Qualitative Risk Assessment for Antimicrobial Resistance among Humans from Salmon Fillet Consumption Due to the High Use of Antibiotics against Bacterial Infections in Farmed Salmon
Abstract
:1. Introduction
2. Methods and Materials
2.1. Process for the Identification and Selection of Experts
- (1)
- Academia: researchers who have at least two peer-reviewed papers in the areas of antibiotic resistance, salmon pathogens, epidemiology of aquaculture production, and fish immunology.
- (2)
- Public sector: professionals (veterinarians, aquaculture engineers, and marine biologists) working on public and/or animal health in governmental organizations, including the National Fisheries and Aquaculture Service (Servicio Nacional de Pesca y Acuicultura; SERNAPESA); Subsecretary of Fisheries and Aquaculture (Subsecretaria de Pesca y Acuicultura; SUBPESCA); Fisheries Development Institute (Instituto de Fomento Pesquero; IFOP); Agricultural and Livestock Service (Servicio Agrícola Ganadero; SAG); Chilean Food Safety Agency (Agencia Chilena Para la Inocuidad Alimentaria; ACHIPIA); and the Institute of Public Health (Instituto de Salud Pública; ISP).
- (3)
- Salmon industry: members of the Salmon Technological Institute (INTENSAL, an organization for scientific and technical support to the production and supply companies of the entire national salmon industry composed of national and international professionals from different areas of the public and private sectors); veterinarians, biochemists, and biologists associated with diagnostic laboratories for aquaculture production in Chile.
2.2. Discussion for Identification of Nodes and Expert Elicitation
2.2.1. Identification of the Hazards of AMR in Salmon Farming
2.2.2. Evaluation of the Levels of Exposure of an Individual or Population to These Potential Hazards
- (1)
- Frequency of oral or medicated feed administration of oxytetracycline and/or florfenicol to treat specific bacterial infections of farmed salmon.
- (2)
- Proportion of commensal bacteria that are capable of developing resistance along with the mechanisms and pathways of direct and indirect transfer of ARGs to human pathogenic bacteria.
- (3)
- Human infections caused by oxytetracycline and/or florfenicol resistant bacteria acquired by people through salmon consumption.
- (4)
- The pharmacokinetic and pharmacodynamic aspects of these antibiotics.
- (5)
- Number of fish treated with antibiotics in Chile.
- (6)
- The possibility of using antibiotics without a veterinarian prescription.
- (7)
- The probability of co-selection, cross-resistance (a single antibiotic resistance mechanism conferring resistance to more than one antibiotic), or co-resistance (multiple antibiotic resistance mechanisms) with other antibiotic classes.
- (8)
- The decrease in antibiotic usage in Chile in the last decade [4] associated with the improvement in farming practices and better vaccines.
- (9)
- The potential link with virulence genes and ARGs.
- (10)
- Surveillance data on fish pathogens and trends of ARB and ARGs in animals, products and subproducts of animal origin, or livestock wastes.
