Magnetite Nanoparticles Functionalized with Therapeutic Agents for Enhanced ENT Antimicrobial Properties
Abstract
:1. Introduction
2. Results
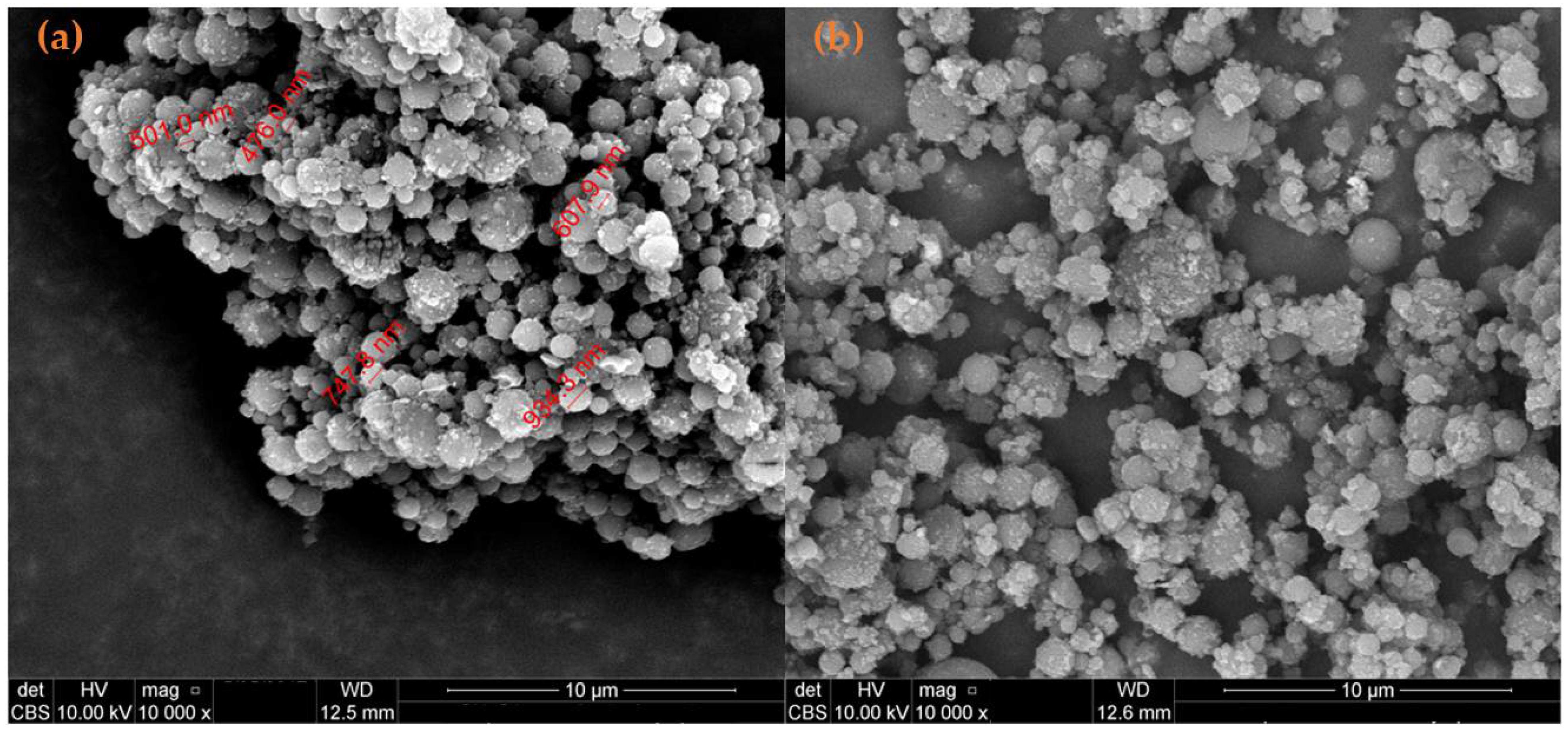
2.1. Physicochemical Characterization of Fe3O4-STR and Fe3O4-NEO Nanoparticles
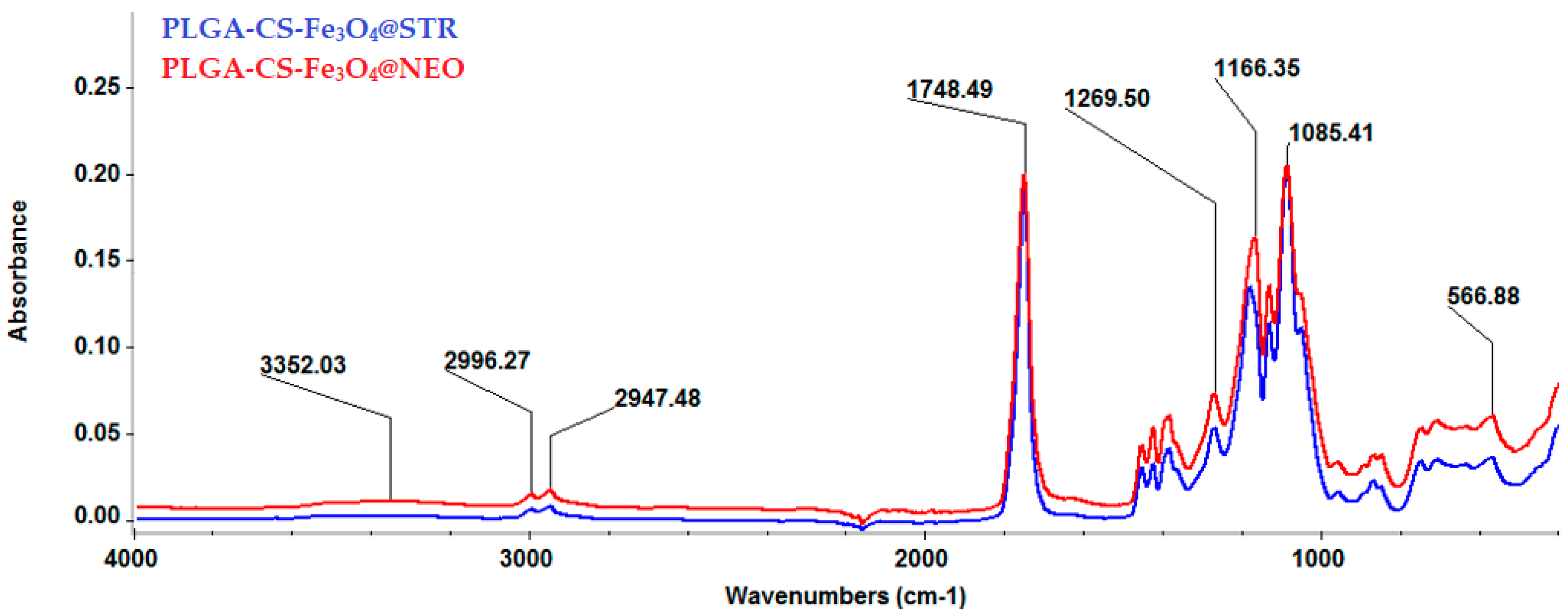
2.2. Physicochemical Characterization of Composites
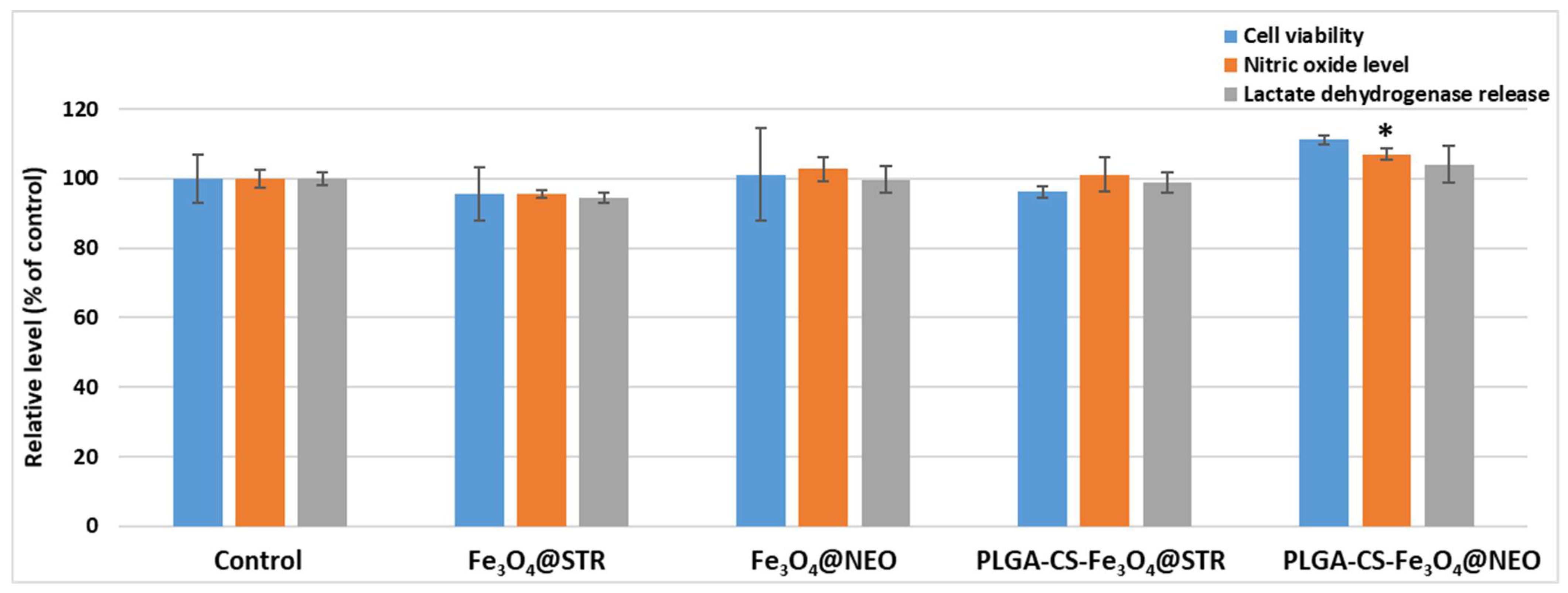
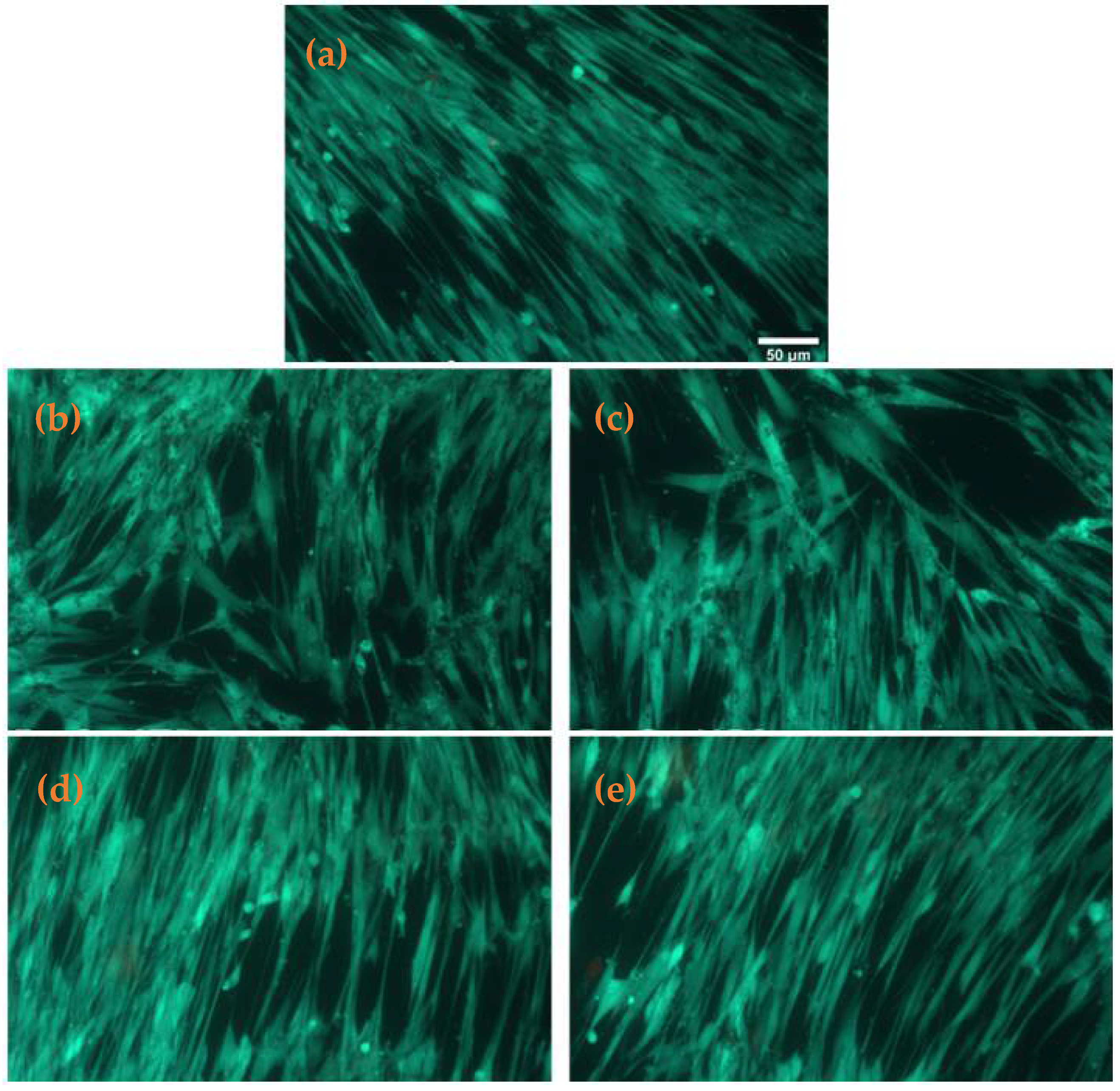
2.3. Biological Evaluation
2.3.1. Cell Viability
2.3.2. Antimicrobial Tests
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Fe3O4@STR and Fe3O4@NEO Nanoparticles
4.3. Preparation of Spheres
4.4. Characterization Methods
4.4.1. XRD
4.4.2. SEM
4.4.3. TEM
4.4.4. FT-IR
4.4.5. TGA-DSC
4.5. Biological Characterization
4.5.1. Cell Culture
4.5.2. Cell Viability and Toxicity Tests
4.5.3. Antimicrobial Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacVane, S.H. Antimicrobial resistance in the intensive care unit: A focus on gram-negative bacterial infections. J. Intensive Care Med. 2017, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Bereket, W.; Hemalatha, K.; Getenet, B.; Wondwossen, T.; Solomon, A.; Zeynudin, A.; Kannan, S. Update on bacterial nosocomial infections. Eur. Rev. Med. Pharm. Sci. 2012, 16, 1039–1044. [Google Scholar]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef] [PubMed]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balakrishnan, K.; Casimeer, S.C.; Ghidan, A.Y.; Al Antary, T.M.; Singaravelu, A. Exploration of Antioxidant, Antibacterial Activities of Green Synthesized Hesperidin Loaded PLGA Nanoparticles. Biointerface Res. Appl. Chem. 2021, 11, 14520–14528. [Google Scholar] [CrossRef]
- Varela, M.F.; Stephen, J.; Lekshmi, M.; Ojha, M.; Wenzel, N.; Sanford, L.M.; Hernandez, A.J.; Parvathi, A.; Kumar, S.H. Bacterial Resistance to Antimicrobial Agents. Antibiotics 2021, 10, 593. [Google Scholar] [CrossRef]
- Nag, M.; Lahiri, D.; Mukherjee, D.; Banerjee, R.; Garai, S.; Sarkar, T.; Ghosh, S.; Dey, A.; Ghosh, S.; Pattnaik, S.; et al. Functionalized Chitosan Nanomaterials: A Jammer for Quorum Sensing. Polymers 2021, 13, 2533. [Google Scholar] [CrossRef]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine Fight against Antibacterial Resistance: An Overview of the Recent Pharmaceutical Innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, A.G.; Rdc, R.; Selari, P.; de Araujo, W.L.; de Souza, A.O. Anti-biofilm action of biological silver nanoparticles produced by aspergillus tubingensis and antimicrobial activity of fabrics carrying it. Biointerface Res. Appl. Chem. 2021, 11, 14764–14774. [Google Scholar] [CrossRef]
