Abstract
Vancomycin or daptomycin is administered to hemodialysis patients infected with methicillin-resistant Staphylococcus and Enterococcus species. Although serious concerns regarding nephrotoxicity due to vancomycin have been raised, it might not be a critical issue in hemodialysis patients. Moreover, very few studies have investigated the effectiveness of vancomycin versus daptomycin in patients undergoing hemodialysis. Hence, we retrospectively evaluated the effectiveness and safety of vancomycin and daptomycin in patients undergoing hemodialysis. We investigated the following measures: mortality, clinical and microbiological effectiveness, and incidence of adverse events in hemodialysis patients who received vancomycin or daptomycin from 2014 to 2019. Moreover, we evaluated the covariates related to 30-day mortality. We found that 73 patients received vancomycin, while 34 received daptomycin for the treatment of infections due to methicillin-resistant Staphylococcus aureus, methicillin-resistant coagulase-negative Staphylococci, and Enterococcus faecium. Mortality after vancomycin treatment was significantly lower than daptomycin treatment (4.1% vs. 26.5%, p < 0.01). The clinical and microbiological effectiveness as well as the safety were not significantly different between the two treatments. Although daptomycin treatment with a loading dose was associated with lower mortality, the mortality of the treatment (8.3%) did not differ significantly compared to that of the vancomycin treatment (4.1%). Therefore, our findings suggest that vancomycin remains the first-line treatment for hemodialysis patients; however, a loading dose may be beneficial for patients receiving daptomycin.
1. Introduction
The absolute and relative risks of mortality from infections in hemodialysis patients have greatly increased due to their immunocompromised state, frequent exposure to the hospital environment, and tunneled catheters that allow the formation of biofilms [1,2]. Infection accounts for 12% to 36% of mortality in hemodialysis patients and is second only to cardiovascular disease as a cause of death [3,4]. The most common species detected in hemodialysis patients are Staphylococcus, including methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant coagulase-negative Staphylococci (MRCNS), which account for 69.1% of these microbes [5]. Moreover, it has been reported that infection mortality due to MRSA and MRCNS is 63.2% [5]. Therefore, appropriate antibiotic selection for infections due to MRSA and MRCNS in hemodialysis patients is an important factor for improving mortality.
Current guidelines recommend the use of vancomycin or daptomycin for the empirical treatment of MRSA infections in hemodialysis patients [6]. Although vancomycin is still considered the first-line treatment for MRSA infections owing to its bactericidal action, serious concerns regarding its safety profile, such as nephrotoxicity, have been raised [7]. However, this may not be a critical issue for hemodialysis patients. Moreover, very few studies have investigated the effectiveness of vancomycin versus daptomycin in patients undergoing hemodialysis. Hence, we retrospectively evaluated the effectiveness and safety of vancomycin and daptomycin in hemodialysis patients.
2. Results
2.1. Patients
In total, 107 patients met the inclusion criteria during the study period, wherein 73 received vancomycin (vancomycin trough concentrations, 16.4 ± 4.4 mg/L) and 34 received daptomycin. Among the patients who received daptomycin, 12 received a loading dose (Figure 1).
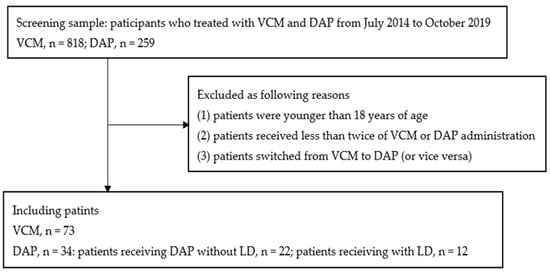
Figure 1.
Flow diagram of study participants. DAP, daptomycin; LD, loading dose; VCM, vancomycin.
The demographic and clinical characteristics stratified by therapeutic regimen are presented in Table 1. Comparing both treatments, the males and patients receiving a loading dose in the vancomycin group had a significantly higher rate than in the daptomycin group (vancomycin vs. daptomycin: males, 87.7% vs. 67.6%, p = 0.01; receiving a loading dose, 82.2% vs. 35.3%, p < 0.01). The others did not differ significantly between the two groups.

Table 1.
Clinical characteristics of the registered patients.
