Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics
Abstract
:1. Introduction
2. Physical Methods to Determine Antimicrobial Effects
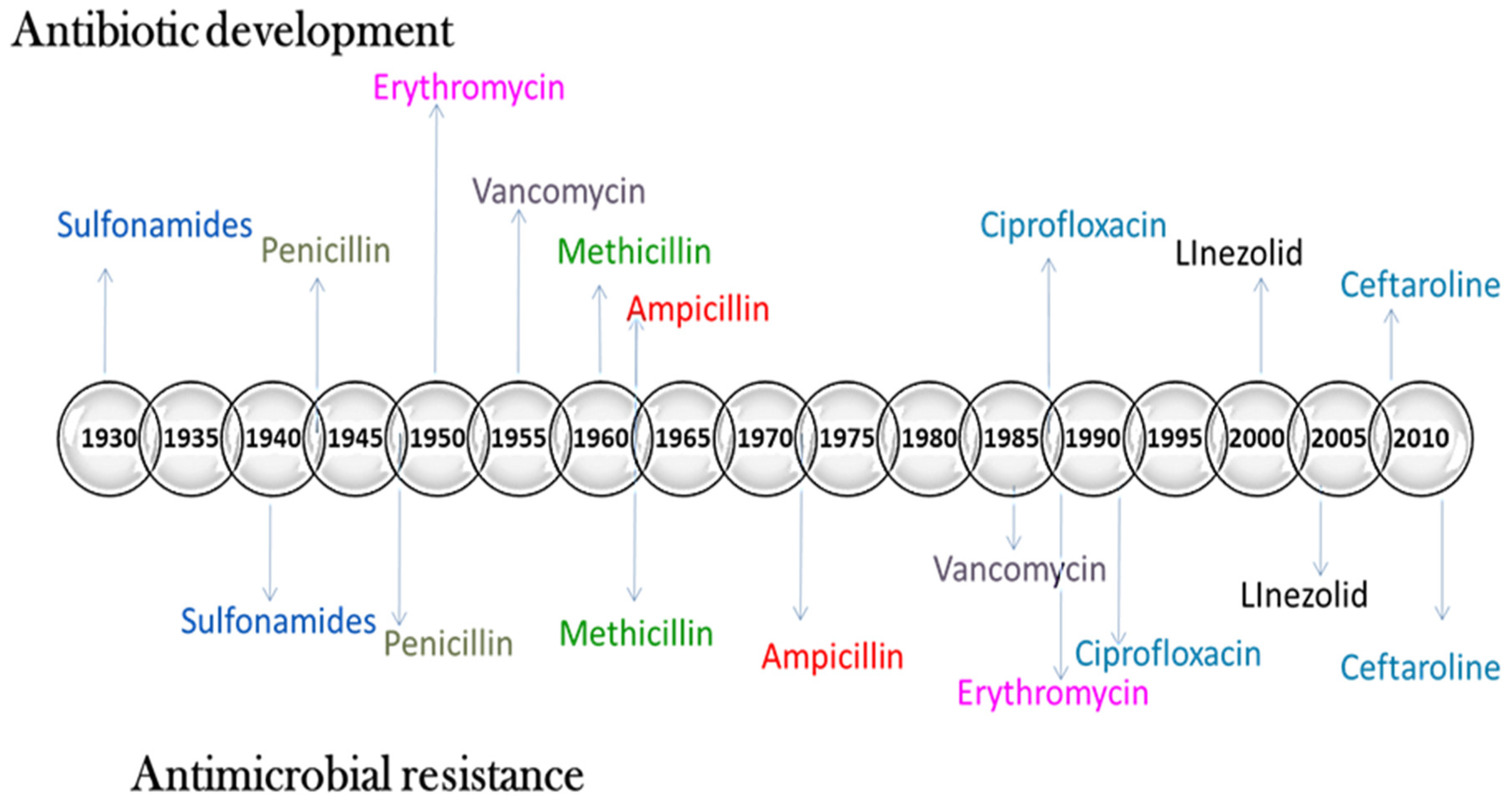
3. Development of Antibiotic Resistance
Limitations of Conventional Antibiotics
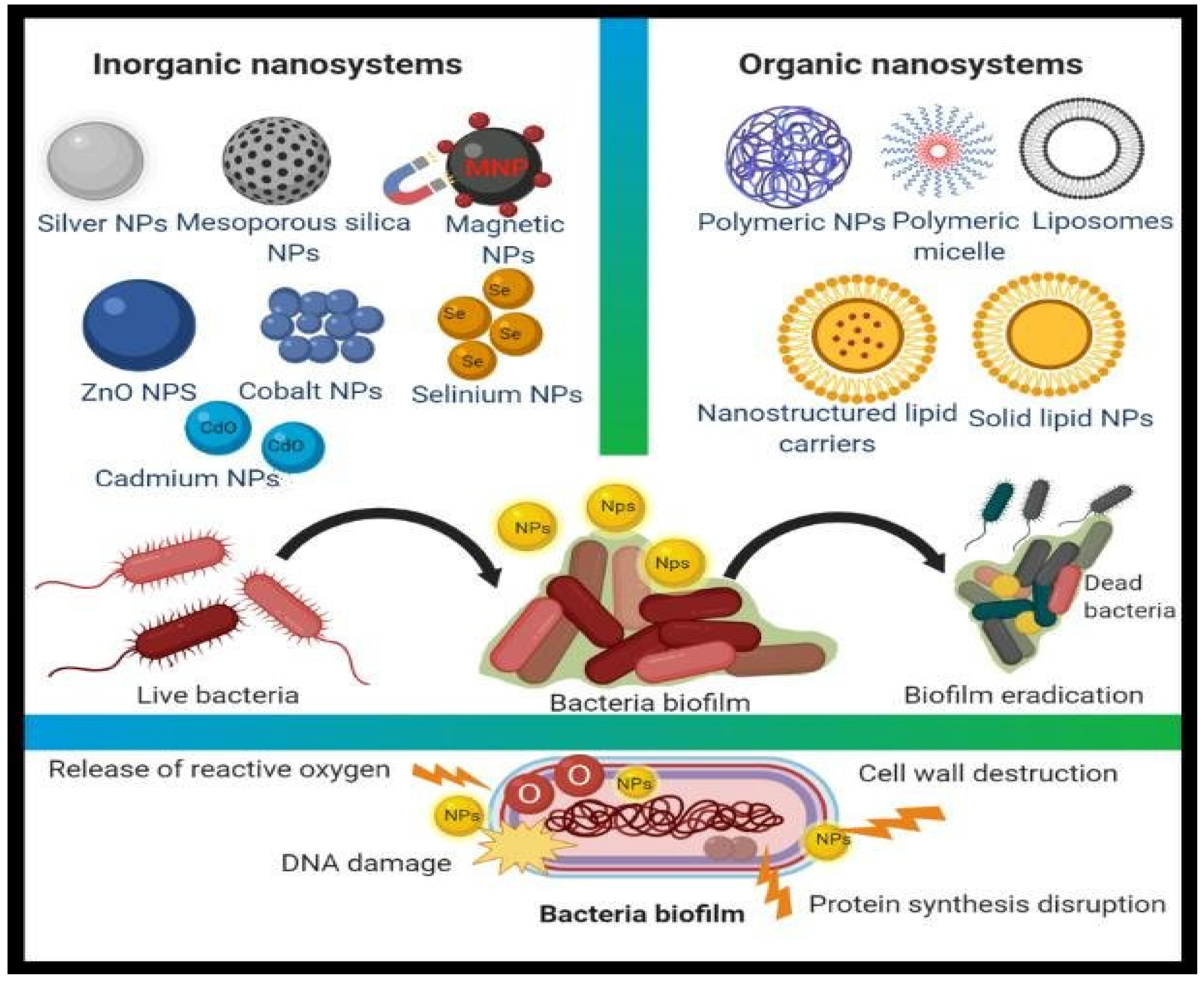
4. Nanoparticles as Antimicrobial Agents
4.1. Synergistic Effect (Antibiotics within Nanoparticles)
4.2. Metal Nanoparticles (Inorganic Nanoparticles)
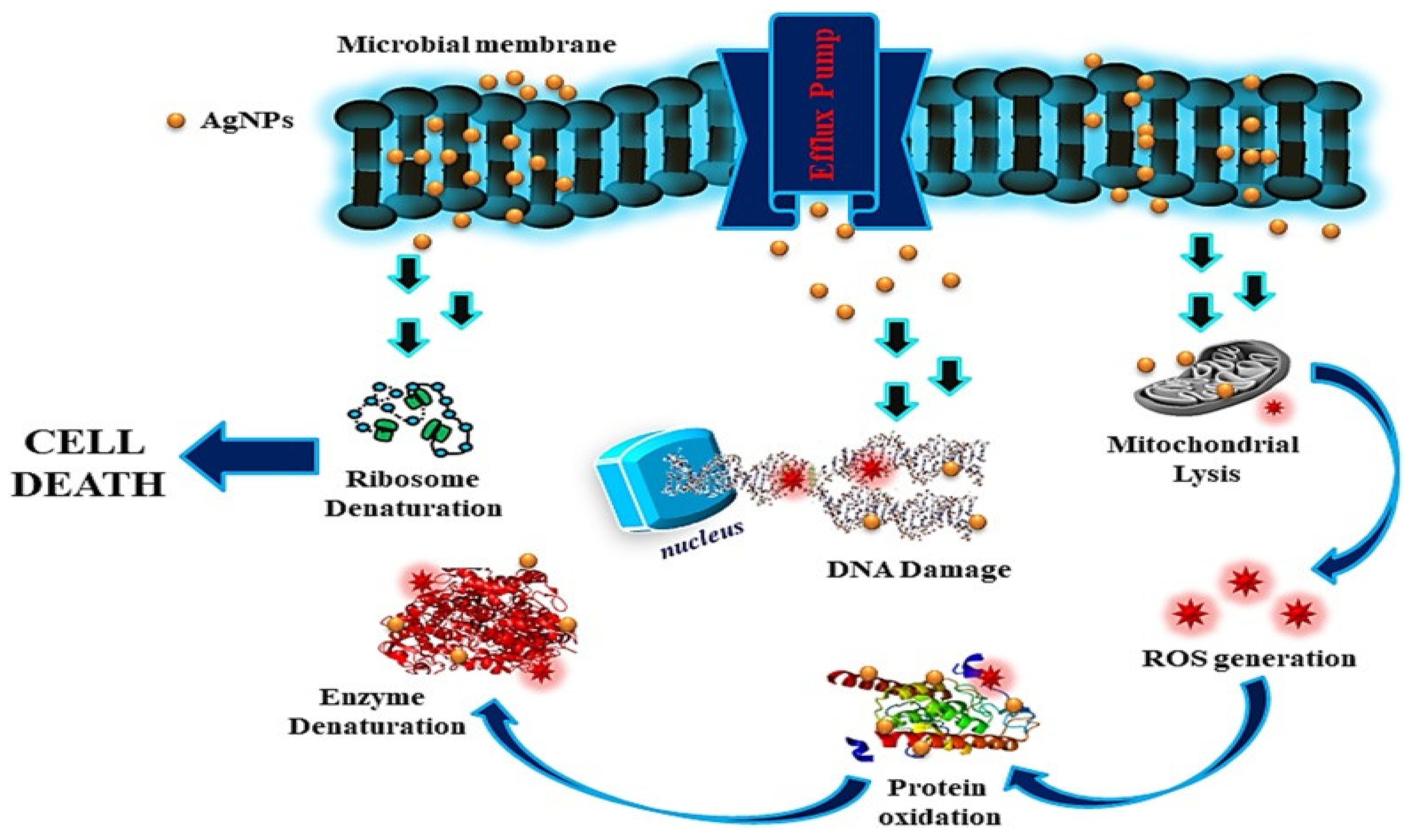
4.2.1. Antimicrobial Role of Silver Nanoparticles
4.2.2. Gold Nanoparticles (Au NPs)
4.3. Copper Nanoparticles (Cu NPs)
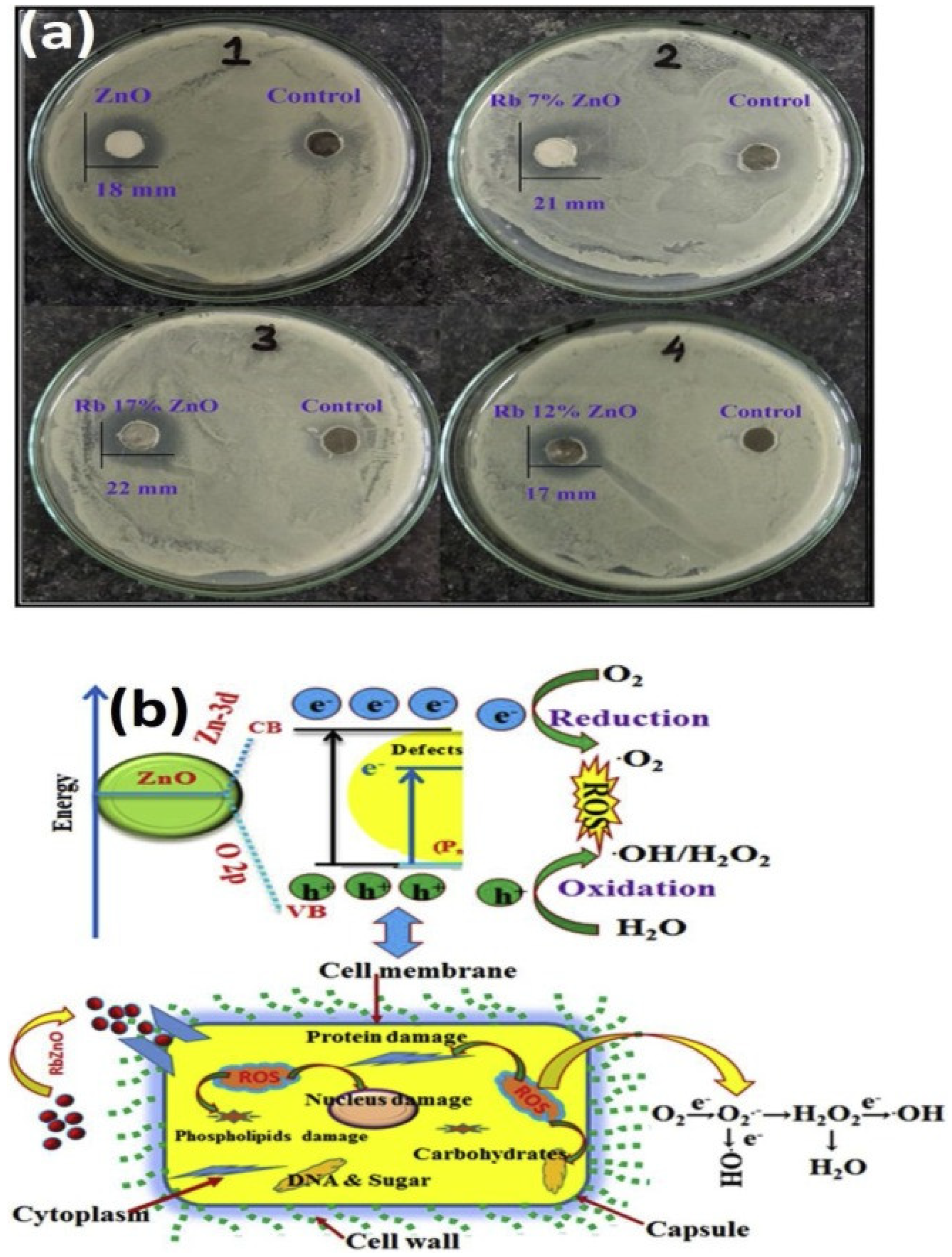
4.4. Zinc Oxide Nanoparticles (ZnO NPs)
4.5. Titanium Dioxide Nanoparticles (TiO2 NPs)
4.6. CuO Nanoparticles (CuO NPs)
5. Organic Nanoparticles
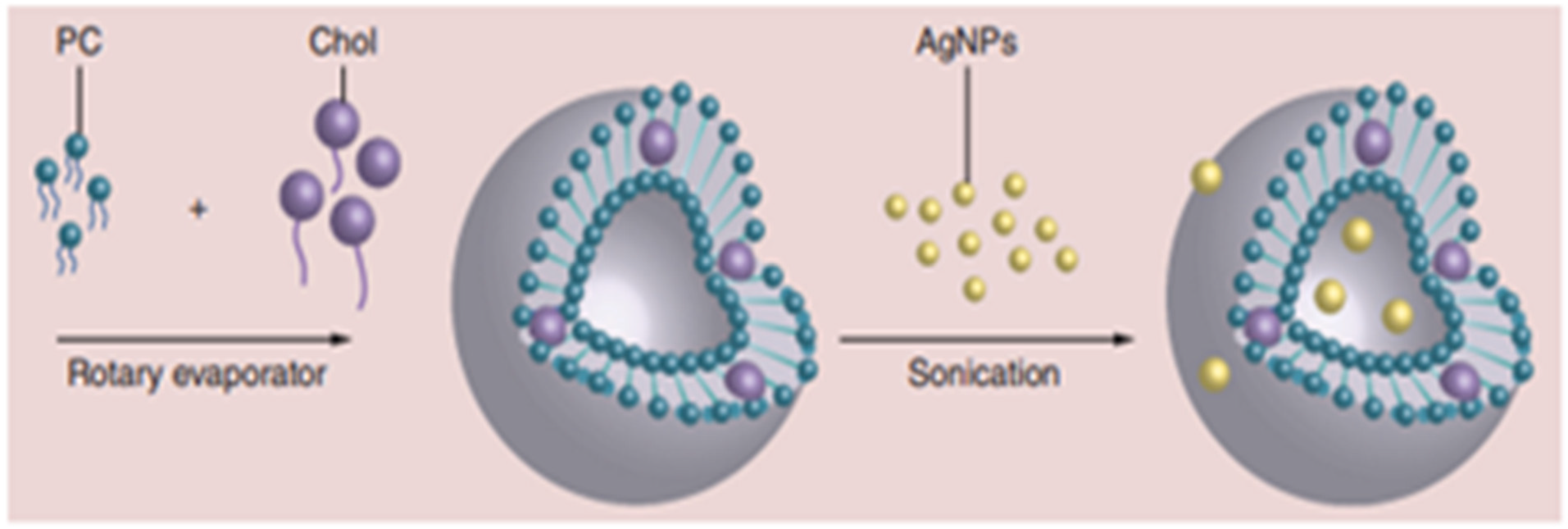
5.1. Liposome
5.2. Cyclodextrin
5.3. Dendrimers
5.4. Chitosan Nanoparticles
5.5. Lignin Nanoparticles
6. Carbon Nanotubes as Antimicrobial Agents
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pereira, D.; Carreira, T.S.; Alves, N.; Sousa, Â.; Valente, J.F.A. Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms. Int. J. Mol. Sci. 2022, 23, 1165. [Google Scholar] [CrossRef] [PubMed]
- Gnanamoorthy, G.; Yadav, V.K.; Yadav, K.K.; Ramar, K.; Alam, J.; Shukla, A.K.; Ali, F.A.A.; Alhoshan, M. Fabrication of different SnO2 nanorods for enhanced photocatalytic degradation and antibacterial activity. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mathpal, M.C.; Ghosh, S.; Inwati, G.K.; Maze, J.R.; Duvenhage, M.-M.; Roos, W.; Swart, H. Plasmonic Au nanoparticles embedded in glass: Study of TOF-SIMS, XPS and its enhanced antimicrobial activities. J. Alloys Compd. 2022, 909, 164789. [Google Scholar] [CrossRef]
- Inwati, G.K.; Kumar, P.; Swart, H.C. Multifunctional properties of hybrid semiconducting nanomaterials and their applications. Nanoscale Compd. Semicond. Optoelectron. Appl. 2022, 7, e06456. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Sheikh, H.I.; Sarkar, T.; Edinur, H.A.; Pati, S.; Ray, R.R. Microbiologically-Synthesized Nanoparticles and Their Role in Silencing the Biofilm Signaling Cascade. Front. Microbiol. 2021, 12, 636588. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. After 2015: Infectious diseases in a new era of health and development. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130426. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; Salamat, M.K.F.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [Green Version]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nature 2017, 543, 15. [Google Scholar] [CrossRef] [Green Version]