2.2.3. Appraisal of the Context
2.3. Risk Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food & Agriculture ORG S.L.: Rome, Italy, 2020; ISBN 978-92-5-132692-3. [Google Scholar]
- Garlock, T.; Asche, F.; Anderson, J.; Bjørndal, T.; Kumar, G.; Lorenzen, K.; Ropicki, A.; Smith, M.D.; Tveterås, R. A global blue revolution: Aquaculture growth across regions, species, and countries. Rev. Fish. Sci. Aquac. 2020, 28, 107–116. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Sernapesca. Informe Sobre Uso de Antimicrobianos En La Salmonicultura Nacional—Año 2020; Servicio Nacional de Pesca y Acuicultura: Santiago, Chile, 2021. [Google Scholar]
- Mardones, F.O.; Paredes, F.; Medina, M.; Tello, A.; Valdivia, V.; Ibarra, R.; Correa, J.; Gelcich, S. Identification of research gaps for highly infectious diseases in aquaculture: The case of the endemic piscirickettsia salmonis in the chilean salmon farming industry. Aquaculture 2018, 482, 211–220. [Google Scholar] [CrossRef]
- Verdugo, C.; Zimin-Veselkoff, N.; Gardner, I.A.; Mardones, F.O. Expert elicitation of the diagnostic performance of two tests for bacterial kidney disease (BKD) surveillance in atlantic salmon (Salmo salar L.) broodstock in Chile. Aquaculture 2020, 525, 735274. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2014; ISBN 978-92-4-156474-8. [Google Scholar]
- Ministerio de Salud, Gobierno de Chile. Minsal Plan Nacional Contra La Resistencia a Los Antimicrobianos; Ministerio de Salud, Gobierno de Chile: Santiago, Chile, 2017. [Google Scholar]
- Servicio Nacional de Pesca y Acuicultura. Sernapesca Manual de Buenas Prácticas En El Uso de Antimicrobianos y Antiparasitarios En La Salmonicultura Chilena; Servicio Nacional de Pesca y Acuicultura: Santiago, Chile, 2015. [Google Scholar]
- Higuera-Llantén, S.; Vásquez-Ponce, F.; Barrientos-Espinoza, B.; Mardones, F.O.; Marshall, S.H.; Olivares-Pacheco, J. Extended antibiotic treatment in salmon farms select multiresistant gut bacteria with a high prevalence of antibiotic resistance genes. PLoS ONE 2018, 13, e0203641. [Google Scholar] [CrossRef] [Green Version]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Caxito, M.; Benavides, J.A.; Munita, J.M.; Rivas, L.; Garcia, P.; Listoni, F.J.P.; Moreno-Switt, A.I.; Paes, A.C. Risk factors associated with faecal carriage of extended-spectrum cephalosporin-resistant Escherichia coli among dogs in Southeast Brazil. Prev. Vet. Med. 2021, 190, 105316. [Google Scholar] [CrossRef]
- Salgado-Caxito, M.; Moreno-Switt, A.I.; Paes, A.C.; Shiva, C.; Munita, J.M.; Rivas, L.; Benavides, J.A. Higher prevalence of extended-spectrum cephalosporin-resistant Enterobacterales in dogs attended for enteric viruses in Brazil before and after treatment with cephalosporins. Antibiotics 2021, 10, 122. [Google Scholar] [CrossRef]
