Impact of Pharmacist-Led Implementation of a Community Hospital-Based Outpatient Parenteral Antimicrobial Therapy on Clinical Outcomes in Thailand
Abstract
:1. Introduction
2. Material and Methods
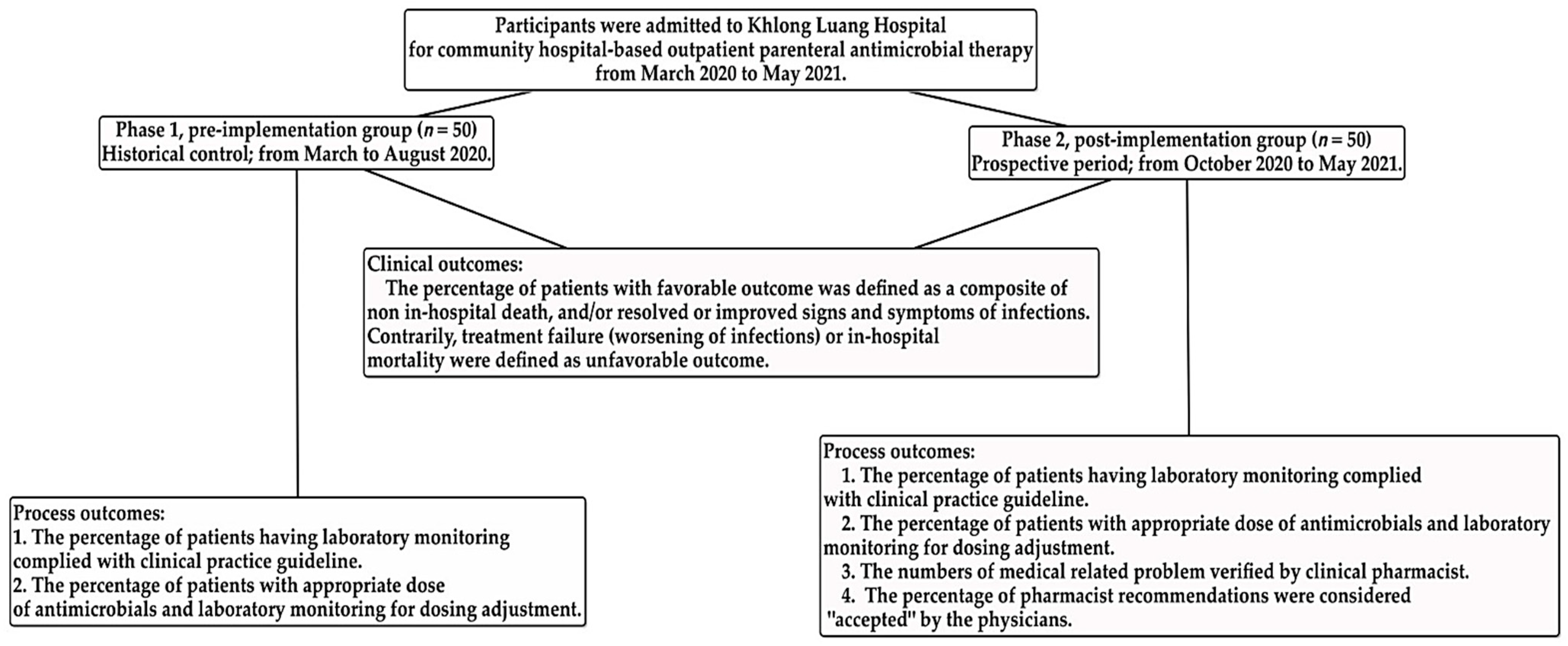
2.1. Participants
2.2. Community Hospital-Based Parenteral Anti-Infective Therapy (CohPAT) Implementation
2.3. Outcome Measurements
Process and Outcome Measurements
2.4. Statistical Analysis
3. Results
3.1. Process and Clinical Outcomes of Implementation
3.2. Description of Pharmacist Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Huang, W.E.; Yang, Q. Clinical Perspective of Antimicrobial Resistance in Bacteria. Infect. Drug Resist. 2022, 15, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Marturano, J.E.; Lowery, T.J. ESKAPE Pathogens in Bloodstream Infections Are Associated With Higher Cost and Mortality but Can Be Predicted Using Diagnoses Upon Admission. Open Forum Infect. Dis. 2019, 6, ofz503. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Takahashi, E.; Hongsuwan, M.; Wuthiekanun, V.; Thamlikitkul, V.; Hinjoy, S.; Day, N.P.; Peacock, S.J.; Limmathurotsakul, D. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. Elife 2016, 5, e18082. [Google Scholar] [CrossRef] [PubMed]
- Poomchaichote, T.; Osterrieder, A.; Prapharsavat, R.; Naemiratch, B.; Ruangkajorn, S.; Thirapantu, C.; Sukrung, K.; Kiatying-Angsulee, N.; Sumpradit, N.; Punnin, S.; et al. “AMR Dialogues”: A public engagement initiative to shape policies and solutions on antimicrobial resistance (AMR) in Thailand. Wellcome Open Res. 2021, 6, 188. [Google Scholar] [CrossRef]
- Ofori-Asenso, R.; Agyeman, A.A. Irrational Use of Medicines-A Summary of Key Concepts. Pharmacy 2016, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Majumder, M.A.A.; Rahman, S.; Cohall, D.; Bharatha, A.; Singh, K.; Haque, M.; Gittens-St Hilaire, M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020, 13, 4713–4738. [Google Scholar] [CrossRef]
- Norris, A.H.; Shrestha, N.K.; Allison, G.M.; Keller, S.C.; Bhavan, K.P.; Zurlo, J.J.; Hersh, A.L.; Gorski, L.A.; Bosso, J.A.; Rathore, M.H.; et al. 2018 Infectious Diseases Society of America Clinical Practice Guideline for the Management of Outpatient Parenteral Antimicrobial Therapy. Clin. Infect. Dis. 2019, 68, e1–e35. [Google Scholar] [CrossRef]