- Inwati, G.K.; Kumar, P.; Singh, M.; Yadav, V.K.; Kumar, A.; Soma, V.R.; Swart, H. Study of photoluminescence and nonlinear optical behaviour of AgCu nanoparticles for nanophotonics. Nano-Struct. Nano-Objects 2021, 28, 100807. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Tortella, G.; Rubilar, O.; Fincheira, P.; Benavides-Mendoza, A. Biostimulation and toxicity: The magnitude of the impact of nanomaterials in microorganisms and plants. J Adv. Res. 2021, 31, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Ogren, M.; Dias, J.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-Silva, F.; Gaspar, M.; Aguiar, S. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Santos, R.; Lima, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial coatings based on chitosan to prevent implant-associated infections: A systematic review. iScience 2021, 24, 103480. [Google Scholar] [CrossRef] [PubMed]
- Inwatia, G.; Raoa, Y.; Singh, M. Single step aqueous synthesis of unsupported PtNi nanoalloys using flower extract as reducing agent and their compositional role to enhance electrocatalytic activity. AIP Conf. Proc. 2017, 1837, 040048. [Google Scholar]
- Palaniappan, N.; Inwati, G.; Singh, M. Biomaterial Co-Cr-Mo Alloys Nano Coating Calcium Phosphate Orthopedic Treatment. IOP Conf. Ser. Mater. Sci. Eng. 2014, 64, 012026. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Inwati, G.K.; Mathpal, M.C.; Maze J., W.; Swart, H. Recent Advances on Ferrites Nanomaterial’s as Photocatalyst for Environment. Adv. Nanostruct. Mater. 2022, 560, 381–409. [Google Scholar] [CrossRef]
- Aldayel, M.; El Semary, N. UV irradiation-promoting effect on the antibacterial activity of cyanobacterial extracts against plant pathogens: A first record. Egypt. J. Biol. Pest Control 2020, 30, 132. [Google Scholar] [CrossRef]
- Ben Salem, I.; Ouesleti, S.; Khammassi, M.A.; Boulila, A.; Mabrouk, Y. Effect of Ionizing Radiation on the Microbiological Safety and Phytochemical Properties of Cooked Malva sylvestris L. BioMed Res. Int. 2018, 2018, 2730713. [Google Scholar] [CrossRef] [Green Version]
- James, C.; Dixon, R.; Talbot, L.; James, S.J.; Williams, N.; Onarinde, B.A. Assessing the Impact of Heat Treatment of Food on Antimicrobial Resistance Genes and Their Potential Uptake by Other Bacteria—A Critical Review. Antibiotics 2021, 10, 1440. [Google Scholar] [CrossRef]
- Elashnikov, R.; Ulbrich, P.; Vokatá, B.; Pavlíčková, V.S.; Švorčík, V.; Lyutakov, O.; Rimpelová, S. Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements. Nanomaterials 2021, 11, 3083. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Vu, Q.H.A.; Pham, T.N.N.; Trinh, K.S. Antibacterial Filtration Using Polyethylene Terephthalate Filters Coated with Copper Nanoparticles. J. Nanomater. 2021, 2021, 6628362. [Google Scholar] [CrossRef]
- Zaman, S.B.; Hussain, M.A.; Nye, R.; Mehta, V.; Mamun, K.T.; Hossain, N. A review on antibiotic resistance: Alarm bells are ringing. Cureus 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huhand, Y.A.J.; Kwon, J. Nanoantibiotics’: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistantera. J. Control. Release 2011, 156, 128–145. [Google Scholar]