- Jans, C.; Sarno, E.; Collineau, L.; Meile, L.; Stärk, K.D.C.; Stephan, R. Consumer exposure to antimicrobial resistant bacteria from food at swiss retail level. Front. Microbiol. 2018, 9, 362. [Google Scholar] [CrossRef]
- Bennani, H.; Mateus, A.; Mays, N.; Eastmure, E.; Stärk, K.D.C.; Häsler, B. Overview of evidence of antimicrobial use and antimicrobial resistance in the food chain. Antibiotics 2020, 9, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derry, C.; Attwater, R.; Booth, S. Rapid health-risk assessment of effluent irrigation on an Australian University Campus. Int. J. Hyg. Environ. Health 2006, 209, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Haga, T. A rapid risk assessment of African Swine Fever introduction and spread in Japan based on expert opinions. J. Vet. Med. Sci. 2018, 80, 1743–1746. [Google Scholar] [CrossRef] [Green Version]
- Estévez, R.A.; Mardones, F.O.; Álamos, F.; Arriagada, G.; Carey, J.; Correa, C.; Escobar-Dodero, J.; Gaete, Á.; Gallardo, A.; Ibarra, R.; et al. Eliciting expert judgements to estimate risk and protective factors for piscirickettsiosis in chilean salmon farming. Aquaculture 2019, 507, 402–410. [Google Scholar] [CrossRef]
- Vose, D.; Acar, J.; Anthony, F.; Franklin, A.; Gupta, R.; Nicholls, T.; Tamura, Y.; Thompson, S.; Threlfall, E.J.; van Vuuren, M.; et al. Antimicrobial resistance: Risk analysis methodology for the potential impact on public health of antimicrobial resistant bacteria of animal origin. Rev. Sci. Tech.-Off. Int. Des Epizoot. 2001, 20, 811–827. [Google Scholar] [CrossRef] [Green Version]
- Knol, A.B.; Slottje, P.; van der Sluijs, J.P.; Lebret, E. The use of expert elicitation in environmental health impact assessment: A seven step procedure. Environ. Health 2010, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Wieland, B.; Dhollander, S.; Salman, M.; Koenen, F. Qualitative risk assessment in a data-scarce environment: A model to assess the impact of control measures on spread of African Swine Fever. Prev. Vet. Med. 2011, 99, 4–14. [Google Scholar] [CrossRef]
- Chereau, F.; Opatowski, L.; Tourdjman, M.; Vong, S. Risk assessment for antibiotic resistance in South East Asia. BMJ 2017, 358, j3393. [Google Scholar] [CrossRef] [Green Version]
- OIE. Chapter 6.5. Risk analysis for antimicrobial resistance arising from the use of antimicrobial agents in aquatic animals. In Aquatic Animal Health Code; World Organisation for Animal Health: Paris, France, 2019; ISBN 978-92-95108-96-7. [Google Scholar]
- Iona, J. Mentimeter; The School Library Association: Swindon, UK, 2018; Volume 66, p. 153. Available online: https://link.gale.com/apps/doc/A555409973/AONE?u=anon~9c9994af&sid=googleScholar&xid=9f9d5f27 (accessed on 12 June 2019).