- Chapman, A.L.; Dixon, S.; Andrews, D.; Lillie, P.J.; Bazaz, R.; Patchett, J.D. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): A UK perspective. J. Antimicrob. Chemother. 2009, 64, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Durojaiye, O.C.; Bell, H.; Andrews, D.; Ntziora, F.; Cartwright, K. Clinical efficacy, cost analysis and patient acceptability of outpatient parenteral antibiotic therapy (OPAT): A decade of Sheffield (UK) OPAT service. Int. J. Antimicrob. Agents 2018, 51, 26–32. [Google Scholar] [CrossRef]
- Suwanpimolkul, G.; Ittiwattanakul, W.; Chantaramaropas, M.; Kitisupornpan, W. Outpatient Parenteral Antibiotic Therapy (OPAT). In Surin Asawitoonthip; Neighbour Media: Bangkok, Thailand, 2018; p. 11. (In Thailand) [Google Scholar]
- Thomnoi, T.; Santimaleeworagun, W. The community hospital-based parenteral antimicrobial therapy (COHPAT) in Thailand: The experience of a model to continously treat bacterial infections at Khlongluanf Hospital. Thai Bull. Pharm. Sci. 2021, 11, 19–31. [Google Scholar]
- Docherty, T.; Schneider, J.J.; Cooper, J. Clinic- and Hospital-Based Home Care, Outpatient Parenteral Antimicrobial Therapy (OPAT) and the Evolving Clinical Responsibilities of the Pharmacist. Pharmacy 2020, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.K.; Burns, B. Systemic Inflammatory Response Syndrome. In StatPearls; Europe PMC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Suleyman, G.; Kenney, R.; Zervos, M.J.; Weinmann, A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J. Clin. Pharm. 2017, 42, 39–43. [Google Scholar] [CrossRef]
- Bauer, A.K.; Mangino, J.; Paolo-Hohman, D.; Goff, A.D. Outpatient Parenteral Antimicrobial Therapy and Antimicrobial Stewardship: Implementation of a Structured Approach to Improve Patient Outcomes. Infect. Dis. Clin. Pract. 2016, 24, 328–331. [Google Scholar] [CrossRef]
- Behzadi, P.; Gajdacs, M. Writing a strong scientific paper in medicine and the biomedical sciences: A checklist and recommendations for early career researchers. Biol. Future 2021, 72, 395–407. [Google Scholar] [CrossRef]
- CmapTools. Available online: https://cmap.ihmc.us/ (accessed on 15 May 2022).
