparticles for improved photocatalytic oxidation: A significant enhancement in photoinduced carrier separation and broad-spectrum light absorption. Sep. Purif. Technol. 2021, 264, 118423. [Google Scholar] [CrossRef]
- Yang, C.; Qin, C.; Zhong, J.; Li, J.; Huang, S.; Wang, Q.; Ma, L. Photocatalytic enhancement mechanism insight for BiVO4 induced by plasma treatment under different atmospheres. J. Alloy. Compd. 2021, 890, 161883. [Google Scholar] [CrossRef]
- Yan, L.; Li, W.; Zhao, Q.; Zhu, Z.; Hu, C.; Liu, B. Enhanced photocatalytic conversion of (3D/2D) BiVO4@Polypyrrole/g-C3N4 ternary composites with Z-scheme band alignment for the Antibiotic removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126783. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Hafeez, H.Y.; Venkataramana, R.; Veluswamy, P.; Choi, H.; Neppolian, B. Design of a highly efficient ternary AgI/rGO/BiVO4 nanocomposite and its direct solar light induced photocatalytic activity. Appl. Surf. Sci. 2019, 487, 1289–1300. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Zhang, L.; Yang, Z.; Zhang, Q.; Cui, H.; Zhu, X.; Wang, J.; Chen, J.; Wang, K. Palygorskite supported BiVO4 photocatalyst for tetracycline hydrochloride removal. Appl. Clay Sci. 2017, 137, 249–258. [Google Scholar] [CrossRef]
- Qin, C.; Liao, H.; Rao, F.; Zhong, J.; Li, J. One-pot hydrothermal preparation of Br-doped BiVO4 with enhanced visible-light photocatalytic activity. Solid State Sci. 2020, 105, 106285. [Google Scholar] [CrossRef]
- Cam, N.T.D.; Pham, H.D.; Pham, T.D.; Phuong, T.T.T.; van Hoang, C.; Tung, M.H.T.; Trung, N.T.; Huong, N.T.; Hien, T.T.T. Novel photocatalytic performance of magnetically recoverable MnFe2O4/BiVO4 for polluted antibiotics degradation. Ceram. Int. 2021, 47, 1686–1692. [Google Scholar] [CrossRef]




| Photocatalyst | Concentration (mg/L) | Catalyst Loading (mg) | Light Source | Lamp | Time (min) | Photodegradation (%) | Ref. |
|---|---|---|---|---|---|---|---|
| • Photodegradation of oxytetracycline antibiotic | |||||||
| BiVO4 | 10 | 50 | Visible | 15 | 240 | 55 | [3] |
| BiVO4 | 10 | 50 | Solar | - | 240 | 83 | [3] |
| BiVO4 | 20 | 100 | Visible | 500 | 70 | 37 | [42] |
| BiVO4 | 20 | 50 | Visible | 1000 | 120 | 61 | [4] |
| BiVO4 | 10 | 50 | Visible | 350 | 35 | 4 | [43] |
| BiVO4 | 10 | 100 | Visible | 1000 | 60 | 36 | [44] |
| BiVO4 | 10 | 100 | Visible | 500 | 60 | 47 | [6] |
| BiVO4/GO | 20 | 100 | Visible | 500 | 70 | 83 | [42] |
| AgCl/BiVO4 | 20 | 100 | Visible | 1000 | 120 | 77 | [4] |
| BiVO4/TiO2 | 10 | 100 | Visible | 1000 | 60 | 68 | [44] |
| C/BiVO4 | 10 | 100 | Visible | 500 | 60 | 89 | [6] |
| Ag/BiVO4/GO | 20 | 100 | Visible | 500 | 70 | 90 | [42] |
| Ag/AgCl/BiVO4 | 20 | 50 | Visible | 1000 | 120 | 98 | [4] |
| BiVO4/TiO2/rGO | 10 | 100 | Visible | 1000 | 60 | 99 | [44] |
| Ag-AgBr/BiVO4/Co3O4 | 10 | 50 | Visible | 350 | 35 | 90 | [43] |
| BiVO4 | 10 | 50 | Visible | 15 | 240 | 62 | This work |