- Hooban, B.; Joyce, A.; Fitzhenry, K.; Chique, C.; Morris, D. The role of the natural aquatic environment in the dissemination of extended spectrum beta-lactamase and carbapenemase encoding genes: A scoping review. Water Res. 2020, 180, 115880. [Google Scholar] [CrossRef]
- Zeballos-Gross, D.; Rojas-Sereno, Z.; Salgado-Caxito, M.; Poeta, P.; Torres, C.; Benavides, J.A. The role of gulls as reservoirs of antibiotic resistance in aquatic environments: A scoping review. Front. Microbiol. 2021, 12, 1938. [Google Scholar] [CrossRef]
- Roche, S.E.; Costard, S.; Meers, J.; Field, H.E.; Breed, A.C. Assessing the risk of nipah virus establishment in australian flying-foxes. Epidemiol. Infect. 2015, 143, 2213–2226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gale, P.; Brouwer, A.; Ramnial, V.; Kelly, L.; Kosmider, R.; Fooks, A.R.; Snary, E.L. Assessing the impact of climate change on vector-borne viruses in the EU through the elicitation of expert opinion. Epidemiol. Infect. 2010, 138, 214–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moutou, F.; Dufour, B.; Ivanov, Y. A qualitative assessment of the risk of introducing foot and mouth disease into Russia and Europe from Georgia, Armenia and Azerbaijan. Rev. Sci. Tech.-Off. Int. Épizooties 2001, 20, 723–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miranda, C.D.; Godoy, F.A.; Lee, M.R. Current status of the use of antibiotics and the antimicrobial resistance in the Chilean Salmon farms. Front. Microbiol. 2018, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Flores-Kossack, C.; Montero, R.; Köllner, B.; Maisey, K. Chilean aquaculture and the new challenges: Pathogens, immune response, vaccination and fish diversification. Fish Shellfish Immunol. 2020, 98, 52–67. [Google Scholar] [CrossRef]
- Lozano-Muñoz, I.; Wacyk, J.; Kretschmer, C.; Vásquez-Martínez, Y.; Martin, M.C.-S. Antimicrobial resistance in chilean marine-farmed salmon: Improving food safety through one health. One Health 2021, 12, 100219. [Google Scholar] [CrossRef]
- Burow, E.; Simoneit, C.; Tenhagen, B.-A.; Käsbohrer, A. Oral antimicrobials increase antimicrobial resistance in porcine E. coli—A systematic review. Prev. Vet. Med. 2014, 113, 364–375. [Google Scholar] [CrossRef]
- Okpara, E.O.; Ojo, O.E.; Awoyomi, O.J.; Dipeolu, M.A.; Oyekunle, M.A.; Schwarz, S. Antimicrobial usage and presence of extended-spectrum β-lactamase-producing enterobacteriaceae in animal-rearing households of selected rural and peri-urban communities. Vet. Microbiol. 2018, 218, 31–39. [Google Scholar] [CrossRef]
- Burridge, L.; Weis, J.S.; Cabello, F.; Pizarro, J.; Bostick, K. Chemical use in salmon aquaculture: A review of current practices and possible environmental effects. Aquaculture 2010, 306, 7–23. [Google Scholar] [CrossRef]