- Mahoney, M.V.; Childs-Kean, L.M.; Khan, P.; Rivera, C.G.; Stevens, R.W.; Ryan, K.L. Recent Updates in Antimicrobial Stewardship in Outpatient Parenteral Antimicrobial Therapy. Curr. Infect. Dis. Rep. 2021, 23, 24. [Google Scholar] [CrossRef]
- Shah, P.J.; Bergman, S.J.; Graham, D.R.; Glenn, S. Monitoring of Outpatient Parenteral Antimicrobial Therapy and Implementation of Clinical Pharmacy Services at a Community Hospital Infusion Unit. J. Pharm. Pract. 2015, 28, 462–468. [Google Scholar] [CrossRef]
- Huck, D.; Ginsberg, J.P.; Gordon, S.M.; Nowacki, A.S.; Rehm, S.J.; Shrestha, N.K. Association of laboratory test result availability and rehospitalizations in an outpatient parenteral antimicrobial therapy programme. J. Antimicrob. Chemother. 2014, 69, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Chapman, A.L.N.; Patel, S.; Horner, C.; Green, H.; Guleri, A.; Hedderwick, S.; Snape, S.; Statham, J.; Wilson, E.; Gilchrist, M.; et al. Updated good practice recommendations for outpatient parenteral antimicrobial therapy (OPAT) in adults and children in the UK. JAC Antimicrob. Resist. 2019, 1, dlz026. [Google Scholar] [CrossRef] [Green Version]
- Chung, E.K.; Beeler, C.B.; Muloma, E.W.; Osterholzer, D.; Damer, K.M.; Erdman, S.M. Development and implementation of a pharmacist-managed outpatient parenteral antimicrobial therapy program. Am. J. Health Syst. Pharm. 2016, 73, e24–e33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allison, G.M.; Muldoon, E.G.; Kent, D.M.; Paulus, J.K.; Ruthazer, R.; Ren, A.; Snydman, D.R. Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin. Infect. Dis. 2014, 58, 812–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heintz, B.H.; Halilovic, J.; Christensen, C.L. Impact of a multidisciplinary team review of potential outpatient parenteral antimicrobial therapy prior to discharge from an academic medical center. Ann. Pharm. 2011, 45, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Petroff, B.J.; Filibeck, D.; Nowobilski-Vasilios, A.; Olsen, R.S.; Rollins, C.J.; Johnson, C. ASHP guidelines on home infusion pharmacy services. Am.J. Health Syst. Pharm. 2014, 71, 325–341. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Rafailidis, P.I.; Konstantelias, A.A.; Falagas, M.E. Predictors of mortality in patients with infections due to multi-drug resistant Gram negative bacteria: The study, the patient, the bug or the drug? J. Infect. 2013, 66, 401–414. [Google Scholar] [CrossRef]
- Huang, V.; Ruhe, J.J.; Lerner, P.; Fedorenko, M. Risk factors for readmission in patients discharged with outpatient parenteral antimicrobial therapy: A retrospective cohort study. BMC Pharm. Toxicol. 2018, 19, 50. [Google Scholar] [CrossRef] [Green Version]
- Md Rezal, R.S.; Hassali, M.A.; Alrasheedy, A.A.; Saleem, F.; Md Yusof, F.A.; Godman, B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: A systematic review of the literature. Expert Rev. Anti-Infect. Ther. 2015, 13, 665–680. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.; Ranjbar, R.; Behzadi, P.; Mohammadian, T. Virulence factors, antibiotic resistance patterns, and molecular types of clinical isolates of Klebsiella Pneumoniae. Expert Rev. Anti-Infect. Ther. 2022, 20, 463–472. [Google Scholar] [CrossRef]