- Ficai, D.; Grumezescu, V.; Fufă, O.M.; Popescu, R.C.; Holban, A.M.; Ficai, A.; Grumezescu, A.M.; Mogoanta, L.; Mogosanu, G.D.; Andronescu, E. Antibiofilm Coatings Based on PLGA and Nanostructured Cefepime-Functionalized Magnetite. Nanomaterials 2018, 8, 633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshal, P.; Jamwal, S.; Kumar, P. Glucagon-like Peptide-1 (GLP-1) and neurotransmitters signaling in epilepsy: An insight review. Neuropharmacology 2018, 136, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.J.N.; Ibrahim, F.B.; Srinivasan, H. Synergistic Interactions of Antimicrobials to Counteract the Drug-Resistant Microorganisms. Biointerface Res. Appl. Chem. 2021, 12, 861–872. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Gherasim, O.; Negut, I.; Banita, S.; Holban, A.M.; Florian, P.; Icriverzi, M.; Socol, G. Nanomagnetite-embedded PLGA Spheres for Multipurpose Medical Applications. Materials 2019, 12, 2521. [Google Scholar] [CrossRef] [Green Version]
- Balaraman, P.; Balasubramanian, B.; Liu, W.-C.; Kaliannan, D.; Durai, M.; Kamyab, H.; Alwetaishi, M.; Maluventhen, V.; Ashokkumar, V.; Chelliapan, S.; et al. Sargassum myriocystum-mediated TiO2-nanoparticles and their antimicrobial, larvicidal activities and enhanced photocatalytic degradation of various dyes. Environ. Res. 2022, 204, 112278. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.-X.; Fakhri, A.; Hosseini, M.; Kamyab, H.; Chelliapan, S. Manganese disulfide-silicon dioxide nano-material: Synthesis, characterization, photocatalytic, antioxidant and antimicrobial studies. J. Photochem. Photobiol. B Biol. 2019, 198, 111579. [Google Scholar] [CrossRef]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application areas and development prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, M.; Fazelian, N.; Fakhri, A.; Kamyab, H.; Yadav, K.K.; Chelliapan, S. Preparation, and structural of new NiS-SiO2 and Cr2S3-TiO2 nano-catalyst: Photocatalytic and antimicrobial studies. J. Photochem. Photobiol. B Biol. 2019, 194, 128–134. [Google Scholar] [CrossRef]
- Israel, L.L.; Galstyan, A.; Holler, E.; Ljubimova, J.Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 2020, 320, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Daoush, W.M. Co-precipitation and magnetic properties of magnetite nanoparticles for potential biomedical applications. J. Nanomed. Res. 2017, 5, 00118. [Google Scholar] [CrossRef]
- Yew, Y.P.; Shameli, K.; Miyake, M.; Khairudin, N.B.B.A.; Mohamad, S.E.B.; Naiki, T.; Lee, K.X. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab. J. Chem. 2020, 13, 2287–2308. [Google Scholar] [CrossRef]
- Andrade, R.G.D.; Veloso, S.R.S.; Castanheira, E. Shape anisotropic iron oxide-based magnetic nanoparticles: Synthesis and biomedical applications. Int. J. Mol. Sci. 2020, 21, 2455. [Google Scholar] [CrossRef] [Green Version]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Antony, V.S.; Sahithya, C.S.; Durga Sruthi, P.; Selvarani, J.; Raji, P.; Prakash, P.; Ponnaiah, P.; Petchi, I.; Pattammadath, S.; Keeyari, S. Itraconazole Coated Super Paramagnetic Iron Oxide Nanoparticles for Antimicrobial Studies. Biointerface Res. Appl. Chem. 2020, 10, 6218–6225. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2021, 199, 16–27. [Google Scholar] [CrossRef]