- Henriksson, P.J.G.; Rico, A.; Troell, M.; Klinger, D.H.; Buschmann, A.H.; Saksida, S.; Chadag, M.V.; Zhang, W. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: A review from a systems perspective. Sustain. Sci. 2018, 13, 1105–1120. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.; Wales, A. Antimicrobial resistance on farms: A review including biosecurity and the potential role of disinfectants in resistance selection. Compr. Rev. Food Sci. Food Saf. 2019, 18, 753–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Guo, J. Disinfection spreads antimicrobial resistance. Science 2021, 371, 474. [Google Scholar] [CrossRef] [PubMed]
- Dang, S.T.T.; Petersen, A.; Van Truong, D.; Chu, H.T.T.; Dalsgaard, A. Impact of medicated feed on the development of antimicrobial resistance in bacteria at integrated pig-fish farms in vietnam. Appl. Environ. Microbiol. 2011, 77, 4494–4498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, M.; Ahmad, F.; Yaqub, B.; Ramzan, A.; Imran, A.; Afzaal, M.; Mirza, S.A.; Mazhar, I.; Younus, M.; Akram, Q.; et al. Current trends of antimicrobials used in food animals and aquaculture. In Antibiotics and Antimicrobial Resistance Genes in the Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 39–69. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Maximum levels of cross-contamination for 24 antimicrobial active substances in non-target feed. Part 1: Methodology, general data gaps and uncertainties. EFSA J. 2021, 19, e06852. [Google Scholar] [CrossRef]
- Moutou, K.; McCarty, I.; Houlihan, D. The effect of dietary flumequine on food consumption and growth in rainbow trout. Aquac. Int. 2001, 9, 95–102. [Google Scholar] [CrossRef]
- Al-Souti, A.; Gallardo, W.; Claereboudt, M.; Mahgoub, O. Attractability and palatability of formulated diets incorporated with chicken feather and algal meals for Juvenile Gilthead Seabream, Sparus Aurata. Aquac. Rep. 2019, 14, 100199. [Google Scholar] [CrossRef]
- Servicio Nacional de Pesca y Acuicultura. Sernapesca NORMA TÉCNICA No4—Procedimiento de Toma de Muestras Para Aislamiento de Piscirickettsia Salmonis y Análisis Para Determinación de Concentración Mínima Inhibitoria (CMI), Mediante Microdilución En Caldo; Servicio Nacional de Pesca y Acuicultura: Santiago, Chile, 2021. [Google Scholar]
- Contreras-Lynch, S.; Smith, P.; Olmos, P.; Loy, M.E.; Finnegan, W.; Miranda, C.D. A novel and validated protocol for performing MIC tests to determine the susceptibility of piscirickettsia salmonis isolates to florfenicol and oxytetracycline. Front. Microbiol. 2017, 8, 1255. [Google Scholar] [CrossRef]
- Hammerum, A.M.; Heuer, O.E.; Emborg, H.-D.; Bagger-Skjøt, L.; Jensen, V.F.; Rogues, A.-M.; Skov, R.L.; Agersø, Y.; Brandt, C.T.; Seyfarth, A.M.; et al. Danish integrated antimicrobial resistance monitoring and research program. Emerg. Infect. Dis. 2007, 13, 1633–1639. [Google Scholar] [CrossRef]