2.2. Microbiological Data
Vancomycin and daptomycin were administered to treat infections caused by MRSA, MRCNS, or Enterococcus faecium (Table 1). The most frequent isolates detected in this study were MRSA (vancomycin, 60.3%; daptomycin, 67.7%; p = 0.46), followed by MRCNS (31.5%; 29.4%; p = 0.83) and E. faecium (8.2%; 0%; p = 0.30). Regarding the minimum inhibitory concentration (MIC) distribution of these strains against vancomycin and daptomycin, the median MICs were 1.0 mg/L (range, 0.5–4.0 mg/L) and 1.0 mg/L (range, 0.25–2.0 mg/L), respectively. Moreover, one and two resistant isolates were detected in patients receiving vancomycin and daptomycin, respectively. However, vancomycin-resistant Enterococcus was not detected in the present study.
2.3. Clinical and Microbiological Effectiveness
The percentage of patients who reached a body temperature of <37.0 °C after the end of the treatment was 55.9% for vancomycin and 44.1% for daptomycin. The percentage of patients who reached CRP levels <60% of the baseline was 55.4% for vancomycin and 44.1% for daptomycin. There were no significant differences between vancomycin and daptomycin treatments (body temperature, p = 0.26; CRP level, p = 0.29; Table 2). Vancomycin showed lower mortality on days 14 (2.7% vs. 11.8%, p = 0.06) and 30 (4.1% vs. 26.5%, p < 0.01; Table 2) as compared to daptomycin. Especially, vancomycin in bacteremia and skin and soft tissue infections showed lower mortality on days 30 as compared to daptomycin (vancomycin vs. daptomycin: bacteremia, 6.7% vs. 27.8%, p = 0.04; skin and soft tissue infections, 3.0% vs. 25.0%, p = 0.02). In a subgroup of patients with bacteremia due to MRSA and E. faecium, there was no significant difference between the two groups (8.3% vs. 33.3%, p = 0.15). Microbiological cure rates showed no significant difference between both groups (78.6% vs. 77.7%, p = 0.94; Table 2).

Table 2.
Clinical and microbiological effectiveness of the registered patients.
2.4. Safety Evaluation
The safety data are listed in Table 3. According to the study criteria, elevated CK and eosinophil counts were observed in two and seven patients, respectively. No patient was diagnosed with eosinophilic pneumonia in this population, whereas four were diagnosed with allergies. Additionally, the total number of patients who were admitted with abnormalities in AST and ALT levels was ten and nine, respectively. However, the incidence of adverse events was not significantly different between the two treatments.

Table 3.
Safety data of the registered patients.
2.5. Comparison between the Survival and Non-Survival Groups
The mortality in our population was 11.2% (survival group, n = 95; non-survival group, n = 12). Table 4 shows the differences in the covariates related to 30-day mortality between the survival and non-survival groups. Receiving a loading dose of vancomycin or daptomycin was associated with a significant reduction in mortality (survival group vs. non-survival group, 72.6% vs. 25.0%, p < 0.01). Receiving the loading dose of vancomycin did not improve mortality (82.9% vs. 66.7%, p = 0.472), whereas that of daptomycin showed a tendency to reduce mortality (44.0% vs. 11.1%, p = 0.08). Moreover, microbiological effect was higher in the survival group than in the non-survival group (81.1% vs. 55.6%, p = 0.08). The other measures did not significantly differ between the two groups. In the logistic analysis (Table 4), covariates significantly related to the mortality were identified: receiving a loading dose (p < 0.01, OR 7.96, 95% CI 2.00–31.72), receiving a loading dose of daptomycin (p = 0.10, OR 6.29, 95% CI 0.68–58.11), and microbiological effectiveness (p = 0.09, OR 3.43, 95% CI 0.81–14.44).

Table 4.
Comparison of survival and non-survival groups.
2.6. Clinical Effectiveness and Safety in Hemodialysis Patients Receiving Vancomycin and Daptomycin Treatments with or without a Loading Dose
Patients receiving a daptomycin treatment without a loading dose were 22, while those with a loading dose were 12. Regarding clinical effectiveness, the 30-day mortality was significantly different among the three groups; the daptomycin treatment with a loading dose showed a lower mortality than that without (vancomycin treatment vs. daptomycin treatment without the loading dose vs. daptomycin treatment with the loading dose, 4.1% vs. 63.4% vs. 8.3%, p < 0.01; Table 5). The other groups did not differ significantly from each other. Regarding safety, there were no significant differences among the three groups (Table 6).