| Characteristic | Pre-Implementation (n = 50 Cases) | Post-Implementation (n = 50 Cases) | p-Value |
|---|---|---|---|
| Age—years (median; IQR) | 63 (20) | 66.5 (18) | 0.603 a |
| Female sex—no. (%) | 19 (38) | 24 (48) | 0.313 b |
| Length of stay in community hospital—days (median; IQR) | 7.5 (7) | 7.5 (9) | 0.663 a |
| Antimicrobial treatment duration in community hospital—days (median; IQR) | 6 (5) | 6.5 (4) | 0.642 a |
| Number of comorbidities—no. (%) | |||
| No underlying disease | 8 (16) | 3 (6) | 0.227 b |
| 1 disease | 18 (36) | 12 (24) | |
| 2 diseases | 12 (24) | 18 (36) | |
| 3 diseases | 7 (14) | 11 (22) | |
| 4 diseases | 5 (10) | 6 (12) | |
| Comorbidities—no. (%) | |||
| Diabetes mellitus | 14 (28) | 17 (34) | 0.517 b |
| Malignancy | 7 (14) | 8 (16) | 0.779 b |
| Chronic kidney disease | 8 (16) | 6 (12) | 0.564 b |
| Cardiovascular disease | 25 (50) | 4 (8) | 0.000 b |
| Cerebrovascular disease | 6 (12) | 10 (20) | 0.275 b |
| Chronic lung disease | 2 (4) | 3 (6) | 1.000 a |
| Liver disease | 1 (2) | 1 (2) | 1.000 a |
| Referring hospital—no. (%) | |||
| Provincial hospital | 31 (62) | 35 (70) | 0.616 b |
| Medical school | 16 (32) | 9 (18) | |
| Others | 3 (6) | 6 (12) | |
| Site of infection—no. (%) | |||
| Lower respiratory | 27 (54) | 15 (30) | 0.015 a |
| Bloodstream | 13 (26) | 19 (38) | 0.198 a |
| Urinary tract | 7 (14) | 13 (26) | 0.134 a |
| Intra-abdomen | 5 (10) | 5 (10) | 1 a |
| Skin and soft tissue | 3 (6) | 11 (22) | 0.021 a |
| Osteoarticular | 3 (6) | 1 (2) | 0.617 c |
| Central nervous system | 1 (2) | 1 (2) | 1 c |
| Cardiovascular system | 1 (2) | 0 (0) | 1 c |
| Causative bacteria—no. (%) | |||
| E. coli | 1 (2) | 11 (22) | 0.002 b |
| K. pneumoniae | 7 (14) | 2 (4) | 0.160 c |
| P. aeruginosa | 2 (4) | 3 (6) | 1.000 c |
| A. baumannii | 5 (10) | 6 (12) | 0.749 b |
| S. aureus | 2 (4) | 3 (6) | 1.000 c |
| Infection with antimicrobial resistant bacteria—no. (%) | |||
| K. pneumoniae (MDR) | 3 (6) | 0 (0) | 0.092 c |
| E. coli (MDR) | 1 (2) | 9 (18) | 0.014 c |
| A. baumannii (CRAB) | 4 (8) | 4 (8) | 1 c |
| S. aureus (MRSA) | 1 (2) | 1 (2) | 1 c |
| Outcome | Pre-Implementation (n = 50 Cases) | Post-Implementation (n = 50 Cases) | p-Value |
|---|---|---|---|
| Dose adjustment and laboratory monitoring—no. (%) | |||
| Appropriate dose of antimicrobials | 39 (78) | 50 (100) | 0.000 |
| Inappropriate dose of antimicrobials or non-laboratory monitoring for dose adjustment | 11 (22) | 0 (0) | |
| Laboratory monitoring * complied with CPG—no. (%) | |||
| Compliance with CPG | 30 (60) | 50 (100) | 0.000 |
| Non-compliance with CPG | 20 (40) | 0 (0) | |
| Clinical outcomes—no. (%) | |||
| Favorable outcome | 37 (74) | 47 (94) | 0.006 |
| Unfavorable outcomes | 13 (26) | 3 (6) | |
| Death | 4 | 0 | |
| Treatment failure | 9 | 3 |
| Characteristic | Unfavorable Outcome (n = 16 Cases) | FavorableOutcome (n = 84 Cases) | OR (95% CI) | aOR (95% CI) |
|---|---|---|---|---|