- Acar, J.F.; Moulin, G. Integrating animal health surveillance and food safety: The issue of antimicrobial resistance. Rev. Sci. Tech.-Off. Int. Épizooties 2013, 32, 383–392. [Google Scholar] [CrossRef]
- Nielsen, L.R.; Alban, L.; Ellis-Iversen, J.; Mintiens, K.; Sandberg, M. Evaluating integrated surveillance of antimicrobial resistance: Experiences from use of three evaluation tools. Clin. Microbiol. Infect. 2020, 26, 1606–1611. [Google Scholar] [CrossRef]
| First Event | Second Event | |||
|---|---|---|---|---|
| Insignificant 1 | Low 2 | Moderate 3 | High 4 | |
| Insignificant | Insignificant | Insignificant | Insignificant | Insignificant |
| Low | Insignificant | Low | Low | Low |
| Moderate | Insignificant | Low | Moderate | Moderate |
| High | Insignificant | Low | Moderate | High |
| First Event | Second Event | |||
|---|---|---|---|---|
| Insignificant 1 | Low 2 | Moderate 3 | High 4 | |
| Insignificant | Insignificant | Low | Low | Moderate |
| Low | Low | Low | Moderate | Moderate |
| Moderate | Low | Moderate | Moderate | High |
| High | Moderate | Moderate | High | High |
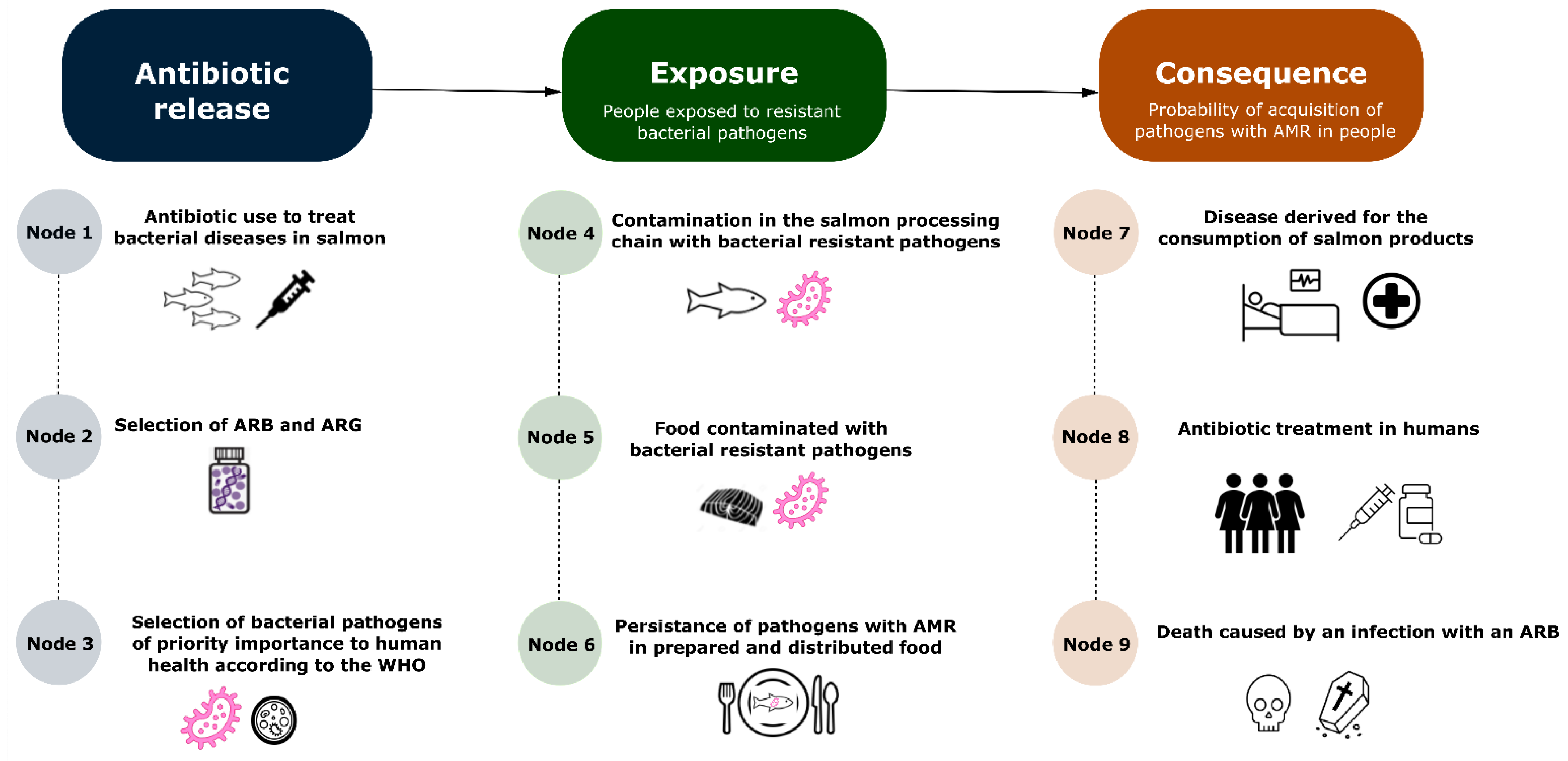
| Node | Definition |
|---|---|
| Node 1 | Probability of the need to use florfenicol or oxytetracycline to treat bacterial infections in farmed salmon. |
| Node 2 | Probability of selection of ARB and ARGs given that the previous event has occurred. |
| Node 3 | Probability that ARB and ARGs have been selected and that these are considered as a priority AMR pathogen for human health by the WHO. |
| Node 4 | Probability that the processing chain has been contaminated with bacteria-resistant pathogens. |
| Node 5 | Probability that salmon fillet has been contaminated with bacteria-resistant pathogens given that the previous event has occurred. |
| Node 6 | Probability of persistence of AMR pathogens in the contaminated salmon fillet after preparation and distribution. |
| Node 7 | Probability of adverse health effects caused by resistant microorganism or resistance determinants due to the consumption of salmon fillet treated with oxytetracycline or florfenicol. |
| Node 8 | Probability of the need to use antibiotics to treat bacterial infections among consumers of salmon fillet treated with antibiotics given that the previous event has occurred. |
| Node 9 | Probability of death caused by a bacterial infection that did not respond to antibiotic therapies and that has originated from the consumption of salmon fillet treated with florfenicol or oxytetracycline. |
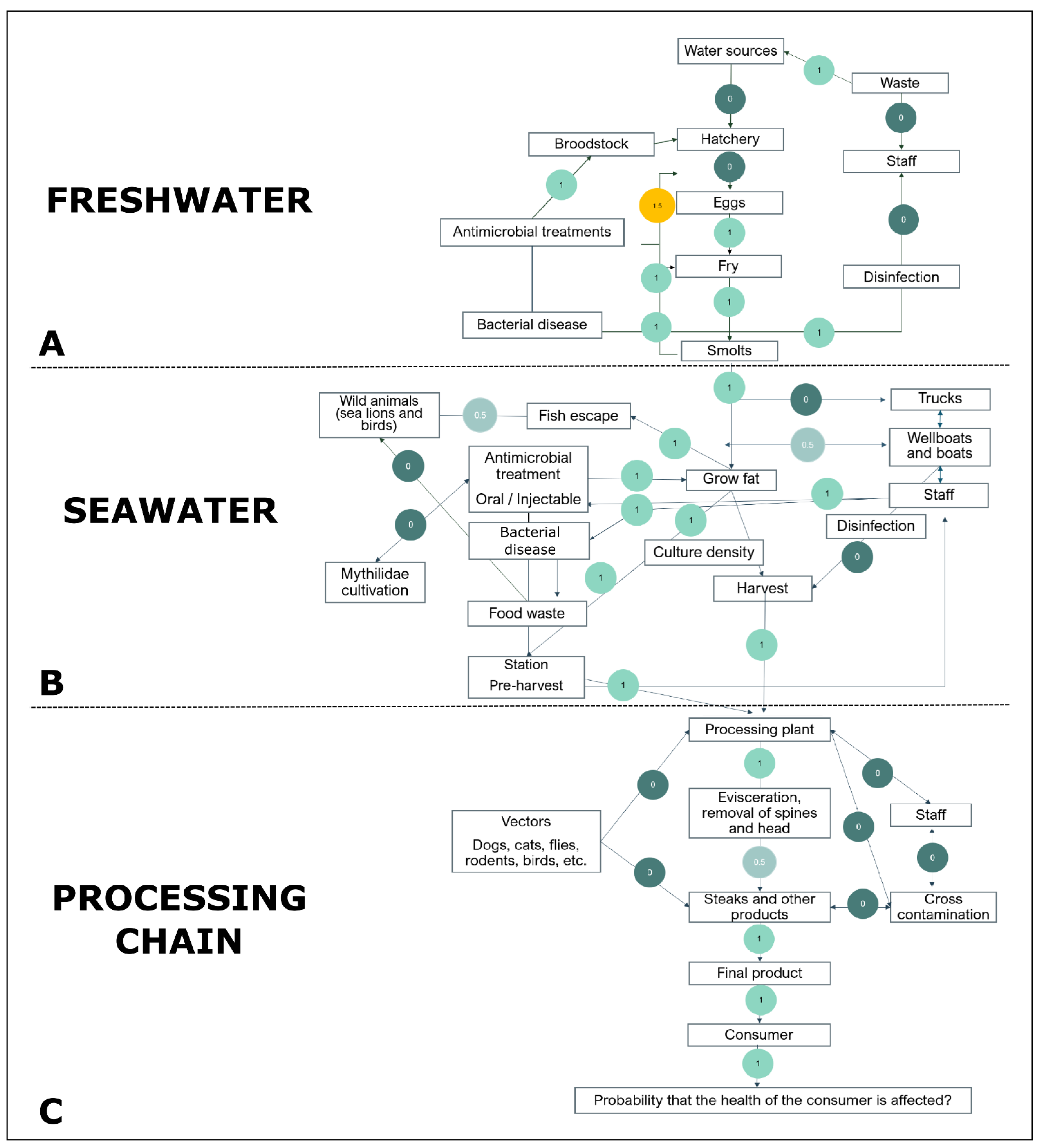
| Phase of the Salmon Production | Scenario | Definition |
|---|---|---|