- Maharramov, A.M.; Ramazanov, M.A.; Hasanova, U.A. Chapter 16—Nanostructures for Antimicrobial Therapy—The Modern Trends in the Treatment of Bacterial Infections. In Antimicrobial Nanoarchitectonics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 445–473. [Google Scholar]
- Barros, C.H.N.; Casey, E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Holban, A.M.; Grumezescu, V.; Grumezescu, A.M.; Vasile, B.Ş.; Truşcă, R.; Cristescu, R.; Socol, G.; Iordache, F. Antimicrobial nanospheres thin coatings prepared by advanced pulsed laser technique. Beilstein J. Nanotechnol. 2014, 5, 872–880. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, V.F.; Francesko, A.; Ribeiro, C.; Bañobre-López, M.; Martins, P.; Lanceros-Mendez, S. Advances in Magnetic Nanoparticles for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700845. [Google Scholar] [CrossRef]
- Wallyn, J.; Anton, N.; Vandamme, T.F. Synthesis, Principles, and Properties of Magnetite Nanoparticles for In Vivo Imaging Applications-A Review. Pharmaceutics 2019, 11, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganapathe, L.S.; Mohamed, M.A.; Mohamad Yunus, R.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Gul, S.; Khan, S.B.; Rehman, I.U.; Khan, M.A.; Khan, M.I. A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics. Front. Mater. 2019, 6, 179. [Google Scholar] [CrossRef] [Green Version]
- Holban, A.M.; Grumezescu, V.; Ficai, A.; Grumezescu, A.M.; Chifiriuc, M.C.; Iordache, F.; Andronescu, E. Highly biocompatible magnetite nanoparticles functionalized with chitosan for improving the efficiency of antibiotics. Univ. Politeh. Buchar. Sci. Bull. 2016, 78, 1454–2331. [Google Scholar]
- Arzani, H.; Adabi, M.; Mosafer, J.; Dorkoosh, F.; Khosravani, M.; Maleki, H.; Nekounam, H.; Kamali, M. Preparation of curcumin-loaded PLGA nanoparticles and investigation of its cytotoxicity effects on human glioblastoma U87MG cells. Biointerface Res. Appl. Chem. 2019, 9, 4225–4231. [Google Scholar]
- Iordache, F.; Oprea, A.E.; Grumezescu, V.; Andronescu, E.; Socol, G.; Grumezescu, A.M.; Popa, M.; Mogoşanu, G.D.; Holban, A.M.; Maniu, H. Poly(lactic-co-glycolic) acid/chitosan microsphere thin films functionalized with Cinnamomi aetheroleum and magnetite nanoparticles for preventing the microbial colonization of medical surfaces. J. Sol-Gel Sci. Technol. 2015, 73, 679–686. [Google Scholar] [CrossRef]
- Rancan, F.; Jurisch, J.; Günday, C.; Türeli, E.; Blume-Peytavi, U.; Vogt, A.; Schaudinn, C.; Günday-Türeli, N. Screening of Surfactants for Improved Delivery of Antimicrobials and Poly-Lactic-co-Glycolic Acid Particles in Wound Tissue. Pharmaceutics 2021, 13, 1093. [Google Scholar] [CrossRef]