Table 5.
Clinical and microbiological effectiveness of patients receiving vancomycin and daptomycin treatments with or without a loading dose.

Table 6.
Safety data of patients receiving vancomycin and daptomycin treatments with or without a loading dose.
3. Discussion
To the best of our knowledge, this is the first study to compare the effectiveness and safety of vancomycin and daptomycin in patients undergoing hemodialysis. In our study, the vancomycin treatment improved mortality without significant adverse events compared with the daptomycin treatment. Moreover, the loading dose, especially that of daptomycin, was one of the covariates related to mortality, which was reduced to 8.3%. Therefore, vancomycin remains as the first-line treatment, while a loading dose of daptomycin may be beneficial in hemodialysis patients.
To date, studies in various populations have investigated and compared the effectiveness and safety of vancomycin and daptomycin [8,9]. A recent meta-analysis indicated that daptomycin was better tolerated than vancomycin in the treatment of MRSA infections without lung involvement [10]. On the other hand, a systematic review of hemodialysis patients reported that there were no studies evaluating the incidence of adverse events attributable to vancomycin [11]. Japanese studies, including the present one, have shown that hemodialysis patients do not frequently develop adverse events caused by vancomycin [12]. These observations highlight the higher tolerability of vancomycin in hemodialysis patients than in those with residual renal function.
In a meta-analysis, the risk of mortality failed to show a statistically significant difference between vancomycin and daptomycin for the treatment of MRSA infections in all patients [10]. However, it has been reported that the clinical failure rate of patients with impaired renal function is lower with vancomycin than with daptomycin [13,14]. Moreover, the present study of hemodialysis patients indicated that vancomycin significantly reduced mortality as compared to daptomycin. Therefore, vancomycin may be more effective than daptomycin in patients with impaired renal function, especially hemodialysis patients. In contrast, there were no significant differences in the clinical and microbiological effectiveness between vancomycin and daptomycin treatments. Moreover, the levels of inflammatory markers before treatment were not significantly different between the two groups. Therefore, the reduction in mortality may be associated with an improvement in the early clinical response. However, the present study did not design to measure the blood concentration of daptomycin even though daptomycin requires a considerable time to achieve steady-state concentrations because of half-life in hemodialysis patients. Further well-designed studies are needed to validate the effectiveness and safety of vancomycin versus daptomycin. Moreover, although caution is advised since the benefits of vancomycin for hemodialysis patients may depend on the MIC, the present as well as other studies [15,16] have found no association between antibiotic treatment and mortality.
A loading dose regimen has been recommended to rapidly reach an effective therapeutic concentration as well as to optimize pharmacokinetic and pharmacodynamic indicators [17,18,19,20]. However, a recent meta-analysis reported that a loading dose of vancomycin did not reduce mortality or improve microbiological effectiveness [21]. In contrast, we previously found in an in vivo study that a loading dose of daptomycin exhibited a higher microbiological activity [22]. Retrospective studies showed that similar treatments also improved clinical symptoms at an early stage [17,23]. In the present study, daptomycin treatment without the loading dose showed a significantly higher mortality than the vancomycin treatment (p < 0.01), while the mortality in the patients receiving daptomycin treatment with the loading dose was equivalent with those of the vancomycin treatment (p = 0.52). Therefore, daptomycin treatment with the loading dose appears to be effective for hemodialysis patients. However, further large-scale studies are needed since the sample size is limited.
Recent practical guideline has recommended a high dose of daptomycin [6]. However, the present retrospective study did not include patients receiving a high dose of daptomycin since previous studies of patients with renal impairment reported that the high dose was associated with a high incidence of creatinine kinase elevation [24]. Therefore, further studies to compare a normal dose with a high dose of daptomycin are needed to validate the effectiveness of a high dose of daptomycin.
Several considerations should be made when interpreting our results. First, this was a non-randomized, single-center, retrospective study. We were unable to investigate the degree of disease severity owing to a lack of data. Selection bias could have been present, and missing data may have influenced the results. Thirty-four patients were treated with daptomycin throughout the study period, and patients receiving a loading dose of daptomycin were 12. The quality of the study is limited by its small sample size. It should be noted that patients, in whom MRSA, MRCNS, or E. faecium were detected, were included in our study. Although we were not able to evaluate the microbiological effectiveness in 22.4% of the patients, more detailed microbiological data regarding the MIC values were reported.