- Inwati, G.K.; Kumar, P.; Roos, W.D.; Swart, H.C. Thermally induced structural metamorphosis of ZnO:Rb nanostructures for antibacterial impacts. Colloids Surf. B Biointerfaces 2020, 188, 110821. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Brown, C.L.; Lux, L.J.; Brownley, K.A.; Van Dorn, R.A.; Edlund, M.J.; Coker-Schwimmer, E.; Weber, R.; Sheitman, B.; Zarzar, T.; et al. Preventing and De-escalating Aggressive Behavior Among Adult Psychiatric Patients: A Systematic Review of the Evidence. Psychiatr. Serv. 2017, 68, 819–831. [Google Scholar] [CrossRef] [Green Version]
- Gasser, M.; Zingg, W.; Cassini, A.; Kronenberg, A. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in Switzerland. Lancet Infect. Dis. 2019, 19, 17–18. [Google Scholar] [CrossRef] [Green Version]
- Ameen, F.; Alsamhary, K.; Alabdullatif, J.A.; Alnadhari, S. A review on metal-based nanoparticles and their toxicity to beneficial soil bacteria and fungi. Ecotoxicol. Environ. Saf. 2021, 213, 112027. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K. Advances in Nanostructures for Antimicrobial Therapy. Materials 2022, 15, 2388. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Mathpal, M.C.; Inwati, G.; Ghosh, S.; Kumar, V.; Roos, W.; Swart, H. Optical and surface properties of Zn doped CdO nanorods and antimicrobial applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 605, 125369. [Google Scholar] [CrossRef]
- Rajendran, S.; Inwati, G.K.; Yadav, V.K.; Choudhary, N.; Solanki, M.B.; Abdellattif, M.H.; Yadav, K.K.; Gupta, N.; Islam, S.; Jeon, B.-H. Enriched Catalytic Activity of TiO2 Nanoparticles Supported by Activated Carbon for Noxious Pollutant Elimination. Nanomaterials 2021, 11, 2808. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Inwati, G.K.; Mathpal, M.C.; Ghosh, S.; Roos, W.; Swart, H. Defects induced Enhancement of Antifungal activities of Zn doped CuO nanostructures. Appl. Surf. Sci. 2021, 560, 150026. [Google Scholar] [CrossRef]
- Varier, K.M.; Gudeppu, M.; Chinnasamy, A.; Thangarajan, S.; Balasubramanian, J.; Li, Y.; Gajendran, B. Nanoparticles: Antimicrobial Applications and Its Prospects. In Advanced Nanostructured Materials for Environmental Remediation; Naushad, M., Rajendran, S., Gracia, F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 321–355. [Google Scholar]
- Hari, N.; Thomas, T.K.; Nair, A.J. Comparative Study on the Synergistic Action of Differentially Synthesized Silver Nanoparticles with β-Cephem Antibiotics and Chloramphenicol. J. Nanosci. 2014, 2014, 201482. [Google Scholar] [CrossRef] [Green Version]
- Bankier, C.; Matharu, R.; Cheong, Y.K.; Ren, G.G.; Cloutman-Green, E.; Ciric, L. Synergistic Antibacterial Effects of Metallic Nanoparticle Combinations. Sci. Rep. 2019, 9, 16074. [Google Scholar] [CrossRef] [Green Version]