- Ikono, R.; Vibriani, A.; Wibowo, I.; Saputro, K.E.; Muliawan, W.; Bachtiar, B.M.; Mardliyati, E.; Bachtiar, E.W.; Rochman, N.T.; Kagami, H.; et al. Nanochitosan antimicrobial activity against Streptococcus mutans and Candida albicans dual-species biofilms. BMC Res. Notes 2019, 12, 383. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Wang, X.; Guo, X.; Yang, P.; Yu, J.; Shui, Y.; Chen, C.; Li, X.; Xu, Q.; Xu, L.; et al. Comparison of Antimicrobial Activity of Chitosan Nanoparticles against Bacteria and Fungi. Coatings 2021, 11, 769. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Fawzy, A.; Abdallah, M.; Alqarni, N. Oxidative degradation of neomycin and streptomycin by cerium(IV) in sulphuric and perchloric acid solutions. J. Mol. Liq. 2020, 312, 113439. [Google Scholar] [CrossRef]
- Kudo, F. 2.22—Biosynthesis of Aminoglycoside Antibiotics. In Comprehensive Natural Products III.; Liu, H.-W., Begley, T.P., Eds.; Elsevier: Oxford, UK, 2020; pp. 588–612. [Google Scholar]
- Jospe-Kaufman, M.; Siomin, L.; Fridman, M. The relationship between the structure and toxicity of aminoglycoside antibiotics. Bioorganic Med. Chem. Lett. 2020, 30, 127218. [Google Scholar] [CrossRef] [PubMed]
- Duskey, J.T.; Baraldi, C.; Gamberini, M.C.; Ottonelli, I.; Da Ros, F.; Tosi, G.; Forni, F.; Vandelli, M.A.; Ruozi, B. Investigating Novel Syntheses of a Series of Unique Hybrid PLGA-Chitosan Polymers for Potential Therapeutic Delivery Applications. Polymers 2020, 12, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibarra, J.; Melendres, J.; Almada, M.; Burboa, M.G.; Taboada, P.; Juárez, J.; Valdez, M.A. Synthesis and characterization of magnetite/PLGA/chitosan nanoparticles. Mater. Res. Express 2015, 2, 095010. [Google Scholar] [CrossRef]
- Dizaji, B.F.; Azerbaijan, M.H.; Sheisi, N.; Goleij, P.; Mirmajidi, T.; Chogan, F.; Irani, M.; Sharafian, F. Synthesis of PLGA/chitosan/zeolites and PLGA/chitosan/metal organic frameworks nanofibers for targeted delivery of Paclitaxel toward prostate cancer cells death. Int. J. Biol. Macromol. 2020, 164, 1461–1474. [Google Scholar] [CrossRef]
- Cricchio, V.; Best, M.; Reverchon, E.; Maffulli, N.; Phillips, G.; Santin, M.; Della Porta, G. Novel Superparamagnetic Microdevices Based on Magnetized PLGA/PLA Microparticles Obtained by Supercritical Fluid Emulsion and Coating by Carboxybetaine-Functionalized Chitosan Allowing the Tuneable Release of Therapeutics. J. Pharm. Sci. 2017, 106, 2097–2105. [Google Scholar] [CrossRef]
- Kiamohamadi, L.; Khoei, S.; Khoee, S.; Asadi, L. The effect of two different polymeric-coated magnetite nano-graphene oxide as 5-fluorouracil carrier and radiofrequency hyperthermia on colon cancer in vitro. Iran. J. Med. Phys. 2018, 15, 166. [Google Scholar]
- Ghanbari, M.; Shamspur, T.; Fathirad, F.; Mahani, S.E. In Situ Preparation of Magnetic Fe3O4 Nanoparticles in Presence of PLGA and PVA as Magnetite Nanocarrier for Targeted Drug Delivery. J. Pharm. Drug Deliv. Res. 2017, 6, 2. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Niculescu, A.G.; Slave, Ș.; Bîrcă, A.C.; Dorcioman, G.; Grumezescu, V.; Holban, A.M.; Oprea, O.C.; Vasile, B.; Grumezescu, A.M.; et al. Anti-Biofilm Coatings Based on Chitosan and Lysozyme Functionalized Magnetite Nanoparticles. Antibiotics 2021, 10, 1269. [Google Scholar] [CrossRef]