4. Patients and Methods
4.1. Patient Population
We retrospectively reviewed the medical records of hemodialysis patients treated with vancomycin or daptomycin at the Aichi Medical University Hospital from July 2014 to October 2019. Patients were excluded from the study for the following reasons: (1) patients were younger than 18 years of age, (2) patients received less than twice of vancomycin or daptomycin administration, and (3) patients switched from vancomycin to daptomycin (or vice versa). The study was reviewed and approved by the ethics committee of the Aichi Medical University (No. 2020-058).
4.2. Treatment Regimen
Vancomycin was administered to achieve a target trough of 10–20 mg/L on the day of the second hemodialysis according to Japanese therapeutic drug monitoring guidelines [25]. Daptomycin was administered thrice per week according to daptomycin prescription information (4–6 mg/kg/day). Some of the patients received a dose of 4–6 mg/kg daptomycin thrice per week with a loading dose (>8 mg/kg) on day 1 based on our previous study results [17,23].
4.3. Data Collection
At least 3 days before the start of treatment, treatment data, including patient demographics, hospitalization history, source of infection, and laboratory data, were retrospectively collected through chart review. The clinical outcomes and antibiotic-related adverse reactions were recorded for each patient.4.4. Microbiological Data
Identification and susceptibility tests for the isolated organisms were conducted at the Department of Laboratory at Aichi Medical University Hospital. All isolates were susceptibility-tested using the broth microdilution method as described by the Clinical and Laboratory Standards Institute (CLSI) [26]. Vancomycin-resistant pathogens were defined as isolates with an MIC of >4 mg/L for Staphylococcus and >8 mg/L for Enterococcus. Daptomycin-resistant pathogens were defined as isolates with an MIC > 2 mg/L for Staphylococcus and >4 mg/L for Enterococcus. For the purpose of this study, all specimens (blood, wound, abscess, pus, or tissue) cultured at the microbiology laboratory during the study periods were included.
4.4. Clinical and Microbiological Effectiveness
The evaluation was performed in accordance with previous studies [17,22]. Data regarding inflammatory markers of body temperature and C-reactive protein (CRP) level at least 3 days before the start (baseline level) and after the end of antibiotic treatment were collected. For clinical effectiveness, we evaluated the percentages of patients who reached a body temperature <37.0 °C and CRP <60% of the baseline level. Survival was defined as survival at 14 and 30 days after antibiotic treatment. A microbiological cure was defined as effective when bacteria disappeared during and after antibiotic treatment.
4.5. Safety Evaluation
We evaluated abnormality with CTCAE version 4.03 the Common Terminology Criteria for Adverse Events (CTCAE). The abnormality was defined as follows: aspartate aminotransferase (AST), >2 × upper baseline (35 U/L); alanine aminotransferase (ALT), >2 × upper baseline (30 U/L); creatinine kinase (CK), >1.3 × upper baseline (200 U/L); and eosinophil granulocyte count, >500/μL. In addition, we assessed the onset of eosinophilic pneumonia and rash (allergies).
4.6. Evaluation of Covariate Related to 30-Day Mortality
The following factors were compared between survival and non-survival groups: loading dose, initial vancomycin trough concentrations, microbiological effectiveness, bacteremia, and detection of vancomycin- or daptomycin-resistant pathogens, MRSA, MRCNS, and E. faecium. Moreover, logistic regression analysis was performed to determine the odds ratio (OR) for 30-day mortality. Factors showing a p-value of 0.1 were considered candidate predictors significantly related to mortality.
4.7. Evaluation of a Loading Dose of Daptomycin for Clinical Effectiveness and Safety
Previously, we revealed that receiving a loading dose of daptomycin was a significant covariate for mortality in hemodialysis patients [23]. However, a logistic regression analysis showed that receiving a loading dose of daptomycin was identified as a positive covariate related to mortality. Thus, we compared the influence of daptomycin treatment with a loading dose of vancomycin and daptomycin treatment without a loading dose on clinical and microbiological outcomes in hemodialysis patients.
4.8. Statistical Analysis
The data regarding the clinical characteristics of the patients were expressed as median values (minimum–maximum). Statistical significance was evaluated using the chi-square test for categorical data and the unpaired t-test for continuous data. Statistical analysis was performed using JMP, version 10.0 (SAS, Tokyo, Japan). A p-value of <0.05 was required to achieve statistical significance.