- Malik, P.; Inwati, G.K.; Mukherjee, T.K.; Singh, S.; Singh, M. Green silver nanoparticle and Tween-20 modulated pro-oxidant to antioxidant curcumin transformation in aqueous CTAB stabilized peanut oil emulsions. J. Mol. Liq. 2019, 291, 111252. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Pei, J.; Fu, B.; Jiang, L.; Sun, T. Biosynthesis, characterization, and anticancer effect of plant-mediated silver nanoparticles using Coptis chinensis. Int. J. Nanomed. 2019, 14, 1969–1978. [Google Scholar] [CrossRef] [Green Version]
- Malik, P.; Katyal, V.; Malik, V.; Asatkar, A.; Inwati, G.; Mukherjee, T.K. Nanobiosensors: Concepts and variations. Int. Sch. Res. Not. 2013, 2013, 9. [Google Scholar] [CrossRef]
- Prasher, P.; Singh, M.; Mudila, H. Silver nanoparticles as antimicrobial therapeutics: Current perspectives and future challenges. 3 Biotech 2018, 8, 411. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated Absorption and Scattering Properties of Gold Nanoparticles of Different Size, Shape, and Composition: Applications in Biological Imaging and Biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, T.J.; Wu, K.; Sehmi, S.K.; Noimark, S.; Peveler, W.J.; Du Toit, H.; Voelcker, N.H.; Allan, E.; MacRobert, A.J.; Gavriilidis, A.; et al. Thiol-Capped Gold Nanoparticles Swell-Encapsulated into Polyurethane as Powerful Antibacterial Surfaces Under Dark and Light Conditions. Sci. Rep. 2016, 6, 39272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanamoorthy, G.; Ramar, K.; Ali, D.; Yadav, V.K.; Ahamed, A.J.; Kumar, G. Synthesis and effective performance of Photocatalytic and Antimicrobial activities of Bauhinia tomentosa Linn plants using of gold nanoparticles. Opt. Mater. 2022, 123, 111945. [Google Scholar] [CrossRef]
- Salvadori, M.R.; Ando, R.A.; Nascimento, C.A.O.D.; Corrêa, B. Intracellular Biosynthesis and Removal of Copper Nanoparticles by Dead Biomass of Yeast Isolated from the Wastewater of a Mine in the Brazilian Amazonia. PLoS ONE 2014, 9, e87968. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; El Zowalaty, M.E.; Shameli, K.; Zainuddin, N.; Salama, M.; Ibrahim, N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int. J. Nanomed. 2013, 8, 4467–4479. [Google Scholar] [CrossRef] [Green Version]
- Kruk, T.; Szczepanowicz, K.; Stefańska, J.; Socha, R.; Warszyński, P. Synthesis and antimicrobial activity of monodisperse copper nanoparticles. Colloids Surf. B Biointerfaces 2015, 128, 17–22. [Google Scholar] [CrossRef]
- Armijo, L.M.; Wawrzyniec, S.J.; Kopciuch, M.; Brandt, Y.I.; Rivera, A.C.; Withers, N.J.; Cook, N.C.; Huber, D.L.; Monson, T.C.; Smyth, H.D.C.; et al. Antibacterial activity of iron oxide, iron nitride, and tobramycin conjugated nanoparticles against Pseudomonas aeruginosa biofilms. J. Nanobiotechnol. 2020, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, S.; Krishnakumar, C.; Arulmozhi, P.; Mahadevan, S.; Parameswari, N. Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Glycosmis pentaphylla (Retz.) DC. Microb. Pathog. 2018, 116, 44–48. [Google Scholar] [CrossRef]