- Mohammed, H.B.; Rayyif, S.M.I.; Curutiu, C.; Birca, A.C.; Oprea, O.C.; Grumezescu, A.M.; Ditu, L.M.; Gheorghe, I.; Chifiriuc, M.C.; Mihaescu, G.; et al. Eugenol-Functionalized Magnetite Nanoparticles Modulate Virulence and Persistence in Pseudomonas aeruginosa Clinical Strains. Molecules 2021, 26, 2189. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jusu, S.M.; Obayemi, J.D.; Salifu, A.A.; Nwazojie, C.C.; Uzonwanne, V.; Odusanya, O.S.; Soboyejo, W.O. Drug-encapsulated blend of PLGA-PEG microspheres: In vitro and in vivo study of the effects of localized/targeted drug delivery on the treatment of triple-negative breast cancer. Sci. Rep. 2020, 10, 14188. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, A.; Karlioti, G.; Balla, E.; Daniilidis, V.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Christodoulou, E.; Koumentakou, I.; Karavas, E.; et al. Poly(Lactic Acid)-Based Microparticles for Drug Delivery Applications: An Overview of Recent Advances. Pharmaceutics 2022, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Güliz, A.K.; Şengel, T.Y.; Şanlier, Ş.H. Amoxicillin Loaded Magnetic Nanoparticles Developed for Treatment of Osteomyelitis. Hacet. J. Biol. Chem. 2020, 48, 137–145. [Google Scholar]
- Rayegan, A.; Allafchian, A.; Sarsari, I.A.; Kameli, P. Synthesis and characterization of basil seed mucilage coated Fe3O4 magnetic nanoparticles as a drug carrier for the controlled delivery of cephalexin. Int. J. Biol. Macromol. 2018, 113, 317–328. [Google Scholar] [CrossRef]
- Lage, W.C.; Sachs, D.; Ribeiro, T.A.N.; Tebaldi, M.L.; de Moura, Y.d.R.S.; Domingues, S.C.; Soares, D.C.F. Mesoporous iron oxide nanoparticles loaded with ciprofloxacin as a potential biocompatible antibacterial system. Microporous Mesoporous Mater. 2021, 321, 111127. [Google Scholar] [CrossRef]
- Ivashchenko, O.; Woźniak, A.; Coy, E.; Peplinska, B.; Gapinski, J.; Jurga, S. Release and cytotoxicity studies of magnetite/Ag/antibiotic nanoparticles: An interdependent relationship. Colloids Surf. B Biointerfaces 2017, 152, 85–94. [Google Scholar] [CrossRef]
- Grumezescu, V.; Negut, I.; Grumezescu, A.M.; Ficai, A.; Dorcioman, G.; Socol, G.; Iordache, F.; Truşcă, R.; Vasile, B.S.; Holban, A.M. MAPLE fabricated coatings based on magnetite nanoparticles embedded into biopolymeric spheres resistant to microbial colonization. Appl. Surf. Sci. 2018, 448, 230–236. [Google Scholar] [CrossRef]
- Rashid, M.; Rabbi, M.A.; Ara, T.; Hossain, M.M.; Islam, M.S.; Elaissari, A.; Ahmad, H.; Rahman, M.M. Vancomycin conjugated iron oxide nanoparticles for magnetic targeting and efficient capture of Gram-positive and Gram-negative bacteria. RSC Adv. 2021, 11, 36319–36328. [Google Scholar] [CrossRef]