5. Conclusions
In conclusion, our study indicated that vancomycin significantly reduced mortality in hemodialysis patients as compared to daptomycin. Although daptomycin treatment with a loading dose was associated with lower mortality, the mortality of the treatment did not differ significantly compared with that of vancomycin. Therefore, our findings suggest that vancomycin remains as the first-line treatment for hemodialysis patients, but a loading dose may be beneficial for patients receiving daptomycin.
Author Contributions
Conceptualization, H.K. and M.H.; Methodology, H.K. and M.H.; Software, H.K.; Validation, N.A. and J.H.; formal analysis, H.K. and M.K.; investigation, H.K. and M.K.; resources, H.K. and M.K.; data curation, H.K. and M.K.; writing—original draft preparation, H.K.; writing—review and editing, M.H., M.K., Y.Y., T.U., N.A., J.H., T.I., and H.M.; visualization, H.K. and M.H.; supervision, H.M.; project administration, H.K.; funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the ethics committee of Aichi Medical University (No. 2020-058).
Informed Consent Statement
Patient consent was waived because only leftover materials, otherwise discarded, were used, and no additional intervention or change in treatment plan was implemented.
Data Availability Statement
All data are available in the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vandecasteele, S.J.; Boelaert, J.R.; De Vriese, A.S. Staphylococcus aureus infections in hemodialysis: What a nephrologist should know. Clin. J. Am. Soc. Nephrol. 2009, 4, 1388–1400. [Google Scholar] [CrossRef]
- Ramanathan, V.; Riosa, S.; Al-Sharif, A.H.; Mansouri, M.D.; Tranchina, A.; Kayyal, T.; Abreo, A.P.; Aslam, S.; Nassar, G.; Darouiche, R.O. Characteristics of biofilm on tunneled cuffed hemodialysis catheters in the presence and absence of clinical infection. Am. J. Kidney Dis. 2012, 60, 976–982. [Google Scholar] [CrossRef]
- Bloembergen, W.E.; Prt, F.K. Epidemiological perspective on infections in chronic dialysis patients. Adv. Ren. Replace Ther. 1996, 3, 201–207. [Google Scholar] [CrossRef]
- Nassar, G.M.; Ayus, J.C. Infectious complications of the hemodialysis access. Kidney Int. 2001, 60, 1–13. [Google Scholar] [CrossRef]
- Iwabuchi, H.; Nakahara, T.; Okamoto, M.; Asano, M.; Oguchi, K.; Kurokawa, K.; Yamanishi, H.; Tateda, K.; Yamaguchi, K. Sepsis in hemodialysis patients. Jpn. Ass. Dial. Phys. 2011, 44, 617–622. [Google Scholar] [CrossRef][Green Version]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Gaudard, P.; Saour, M.; Morquin, D.; David, H.; Eliet, J.; Villiet, M.; Daures, J.P.; Colson, P. Acute kidney injury during daptomycin versus vancomycin treatment in cardiovascular critically ill patients: A propensity score matched analysis. BMC Infect. Dis. 2019, 19, 438. [Google Scholar] [CrossRef]
- Dvorchik, B.H.; Brazier, D.; DeBruin, M.F.; Arbeit, R.D. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 2003, 47, 1318–1323. [Google Scholar] [CrossRef]
- Arbeit, R.D.; Maki, D.; Tally, F.P.; Camponaro, E.; Eisenstein, B.I.; Daptomycin 98-01 and 99-01 Investigators. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 2004, 38, 1673–1681. [Google Scholar] [CrossRef]
- Maraolo, A.E.; Giaccone, A.; Saracino, A.; Bavaro, D.F. Daptomycin versus vancomycin for the treatment of methicillin-resistant Staphylococcus aureus bloodstream infection with or without endocarditis: A systematic review and meta-analysis. Antibiotics 2021, 10, 1014. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.; Upjohn, M.; Buising, K.; Pedagogos, E.; Nelson, C.; Kirkpatrick, C.M.; Kong, D.C.M. Vancomycin dosing in chronic high-flux haemodialysis: A systematic review. Int. J. Antimicrob. Agents 2018, 51, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nakatani, T.; Katori, H.; Hara, S.; Hayashi, M.; Takaichi, K.; Nakata, K.; Kasuya, Y. Vancomycin pharmacokinetics and dosing recommendations in patients undergoing hemodialysis. Jpn. J. Chemother. 2003, 51, 693–702. [Google Scholar]
- Cubicin (Daptomycin for Injection) [Prescribing Information]; Cubist Pharmaceuticals Inc.: Lexington, MA, USA, 2006.