| BiVO4 | 10 | 50 | Solar | - | 240 | 93 | This work |
| • Photodegradation of tetracycline antibiotic | |||||||
| BiVO4 | 20 | 50 | Visible | 300 | 60 | 60 | [45] |
| BiVO4 | 10 | 100 | Visible | 300 | 90 | 56 | [46] |
| BiVO4 | 20 | 100 | Visible | 300 | 80 | 20 | [47] |
| BiVO4 | 15 | 200 | Visible | 32 | 180 | 52 | [7] |
| BiVO4 | 20 | 100 | Visible | 300 | 60 | 41 | [8] |
| BiVO4 | 20 | 50 | Visible | 300 | 240 | 25 | [48] |
| BiVO4 | 20 | 100 | Visible | 300 | 120 | 34 | [2] |
| BiVO4 | 30 | 50 | Visible | 300 | 54 | 54 | [49] |
| BiVO4 | 10 | 100 | Visible | 1000 | 60 | 34 | [44] |
| BiVO4 | 20 | 30 | Visible | 300 | 25 | 42 | [50] |
| BiVO4 | 30 | 50 | Visible | 500 | 240 | 59 | [51] |
| BiVO4 | 20 | 50 | Visible | 500 | 240 | 20 | [52] |
| ZnO/BiVO4 | 20 | 50 | Visible | 300 | 60 | 85 | [45] |
| MnFe2O4/BiVO4 | 10 | 400 | Visible | 30 | 120 | 92 | [53] |
| Ag/BiVO4 | 20 | 100 | Visible | 300 | 80 | 65 | [47] |
| Ti/BiVO4 | 15 | 200 | Visible | 32 | 180 | 60 | [7] |
| g-C3N4/BiVO4 | 20 | 100 | Visible | 300 | 60 | 60 | [8] |
| H2-BiVO4 | 20 | 50 | Visible | 300 | 240 | 75 | [48] |
| BiVO4/rGH-3 | 20 | 100 | Visible | 300 | 120 | 73 | [2] |
| CuO/BiVO4 | 80 | 100 | Visible | 300 | 50 | 28 | [9] |
| BiVO4/TiO2 | 10 | 100 | Visible | 1000 | 60 | 73 | [44] |
| 30%AgI/BiVO4 | 20 | 30 | Visible | 300 | 25 | 62 | [50] |
| BiVO4/ Pal | 30 | 50 | Visible | 500 | 240 | 82 | [51] |
| 3%Br/BiVO4 | 20 | 50 | Visible | 500 | 240 | 79 | [52] |
| Fe3O4/BiVO4/Cds | 10 | 100 | Visible | 300 | 90 | 75 | [46] |
| N-GNDs/BiVO4 | 20 | 100 | Visible | 300 | 80 | 85 | [47] |
| g-C3N4/BiVO4+PMS | 20 | 100 | Visible | 300 | 60 | 76 | [8] |
| BiVO4@PPy/g-C3N4 | 30 | 50 | Visible | 300 | 120 | 90 | [49] |
| CuO/BiVO4+PMS | 80 | 50 | Visible | 300 | 50 | 68 | [9] |
| BiVO4/TiO2/rGO | 10 | 100 | Visible | 1000 | 60 | 96 | [44] |
| 30%AgI/rGO/BiVO4 | 20 | 30 | Visible | 300 | 25 | 84 | [50] |
| BiVO4 | 10 | 50 | Visible | 15 | 240 | 59 | This work |
| BiVO4 | 10 | 50 | Solar | - | 240 | 72 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hemavibool, K.; Sansenya, T.; Nanan, S. Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation. Antibiotics 2022, 11, 761. https://doi.org/10.3390/antibiotics11060761
Hemavibool K, Sansenya T, Nanan S. Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation. Antibiotics. 2022; 11(6):761. https://doi.org/10.3390/antibiotics11060761
Chicago/Turabian StyleHemavibool, Khuanjit, Theepakorn Sansenya, and Suwat Nanan. 2022. "Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation" Antibiotics 11, no. 6: 761. https://doi.org/10.3390/antibiotics11060761
APA StyleHemavibool, K., Sansenya, T., & Nanan, S. (2022). Enhanced Photocatalytic Degradation of Tetracycline and Oxytetracycline Antibiotics by BiVO4 Photocatalyst under Visible Light and Solar Light Irradiation. Antibiotics, 11(6), 761. https://doi.org/10.3390/antibiotics11060761