- Vardanyan, R.S.; Hruby, V.J. 32—Antibiotics. In Synthesis of Essential Drugs; Vardanyan, R.S., Hruby, V.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 425–498. [Google Scholar]
- Hu, Y.; Liu, L.; Zhang, X.; Feng, Y.; Zong, Z. In Vitro Activity of Neomycin, Streptomycin, Paromomycin and Apramycin against Carbapenem-Resistant Enterobacteriaceae Clinical Strains. Front. Microbiol. 2017, 8, 2275. [Google Scholar] [CrossRef] [Green Version]
- Story, S.; Skriba, M.J.; Maiti, K.; Nihar, R.; Degtyareva, N.N.; Green, K.D.; Khodaverdian, V.; Oyelere, A.K.; Garneau-Tsodikova, S.; Arya, D.P. Synthesis, antimicrobial activity, attenuation of aminoglycoside resistance in MRSA, and ribosomal A-site binding of pyrene-neomycin conjugates. Eur. J. Med. Chem. 2019, 163, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Nekobahr, E.; Akhtari, J.; Saeedi, M.; Akbari, J.; Fathi, F. Synthesis and characterization of magnetite nanoparticles by co-precipitation method coated with biocompatible compounds and evaluation of in-vitro cytotoxicity. Toxicol. Rep. 2021, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, I.; Moustafa, M.M.; Nassar, M.Y. Facile controllable synthesis of magnetite nanoparticles via a co-precipitation approach. Egypt. J. Chem. 2022, 65, 9. [Google Scholar] [CrossRef]
- Klencsár, Z.; Ábrahám, A.; Szabó, L.; Szabó, E.G.; Stichleutner, S.; Kuzmann, E.; Homonnay, Z.; Tolnai, G. The effect of preparation conditions on magnetite nanoparticles obtained via chemical co-precipitation. Mater. Chem. Phys. 2019, 223, 122–132. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X.B. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Donalisio, M.; Argenziano, M.; Rittà, M.; Bastiancich, C.; Civra, A.; Lembo, D.; Cavalli, R. Acyclovir-loaded sulfobutyl ether-β-cyclodextrin decorated chitosan nanodroplets for the local treatment of HSV-2 infections. Int. J. Pharm. 2020, 587, 119676. [Google Scholar] [CrossRef] [PubMed]
- Fatouh, A.M.; Elshafeey, A.H.; Abdelbary, A. Galactosylated Chitosan Coated Liposomes of Ledipasvir for Liver Targeting: Chemical Synthesis, Statistical Optimization, In-vitro and In-vivo evaluation. J. Pharm. Sci. 2021, 110, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, Y.; Wang, L.; Li, G.; Gao, J.; Wang, Y. Development of L-carnosine functionalized iron oxide nanoparticles loaded with dexamethasone for simultaneous therapeutic potential of blood brain barrier crossing and ischemic stroke treatment. Drug Deliv. 2021, 28, 380–389. [Google Scholar] [CrossRef]
- Tzankova, V.; Aluani, D.; Kondeva-Burdina, M.; Yordanov, Y.; Odzhakov, F.; Apostolov, A.; Yoncheva, K. Hepatoprotective and antioxidant activity of quercetin loaded chitosan/alginate particles in vitro and in vivo in a model of paracetamol-induced toxicity. Biomed. Pharmacother. 2017, 92, 569–579. [Google Scholar] [CrossRef]