| Freshwater/Seawater | Antimicrobial treatments | The use of antibiotics to eliminate or inhibit the growth of bacteria in a living organism. The main antibiotics used in salmon farming are florfenicol and oxytetracycline. |
| Freshwater/Seawater | Bacterial diseases | Refers to the main bacterial diseases in farmed salmon, including Salmon Rickettsial Syndrome (caused by Piscirickettsia salmonis) and Bacterial Kidney Disease (caused by Renibacterium salmoninarum). |
| Freshwater/Seawater | Disinfection | A sanitary practice to prevent infectious diseases by using bactericidal or bacteriostatic products to eliminate or inhibit the growth of bacteria on inert surfaces. |
| Freshwater/Seawater/Processing chain | Staff | All professionals working in at least one stage within the salmon production either in the salmon farms or in the processing chain. |
| Freshwater | Broodstock | A group of mature salmon individuals used for breeding. |
| Freshwater | Eggs | Farmed salmon eggs recovered from breeding. |
| Freshwater | Fry | Farmed salmon fry, from the hatched eggs in alevin to the onset of smoltification. |
| Freshwater | Hatchery | Incubation period of farmed salmon eggs recovered from breeding. |
| Freshwater | Smolts | Period when juvenile farmed salmon initiate their physiological adaptation to live in a marine (saltwater) environment. |
| Freshwater | Waste | Refers to the liquid wastes produced by industry that could be released into aquatic environments. |
| Freshwater | Water sources | Rivers that supply salmon farms and, eventually, other production systems (i.e., livestock and agricultural irrigation) and communities. |
| Seawater | Culture density | Refers to the number of farmed salmon by the size of the cages in the seawater phase. |
| Seawater | Fish escape | Refers to farmed salmon that manage to escape from the pens into the local aquatic environment. |
| Seawater | Food waste | Refers to residues of medicated feed used for antibiotic administration in salmon farming. |
| Seawater | Grow fat | Rearing and fattening period of farmed salmon during the seawater phase. |
| Seawater | Harvest | Process in which salmon that finish the Grow fat step are extracted for human consumption. |
| Seawater | Mythilidae cultivation | Mytilidae farms that are located near the salmon farms. |
| Seawater | Pre-harvest station | An empty farm typically nearby a processing plant that receives from another farm market-size fish a few hours before harvest at the processing plant. Sometimes called a collection center. |
| Seawater | Trucks | Refers to the trucks used to transport farmed salmon. |
| Seawater | Wellboats and boats | Refers to the wellboats (a fishing vessel) used for storing and transporting live farmed salmon and the boats used to visit the salmon farm pens. |