- Tiwari, V.; Mishra, N.; Gadani, K.; Solanki, P.; Shah, N.A.; Tiwari, M. Mechanism of Anti-bacterial Activity of Zinc Oxide Nanoparticle Against Carbapenem-Resistant Acinetobacter baumannii. Front. Microbiol. 2018, 9, 1218. [Google Scholar] [CrossRef] [Green Version]
- Gumienna, M.; Górna, B. Antimicrobial Food Packaging with Biodegradable Polymers and Bacteriocins. Molecules 2021, 26, 3735. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial Activity of TiO2 Photocatalyst Alone or in Coatings on E. coli: The Influence of Methodological Aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
- Arora, B.; Murar, M.; Dhumale, V. Antimicrobial potential of TiO2 nanoparticles against MDR Pseudomonas aeruginosa. J. Exp. Nanosci. 2015, 10, 819–827. [Google Scholar] [CrossRef] [Green Version]
- Inwati, G.; Rao, Y.; Singh, M. In Situ Growth of Low-Dimensional Silver Nanoclusters with Their Tunable Plasmonic and Thermodynamic Behavior. ACS Omega 2017, 2, 5748–5758. [Google Scholar] [CrossRef] [PubMed]
- Inwati, G.; Rao, Y.; Singh, M. Thermodynamically induced in Situ and Tunable Cu Plasmonic Behaviour. Sci. Rep. 2018, 8, 3006. [Google Scholar] [CrossRef]
- Inwati, G.K.; Rao, Y.; Singh, M. In Situ Free Radical Growth Mechanism of Platinum Nanoparticles by Microwave Irradiation and Electrocatalytic Properties. Nanoscale Res. Lett. 2016, 11, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnanamoorthy, G.; Yadav, V.K.; Ali, D.; Narayanan, V.; Katubi KM, S.; Alarifi, S. Trigger action of copper aminophosphate (X-CuAP) nanoparticles for enhanced electrochemical, photocatalyst and biological properties. Opt. Mater. 2021, 117, 111113. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Yadav, V.K.; Narayanan, V. Well organized assembly of (X)-CuSnO3 nanoparticles enhanced photocatalytic and anti-bacterial properties. J. Water Process Eng. 2020, 36, 101258. [Google Scholar] [CrossRef]
- Pawar, S.H.; Rohiwal, S.S.; Yakhmi, J. Organic-inorganic antimicrobial nanostructures for health care applications. Biomater. Tissue Eng. Bull. 2017, 4, 66–80. [Google Scholar]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Moribe, K.; Maruyama, K. Pharmaceutical Design of the Liposomal Antimicrobial Agents for Infectious Disease. Curr. Pharm. Des. 2002, 8, 441–454. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Eid, K.A.; Azzazy, H.M. Sustained broad-spectrum antibacterial effects of nanoliposomes loaded with silver nanoparticles. Nanomedicine 2013, 9, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Nagore, P.; Ghotekar, S.; Mane, K.; Ghoti, A.; Bilal, M.; Roy, A. Structural Properties and Antimicrobial Activities of Polyalthia longifolia Leaf Extract-Mediated CuO Nanoparticles. BioNanoScience 2021, 11, 579–589. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Silver Nanoparticles Synthesis from a Pharmaceutically Important Medicinal Plant Plumbago Zeylanica. MOJ Bioequivalence Bioavailab. 