| Age ≥60 years—no. (%) | 11 (68.8) | 52 (61.9) | 1.35 (0.43–4.26) | |
| Female sex—no. (%) | 6 (37.5) | 37 (44) | 0.76 (0.25–2.29) | |
| Comorbidities ≥3 diseases—no. (%) | 3 (18.8) | 26 (31) | 0.52 (0.14–1.96) | |
| Comorbidities—no. (%) | ||||
| Diabetes mellitus | 2 (12.5) | 29 (34.5) | 0.27 (0.06–1.27) | |
| Malignancy | 3 (18.8) | 12 (14.3) | 1.39 (0.34–5.59) | |
| Chronic kidney disease | 1 (6.3) | 13 (15.5) | 0.36 (0.04–3.00) | |
| Cardiovascular disease | 6 (37.5) | 23 (27.4) | 1.59 (0.52–4.88) | |
| Cerebrovascular disease | 1 (6.3) | 15 (17.9) | 0.31 (0.04–2.50) | |
| Chronic lung disease | 1 (6.3) | 4 (4.8) | 1.33 (0.14–12.77) | |
| Liver disease | 1 (6.3) | 1 (1.2) | 5.53 (0.33–93.37) | |
| Site of infections—no. (%) | ||||
| Lower respiratory tract | 11 (68.8) | 28 (33.3) | 4.4 (1.39–13.9) | 3.68 (1.13–12.06) |
| Bloodstream | 4 (25) | 26 (31) | 0.74 (0.22–2.53) | |
| Urinary tract | 0 (0) | 20 (23.8) | 0.10 (0.01–1.66) a | |
| Intra-abdomen | 2 (12.5) | 8 (9.5) | 1.36 (0.26–7.07) | |
| Skin and soft tissue | 2 (12.5) | 13 (15.5) | 0.78 (0.16–3.85) | |
| Causative bacteria—no. (%) | ||||
| E. coli | 0 (0) | 12 (14.3) | 0.18 (0.01–3.12) a | |
| K. pneumoniae | 2 (12.5) | 7 (8.3) | 1.57 (0.30–8.36) | |
| P. aeruginosa | 0 (0) | 5 (6) | 0.44 (0.02–8.31) a | |
| A. baumannii | 13 (18.8) | 8 (9.5) | 2.19 (0.51–9.36) | |
| S. maltophilia | 1 (6.3) | 0 (0) | 16.35 (0.64–420.18) a | |
| S. aureus | 0 (0) | 5 (6) | 0.44 (0.02–8.31) a | |
| Infection with antimicrobial resistant bacteria—no. (%) | ||||
| E. coli (MDR) | 0 (0) | 10 (11.9) | 0.22 (0.01–3.86) a | |
| K. pneumoniae (MDR) | 1 (6.3) | 2 (2.4) | 2.73 (0.23–32.08) | |
| A. baumannii (CRAB) | 2 (12.5) | 6 (7.1) | 1.86 (0.34–10.15) | |
| S. aureus (MRSA) | 0 (0) | 2 (2.4) | 1.00 (0.05–21.80) a | |
| Post-implementation period | 3 (18.8) | 47 (56) | 0.18 (0.05–0.67) | 0.21 (0.06–0.83) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomnoi, T.; Komenkul, V.; Prawang, A.; Santimaleeworagun, W. Impact of Pharmacist-Led Implementation of a Community Hospital-Based Outpatient Parenteral Antimicrobial Therapy on Clinical Outcomes in Thailand. Antibiotics 2022, 11, 760. https://doi.org/10.3390/antibiotics11060760
Thomnoi T, Komenkul V, Prawang A, Santimaleeworagun W. Impact of Pharmacist-Led Implementation of a Community Hospital-Based Outpatient Parenteral Antimicrobial Therapy on Clinical Outcomes in Thailand. Antibiotics. 2022; 11(6):760. https://doi.org/10.3390/antibiotics11060760
Chicago/Turabian StyleThomnoi, Teeranuch, Virunya Komenkul, Abhisit Prawang, and Wichai Santimaleeworagun. 2022. "Impact of Pharmacist-Led Implementation of a Community Hospital-Based Outpatient Parenteral Antimicrobial Therapy on Clinical Outcomes in Thailand" Antibiotics 11, no. 6: 760. https://doi.org/10.3390/antibiotics11060760
APA StyleThomnoi, T., Komenkul, V., Prawang, A., & Santimaleeworagun, W. (2022). Impact of Pharmacist-Led Implementation of a Community Hospital-Based Outpatient Parenteral Antimicrobial Therapy on Clinical Outcomes in Thailand. Antibiotics, 11(6), 760. https://doi.org/10.3390/antibiotics11060760