- Xie, Y.; Yi, Y.; Hu, X.; Shangguan, M.; Wang, L.; Lu, Y.; Qi, J.; Wu, W. Synchronous microencapsulation of multiple components in silymarin into PLGA nanoparticles by an emulsification/solvent evaporation method. Pharm. Dev. Technol. 2016, 21, 672–679. [Google Scholar] [CrossRef]
- Subbiah, L.; Palanisamy, S.; Thamizhmurasu, S.; Mathew Joseph, A.B.; Thangavelu, P.; Ganeshan, M.; Raj, D.B.T.G. Development of Meloxicam-chitosan magnetic nanoconjugates for targeting rheumatoid arthritis joints: Pharmaceutical characterization and preclinical assessment on murine models. J. Magn. Magn. Mater. 2021, 523, 167571. [Google Scholar] [CrossRef]
- Khanal, S.; Adhikari, U.; Rijal, N.P.; Bhattarai, S.R.; Sankar, J.; Bhattarai, N. pH-Responsive PLGA Nanoparticle for Controlled Payload Delivery of Diclofenac Sodium. J. Funct. Biomater. 2016, 7, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afzali, E.; Eslaminejad, T.; Rouholamini, S.E.Y.; Shahrokhi-Farjah, M.; Ansari, M. Cytotoxicity Effects of Curcumin Loaded on Chitosan Alginate Nanospheres on the KMBC-10 Spheroids Cell Line. Int. J. Nanomed. 2021, 16, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, L.P.; Ucakar, B.; Joudiou, N.; Bianco, J.; Danhier, P.; Zhao, M.; Bastiancich, C.; Gallez, B.; Danhier, F.; Préat, V. Magnetic targeting of paclitaxel-loaded poly(lactic-co-glycolic acid)-based nanoparticles for the treatment of glioblastoma. Int. J. Nanomed. 2018, 13, 4509–4521. [Google Scholar] [CrossRef] [Green Version]
- Sivakumar, B.; Aswathy, R.G.; Nagaoka, Y.; Iwai, S.; Hasumura, T.; Venugopal, K.; Kato, K.; Yoshida, Y.; Maekawa, T.; Sakthikumar, D.N. Augmented cellular uptake and antiproliferation against pancreatic cancer cells induced by targeted curcumin and SPION encapsulated PLGA nanoformulation. Mater. Express 2014, 4, 183–195. [Google Scholar] [CrossRef]
- Ashjari, M.; Panahandeh, F.; Niazi, Z.; Abolhasani, M.M. Synthesis of PLGA–mPEG star-like block copolymer to form micelle loaded magnetite as a nanocarrier for hydrophobic anticancer drug. J. Drug Deliv. Sci. Technol. 2020, 56, 101563. [Google Scholar] [CrossRef]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Ismail, M.; Webster, T.J. Synthesis, characterization, controlled release, and antibacterial studies of a novel streptomycin chitosan magnetic nanoantibiotic. Int. J. Nanomed. 2014, 9, 549–557. [Google Scholar] [CrossRef] [Green Version]
- El Zowalaty, M.E.; Al Ali, S.H.H.; Husseiny, M.I.; Geilich, B.M.; Webster, T.J.; Hussein, M.Z. The ability of streptomycin-loaded chitosan-coated magnetic nanocomposites to possess antimicrobial and antituberculosis activities. Int. J. Nanomed. 2015, 10, 3269–3274. [Google Scholar] [CrossRef] [Green Version]
- Grumezescu, A.M.; Cotar, A.I.; Andronescu, E.; Ficai, A.; Ghitulica, C.D.; Grumezescu, V.; Vasile, B.S.; Chifiriuc, M.C. In vitro activity of the new water-dispersible Fe3O4@usnic acid nanostructure against planktonic and sessile bacterial cells. J. Nanoparticle Res. 2013, 15, 1766. [Google Scholar] [CrossRef]