2017, 3, 118–121. [Google Scholar]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Singh, M.; Sharma, R.; Banerjee, U. Biotechnological applications of cyclodextrins. Biotechnol. Adv. 2002, 20, 341–359. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Nikolić, I.; Savic, I.; Popsavin, M.; Rakic, S.J.; Mihajilov-Krstev, T.M.; Ristic, I.S.; Eric, S.P.; Savić-Gajic, I.M. Preparation, characterization and antimicrobial activity of inclusion complex of biochanin A with (2-hydroxypropyl)-β-cyclodextrin. J. Pharm. Pharmacol. 2018, 70, 1485–1493. [Google Scholar] [CrossRef]
- Raina, S.; Roy, A.; Bharadvaja, N. Degradation of dyes using biologically synthesized silver and copper nanoparticles. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100278. [Google Scholar] [CrossRef]
- Ciociola, T.; Giovati, L.; Conti, S.; Magliani, W.; Santinoli, C.; Polonelli, L. Natural and synthetic peptides with antifungal activity. Future Med. Chem. 2016, 8, 1413–1433. [Google Scholar] [CrossRef]
- Kannan, R.; Prabakaran, P.; Basu, R.; Pindi, C.; Senapati, S.; Muthuvijayan, V.; Prasad, E. Mechanistic Study on the Antibacterial Activity of Self-Assembled Poly(aryl ether)-Based Amphiphilic Dendrimers. ACS Appl. Bio Mater. 2019, 2, 3212–3224. [Google Scholar] [CrossRef] [Green Version]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of noncytotoxic chitosan-gold nanocomposites as efficient antibacterial materials. ACS Appl. Mater. Interfaces 2015, 1087–1099. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Inwati, G.; Singh, M. Green synthesis of capped gold nanoparticles and their effect on Gram-positive and Gram-negative bacteria. Future Sci. OA 2017, 3, FSO239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, A.; Singh, V.; Sharma, S.; Ali, D.; Azad, A.K.; Kumar, G.; Bin Emran, T. Antibacterial and Dye Degradation Activity of Green Synthesized Iron Nanoparticles. J. Nanomater. 2022, 2022, 3636481. [Google Scholar] [CrossRef]
- Modi, S.; Prajapati, R.; Inwati, G.K.; Deepa, N.; Tirth, V.; Yadav, V.K.; Yadav, K.K.; Islam, S.; Gupta, P.; Kim, D.-H.; et al. Recent Trends in Fascinating Applications of Nanotechnology in Allied Health Sciences. Crystals 2021, 12, 39. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Matalqah, M.S.; Aiedeh, K.; Mhaidat, N.M.; Alzoubi, K.H.; Bustanji, Y.; Hamad, I. Chitosan Nanoparticles as a Novel Drug Delivery System: A Review Article. Curr. Drug Targets 2020, 21, 1613–1624. [Google Scholar] [CrossRef]
- Mahmood, M.A.; Madni, A.; Rehman, M.; Rahim, M.A.; Jabar, A. Ionically Cross-Linked Chitosan Nanoparticles for Sustained Delivery of Docetaxel: Fabrication, Post-Formulation and Acute Oral Toxicity Evaluation. Int. J. Nanomed. 2019, 14, 10035–10046. [Google Scholar] [CrossRef] [Green Version]