- Fowler, V.G., Jr.; Boucher, H.W.; Corey, G.R.; Abrutyn, E.; Karchmer, A.W.; Rupp, M.E.; Levine, D.P.; Chambers, H.F.; Tally, F.P.; Vigliani, G.A.; et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N. Engl. J. Med. 2006, 355, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Kalimuddin, S.; Chan, Y.F.Z.; Philips, R.; Ong, S.P.; Archuleta, S.; Lye, D.C.; Tan, T.T.; Low, J.G.H. A randomized phase 2B trial of vancomycin versus daptomycin for the treatment of methicillin-resistant Staphylococcus aureus bacteremia due to isolates with high vancomycin minimum inhibitory concentrations—Results of a prematurely terminated study. Trials 2018, 19, 305. [Google Scholar] [CrossRef] [PubMed]
- Moise, P.A.; Culshaw, D.L.; Wong-Beringer, A.; Bensman, J.; Lamp, K.C.; Smith, W.J.; Bauer, K.; Goff, D.A.; Adamson, R.; Leuthner, K.; et al. Comparative Effectiveness of Vancomycin versus Daptomycin for MRSA Bacteremia with Vancomycin MIC >1 mg/L: A Multicenter Evaluation. Clin. Ther. 2016, 38, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hagihara, M.; Nishiyama, N.; Koizumi, Y.; Yamagishi, Y.; Matsuura, K.; Mikamo, H. Clinical effectiveness of daptomycin loading dose in patients infected with Gram-positive pathogens. J. Infect. Chmother. 2017, 23, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Lomaestro, B.M.; Rotschafer, J.C.; Moellering, R.C.; Craig, W.A.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Vancomycin therapeutic guidelines: A summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin. Infect. Dis. 2009, 49, 325–327. [Google Scholar] [CrossRef]
- Canut, A.; Isla, A.; Betriu, C.; Gascon, A.R. Pharmacokinetic-pharmacodynamic evaluation of daptomycin, tigecycline, and linezolid versus vancomycin for the treatment of MRSA infections in four western European countries. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2227–2235. [Google Scholar] [CrossRef]
- Diolez, J.; Venisse, N.; Belmouaz, S.; Bauwens, M.A.; Bridoux, F.; Beraud, G. Pilot Pharmacokinetic Study of High-Dose Daptomycin in Hemodialysis Patients with Infected Medical Devices. Am. J. Kidney Dis. 2017, 70, 732–734. [Google Scholar] [CrossRef]
- Mei, H.; Wang, J.; Che, H.; Wang, R.; Cai, Y. The clinical efficacy and safety of vancomycin loading dose: A systematic review and meta-analysis. Medicine 2019, 98, e17639. [Google Scholar] [CrossRef]
- Kato, H.; Hagihara, M.; Murakami, E.; Suematsu, H.; Nishiyama, N.; Koizumi, Y.; Yamagishi, Y.; Uno, B.; Mikamo, H. Considerations about the use of a loading dose of daptomycin in neutropenic murine thigh model with methicillin-resistant Staphylococcus aureus infection. Chemotherapy 2017, 63, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Hagihara, M.; Shibata, Y.; Asai, N.; Koizumi, Y.; Watarai, M.; Yamagishi, Y.; Mikamo, H. Retrospective study on clinical efficacy and safety for daptomycin intermittent doses with or without loading dose in renal failure patients. J. Infect. Chemother. 2020, 26, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.H.; Shao, C.H.; Chen, C.Y.; Lin, S.W.; Wu, C.C. Safety of high-dose daptomycin in patients with severe renal impairment. Ther. Clin. Risk Manag. 2018, 14, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takesue, Y.; Ohmagari, N.; Mochizuki, T.; Mikamo, H.; Seki, M.; Takakura, S.; Tokimatsu, I.; Takahashi, Y.; Kasahara, K.; et al. Practice guidelines for therapeutic drug monitoring of vancomycin: A consensus review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 365–380. [Google Scholar] [CrossRef]
- Clinical Laboratory Standards Institute. Clinical Laboratory Standards Institute. In Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard; CLSI Publication M07-A10.10th Edition; Clinical Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).