- Grumezescu, A.; Vasile, B.; Holban, A.J.L.A.N. Eugenol functionalized magnetite nanostructures used in anti-infectious therapy. Lett. Appl. Nanobiosci. 2013, 2, 120–123. [Google Scholar]
- Doan, T.; Couet, W.; Olivier, J.C. Formulation and in vitro characterization of inhalable rifampicin-loaded PLGA microspheres for sustained lung delivery. Int. J. Pharm. 2011, 414, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Socol, G.; Grumezescu, A.M.; Holban, A.M.; Ficai, A.; Truşcǎ, R.; Bleotu, C.; Balaure, P.C.; Cristescu, R.; Chifiriuc, M.C. Functionalized antibiofilm thin coatings based on PLA–PVA microspheres loaded with usnic acid natural compounds fabricated by MAPLE. Appl. Surf. Sci. 2014, 302, 262–267. [Google Scholar] [CrossRef]
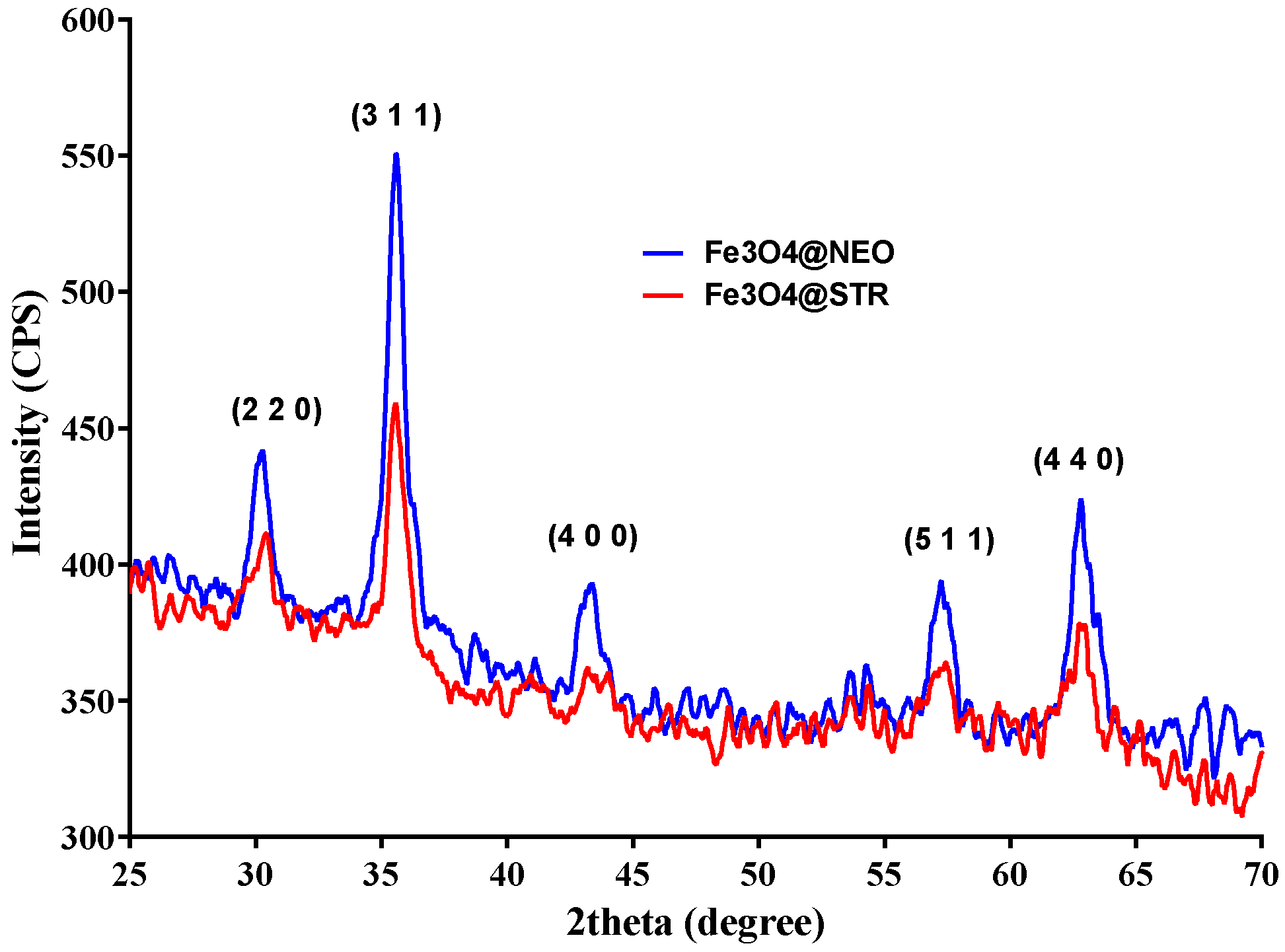
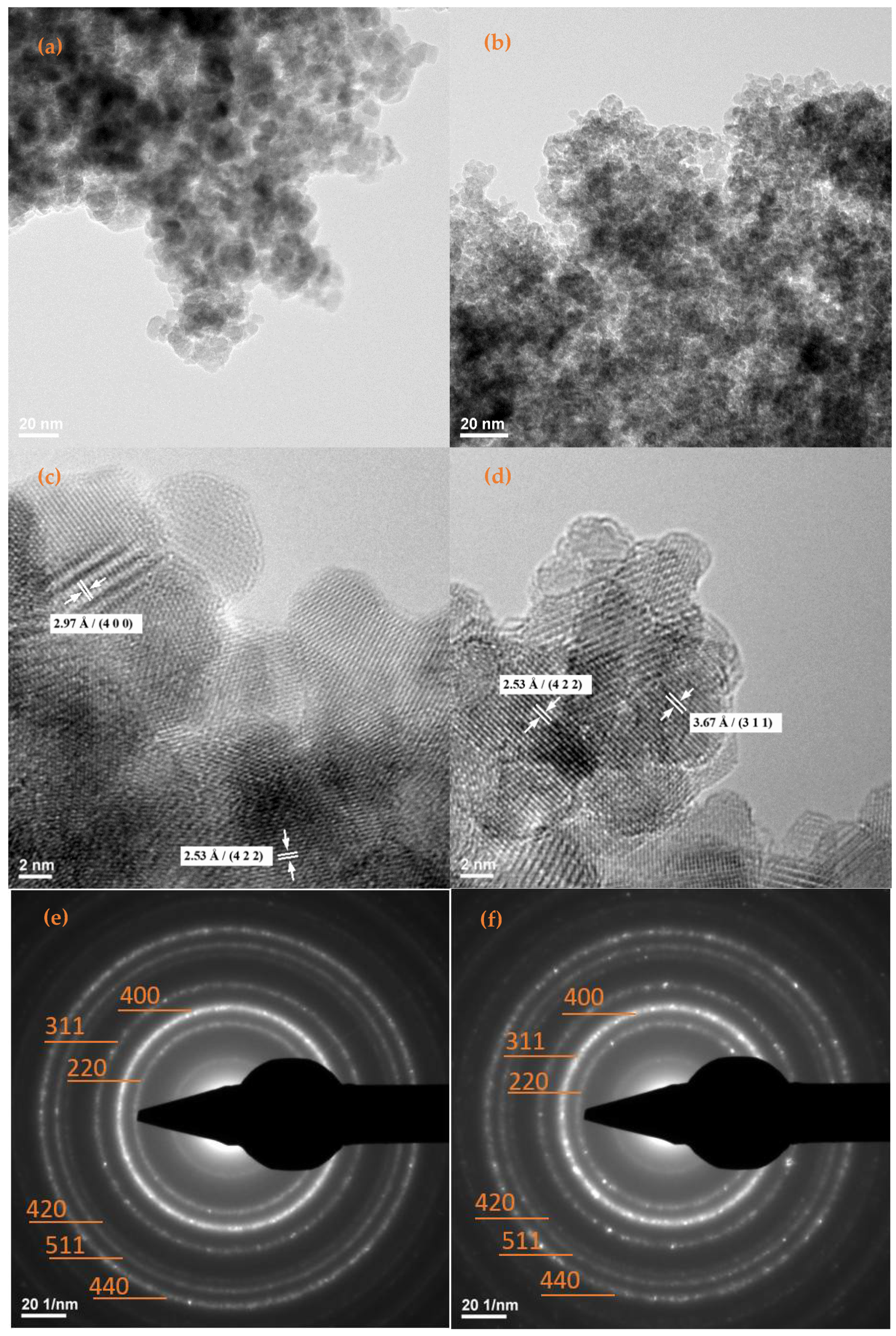
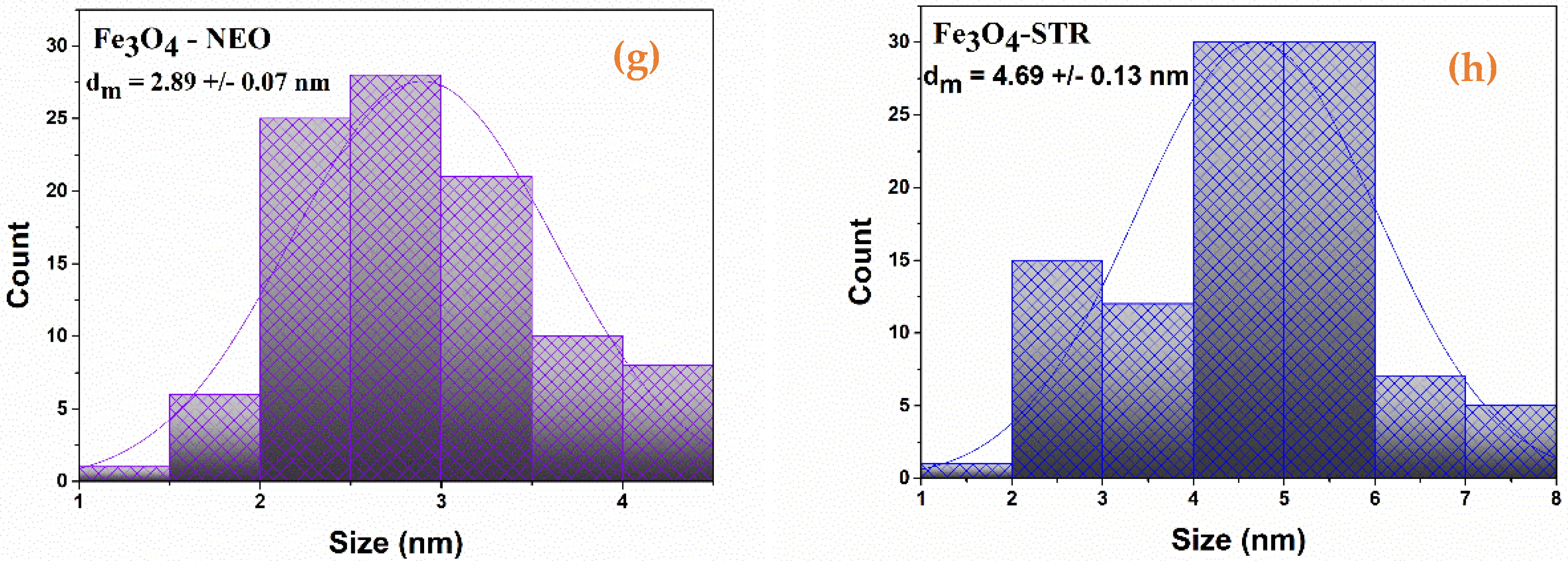
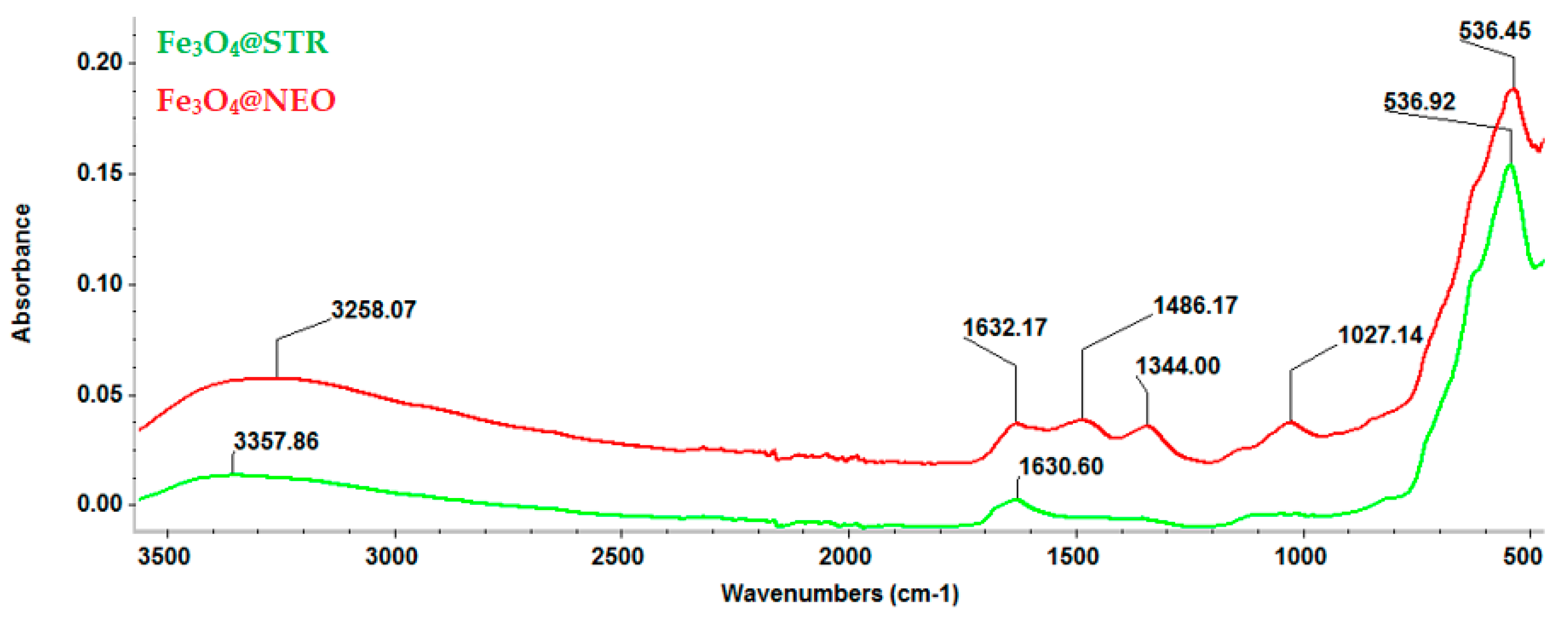
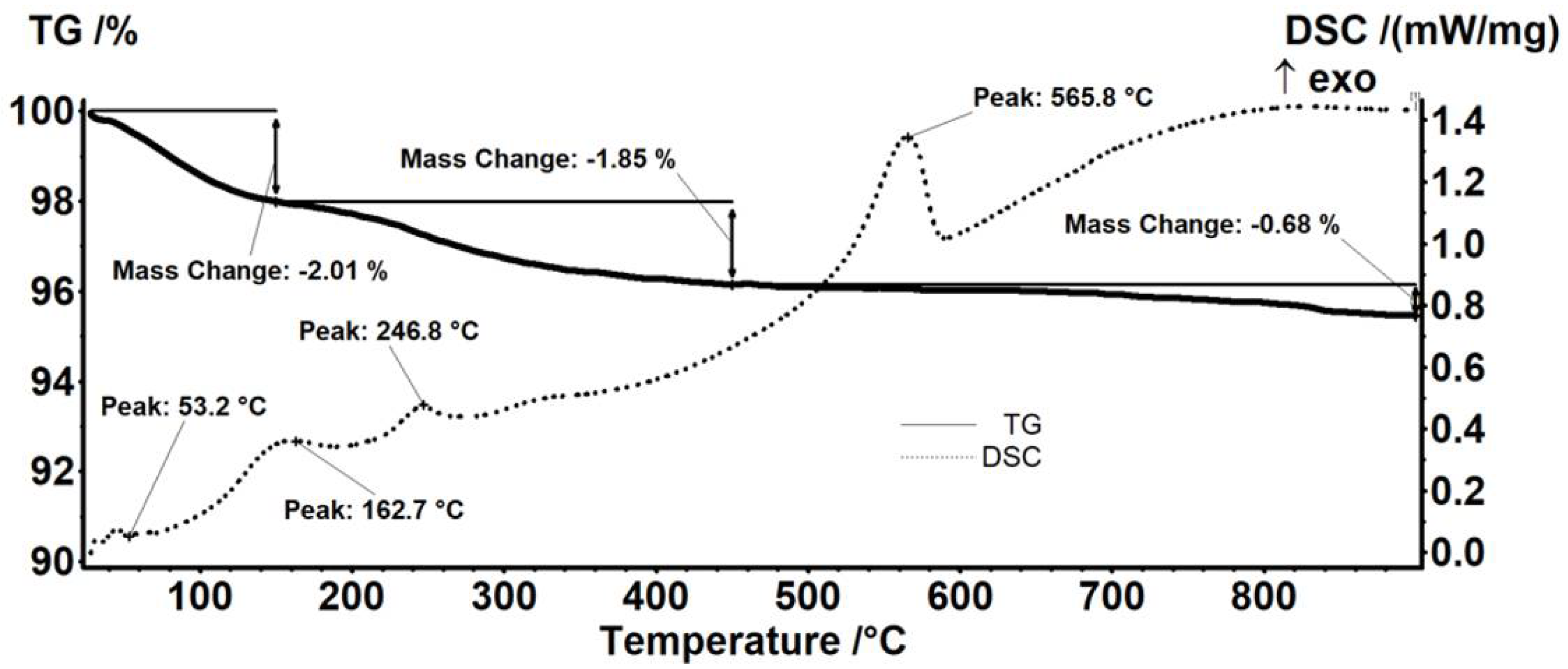
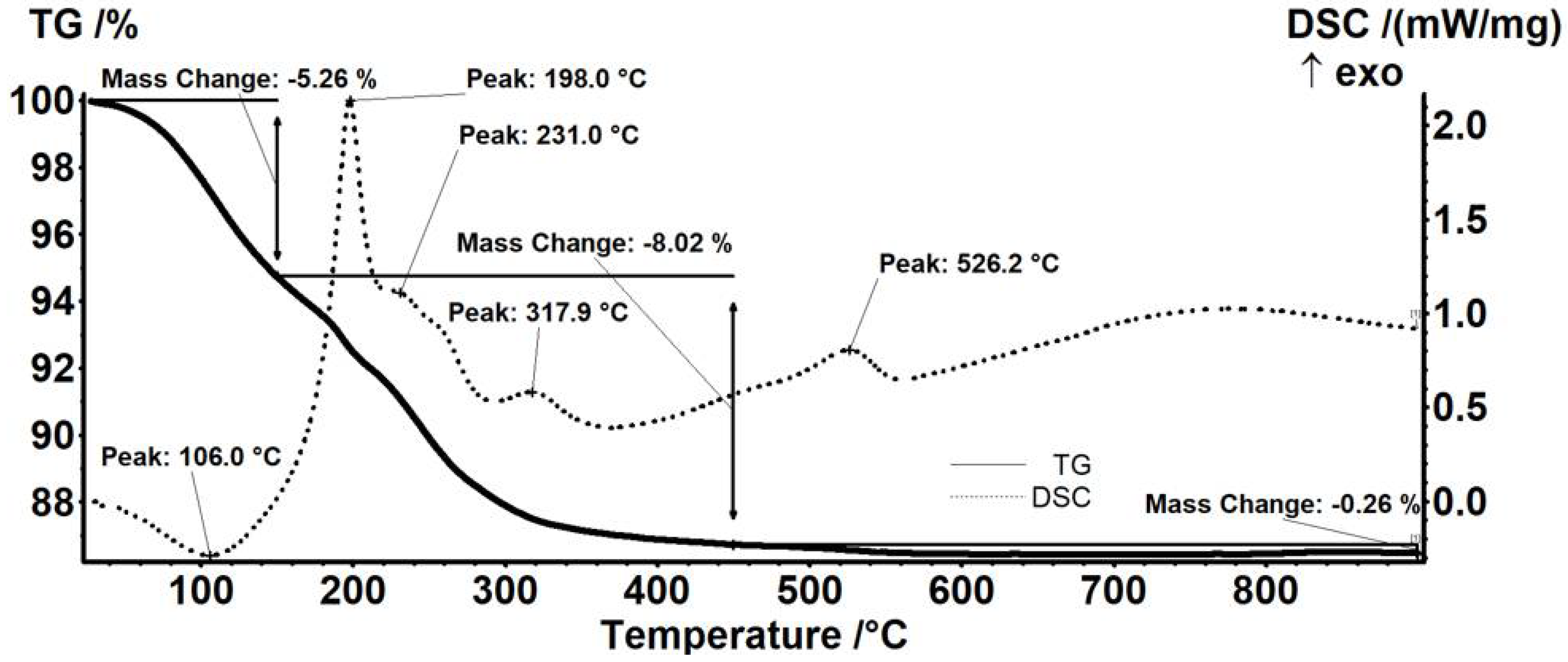
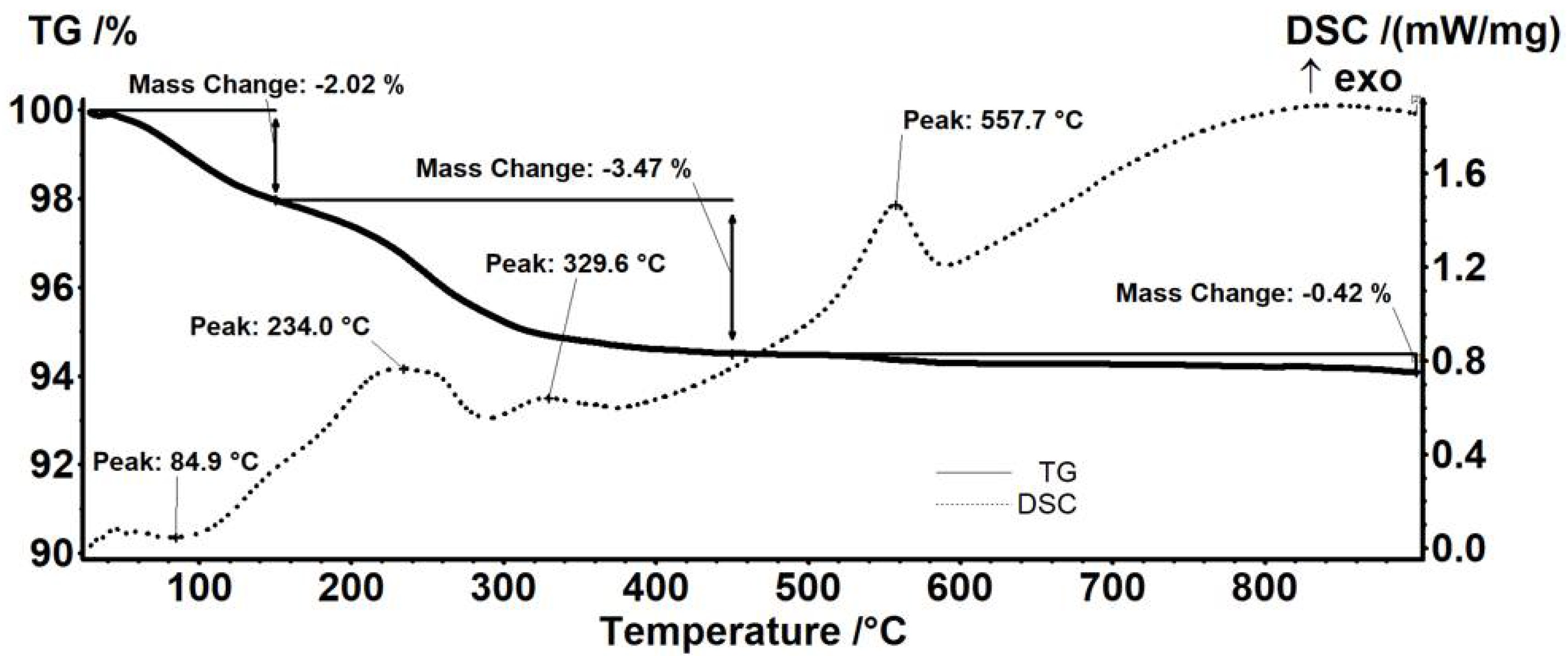

| Sample | Mass Loss RT-150 °C | Mass Loss 150–450 °C | Residual Mass (%) | Endo | Exo | Estimated Load (%) |
|---|---|---|---|---|---|---|
| Fe3O4 | 2.01% | 1.85% | 95.45% | 53.2 °C | 565.8 °C | - |
| Fe3O4@NEO | 5.26% | 8.02% | 86.46% | 106.0 °C | 526.2 °C | 9.42% |
| Fe3O4@STR | 2.02% | 3.47% | 94.08% | 84.9 °C | 557.7 °C | 1.44% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caciandone, M.; Niculescu, A.-G.; Grumezescu, V.; Bîrcă, A.C.; Ghica, I.C.; Vasile, B.Ș.; Oprea, O.; Nica, I.C.; Stan, M.S.; Holban, A.M.; et al. Magnetite Nanoparticles Functionalized with Therapeutic Agents for Enhanced ENT Antimicrobial Properties. Antibiotics 2022, 11, 623. https://doi.org/10.3390/antibiotics11050623
Caciandone M, Niculescu A-G, Grumezescu V, Bîrcă AC, Ghica IC, Vasile BȘ, Oprea O, Nica IC, Stan MS, Holban AM, et al. Magnetite Nanoparticles Functionalized with Therapeutic Agents for Enhanced ENT Antimicrobial Properties. Antibiotics. 2022; 11(5):623. https://doi.org/10.3390/antibiotics11050623
Chicago/Turabian StyleCaciandone, Mara, Adelina-Gabriela Niculescu, Valentina Grumezescu, Alexandra Cătălina Bîrcă, Ionuț Cosmin Ghica, Bogdan Ștefan Vasile, Ovidiu Oprea, Ionela Cristina Nica, Miruna Silvia Stan, Alina Maria Holban, and et al. 2022. "Magnetite Nanoparticles Functionalized with Therapeutic Agents for Enhanced ENT Antimicrobial Properties" Antibiotics 11, no. 5: 623. https://doi.org/10.3390/antibiotics11050623
APA StyleCaciandone, M., Niculescu, A.-G., Grumezescu, V., Bîrcă, A. C., Ghica, I. C., Vasile, B. Ș., Oprea, O., Nica, I. C., Stan, M. S., Holban, A. M., Grumezescu, A. M., Anghel, I., & Anghel, A. G. (2022). Magnetite Nanoparticles Functionalized with Therapeutic Agents for Enhanced ENT Antimicrobial Properties. Antibiotics, 11(5), 623. https://doi.org/10.3390/antibiotics11050623