- Lintinen, K.; Luiro, S.; Figueiredo, P.; Sakarinen, E.; Mousavi, Z.; Seitsonen, J.; Rivière, G.N.S.; Mattinen, U.; Niemelä, M.; Tammela, P.; et al. Antimicrobial Colloidal Silver–Lignin Particles via Ion and Solvent Exchange. ACS Sustain. Chem. Eng. 2019, 7, 15297–15303. [Google Scholar] [CrossRef] [Green Version]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef] [Green Version]
- Yun, J.; Wei, L.; Li, W.; Gong, D.; Qin, H.; Feng, X.; Li, G.; Ling, Z.; Wang, P.; Yin, B. Isolating High Antimicrobial Ability Lignin From Bamboo Kraft Lignin by Organosolv Fractionation. Front. Bioeng. Biotechnol. 2021, 9, 363. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gupta, N.; Kumar, P.; Dashti, M.G.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Choudhary, N.; Algahtani, A.; et al. Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials 2022, 15, 953. [Google Scholar] [CrossRef]
- Sharma, K.K.; Maurya, I.K.; Khan, S.I.; Jacob, M.R.; Kumar, V.; Tikoo, K.; Jain, R. Discovery of a Membrane-Active, Ring-Modified Histidine Containing Ultrashort Amphiphilic Peptide That Exhibits Potent Inhibition of Cryptococcus neoformans. J. Med. Chem. 2017, 60, 6607–662188. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Y.; Sun, Q.; Zhou, C.; Hu, S.; Lenahan, C.; Xu, W.; Deng, Y.; Li, G.; Tao, S. Update on Nanoparticle-Based Drug Delivery System for Anti-inflammatory Treatment. Front. Bioeng. Biotechnol. 2021, 9, 630352. [Google Scholar] [CrossRef] [PubMed]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon nanotubes as anti-bacterial agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef] [PubMed]
- Inwati, G.K.; Yadav, V.K.; Ali, I.H.; Vuggili, S.B.; Kakodiya, S.D.; Solanki, M.K.; Yadav, K.K.; Ahn, Y.; Yadav, S.; Islam, S.; et al. 2D Personality of Multifunctional Carbon Nitrides towards Enhanced Catalytic Performance in Energy Storage and Remediation. Appl. Sci. 2021, 8, 3753. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and anti-adhesive properties of carbon nanotube-based surfaces for medical applications: A systematic review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef] [PubMed]
- Malek, I.; Schaber, C.F.; Heinlein, T.; Schneider, J.J.; Gorb, S.N.; Schmitz, R.A. Vertically aligned multi walled carbon nanotubes prevent biofilm formation of medically relevant bacteria. J. Mater. Chem. B 2016, 4, 5228–5235. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Liu, D.; Mao, Y.; Ding, L. Carbon nanotubes as antimicrobial agents for water disinfection and pathogen control. J. Water Health 2018, 16, 171–180. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Ana, M. Díez-Pascual, State of the Art in the Antibacterial and Antiviral Applications of Carbon-Based Polymeric Nanocomposites. Int. J. Mol. Sci. 2021, 22, 10511. [Google Scholar]
- ClinicalTrials.gov Extension Study of Liposomal Amikacin for Inhalation in Cystic Fibrosis (CF) Patients with Chronic Pseudomonas Aeruginosa (Pa) Infection. Available online: https://clinicaltrials.gov/ct2/show/NCT01316276 (accessed on 12 November 2019).
- ClinicalTrials.gov Study of Dose Escalation of Liposomal Amikacin for Inhalation (ARIKAYCE™)—Extension Phase. Available online: https://www.clinicaltrials.gov/ct2/show/NCT03905642 (accessed on 18 November 2019).
- Ghobrial, O.G.; Derendorf, H.; Hillman, J.D. Pharmacodynamic activity of the lantibiotic MU1140. Int. J. Antimicrob. Agents 2009, 33, 70–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molchanova, N.; Hansen, P.R.; Franzyk, H. Advances in Development of Antimicrobial Peptidomimetics as Potential Drugs. Molecules 2017, 22, 1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Method | Characteristics | Mode of Action | Reference |
|---|---|---|---|
| Non-ionizing radiation (UV radiation) | 260-nanometer UV range was studied as a prominent zone under 200–280-nanometer UV light | Induces thymine–thymine dimmers that subsequently inhibit the replication of DNA | [17] |
| Ionizing radiation | Electromagnetic radiation and particulate matter | Electron beams, as these are particulate in origin, generate high energy electrons, whereas gamma rays, which are electromagnetic, are used to sterilize a wide range of objects in seconds, including needles, bandage packs, edibles, and medications | [18] |
| Heat | Heat leads to oxidative effects and denaturation and coagulation of proteins. | Heat labile microbes are easily killed due to oxidative effects and protein denaturation | [19] |
| Dry heat | Generally used for sterilization purposes | Higher quantities of electrolytes cause irregular protein structures, radical formations, and lethal effects. | [19] |
| Humid hotness | More effective than dry heat Autoclaving is used at 121 °C for 15 min | The heat is under pressure, which increases its penetration power and kills the spores | [20] |
| Filtration | Different range of membrane filters is used, including earthenware filters, membrane filters, ultrafiltration, sintered glass, and nano-ranged filters or air filters | Separates microorganisms instead of killing them | [21] |
| Conventional Antibiotics | Nanoantibiotics | References |
|---|---|---|
| Lost selective membrane permeability | Interrupt transmembrane transport | [22,23] |
| Antibiotics contain specific functional groups to inhibit biomolecules and their synthesis | Metal nanoparticles, such as ZnO NPs, Ag NPs ROS system damage cellular components, such as cell membrane/wall by adsorbing on the surface Inhibit enzyme and DNA synthesis Produce reactive oxygen species (ROS) that damage the cellular components Disturb energy transduction by interrupting transmembrane electron transport chain reaction, Release heavy metal ions with deleterious effects | [24] |
| Resistance to antibiotics is possible, as bacteria develop resistance genes | Offer resistance against genetic molecules in bacterial cells | [22,24] |
| Require high production costs and times | Require less time and feature lower production costs | [23] |
| Nanomaterials | Antibiotics/Drugs | Target Bacteria/Diseases | References |
|---|---|---|---|
| Ag NPs | Ciprofloxacin, vancomycin Clotrimazole | VRE, MRSA MRSA, S. aureus, | [38,39] |
| Au NPs | Vancomycin, ampicillin | MRSA, MRSA, P. aeruginosa, Enterobacter aerogenes, E. coli | [42] |
| ZnO NPs | Ciprofloxacin, ceftazidime | MDRA. baumannii | [49] |
| Fe3O4 NPs | Ampicillin Ampicillin | S. aureus E. coli, P. aeruginosa, MRSA | |
| SWCNTs | Ciprofloxacin | S. aureus, P. aeruginosa, E. coli | [84,85] |
| Chitosan | Streptomycin Ciprofloxacin | Listeria monocytogenes Uropathogenic E. coli | [62] |
| Liposome | Pioglitazone (PIO), dexamethasone plus minocycline | Atherosclerotic plaques Orthopedic/dental implants | [59] |
| Exosome | Curcumin | Septic shock | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modi, S.; Inwati, G.K.; Gacem, A.; Saquib Abullais, S.; Prajapati, R.; Yadav, V.K.; Syed, R.; Alqahtani, M.S.; Yadav, K.K.; Islam, S.; et al. Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics 2022, 11, 708. https://doi.org/10.3390/antibiotics11060708
Modi S, Inwati GK, Gacem A, Saquib Abullais S, Prajapati R, Yadav VK, Syed R, Alqahtani MS, Yadav KK, Islam S, et al. Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics. 2022; 11(6):708. https://doi.org/10.3390/antibiotics11060708
Chicago/Turabian StyleModi, Shreya, Gajendra Kumar Inwati, Amel Gacem, Shahabe Saquib Abullais, Rajendra Prajapati, Virendra Kumar Yadav, Rabbani Syed, Mohammed S. Alqahtani, Krishna Kumar Yadav, Saiful Islam, and et al. 2022. "Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics" Antibiotics 11, no. 6: 708. https://doi.org/10.3390/antibiotics11060708
APA StyleModi, S., Inwati, G. K., Gacem, A., Saquib Abullais, S., Prajapati, R., Yadav, V. K., Syed, R., Alqahtani, M. S., Yadav, K. K., Islam, S., Ahn, Y., & Jeon, B.-H. (2022). Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics, 11(6), 708. https://doi.org/10.3390/antibiotics11060708