| Seawater | Wild animals | Refers to wild animals that can be found in (or near) the aquatic environments of salmon farms. |
| Processing chain | Consumer | The person who purchased and/or consumed farmed salmon. |
| Processing chain | Cross-contamination | Refers to the process by which food comes into contact with external substances, generally harmful to health. |
| Processing chain | Evisceration, removal of spines and head | Refers to the removal of the viscera (i.e., intestines) and inedible parts of a salmon carcass for the preparation of the final product. |
| Processing chain | Final product | Refers to salmon fillets or their subproducts ready to be marketed. |
| Processing chain | Probability that the health of the consumer is affected | Refers to the final product contaminated with AMR pathogens available for sale and consumption. |
| Processing chain | Processing plant | A place where various operations are carried out to process, handle, and store harvested salmon for human consumption. |
| Processing chain | Steaks and other products | Refers to the preparation of final products by cutting the salmon meat into fillets or subproducts before packaging. |
| Processing chain | Vectors | Refers to any animal that can act as a carrier of ARB or ARGs and contaminate the processing chain. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgado-Caxito, M.; Zimin-Veselkoff, N.; Adell, A.D.; Olivares-Pacheco, J.; Mardones, F.O. Qualitative Risk Assessment for Antimicrobial Resistance among Humans from Salmon Fillet Consumption Due to the High Use of Antibiotics against Bacterial Infections in Farmed Salmon. Antibiotics 2022, 11, 662. https://doi.org/10.3390/antibiotics11050662
Salgado-Caxito M, Zimin-Veselkoff N, Adell AD, Olivares-Pacheco J, Mardones FO. Qualitative Risk Assessment for Antimicrobial Resistance among Humans from Salmon Fillet Consumption Due to the High Use of Antibiotics against Bacterial Infections in Farmed Salmon. Antibiotics. 2022; 11(5):662. https://doi.org/10.3390/antibiotics11050662
Chicago/Turabian StyleSalgado-Caxito, Marília, Natalia Zimin-Veselkoff, Aiko D. Adell, Jorge Olivares-Pacheco, and Fernando O. Mardones. 2022. "Qualitative Risk Assessment for Antimicrobial Resistance among Humans from Salmon Fillet Consumption Due to the High Use of Antibiotics against Bacterial Infections in Farmed Salmon" Antibiotics 11, no. 5: 662. https://doi.org/10.3390/antibiotics11050662
APA StyleSalgado-Caxito, M., Zimin-Veselkoff, N., Adell, A. D., Olivares-Pacheco, J., & Mardones, F. O. (2022). Qualitative Risk Assessment for Antimicrobial Resistance among Humans from Salmon Fillet Consumption Due to the High Use of Antibiotics against Bacterial Infections in Farmed Salmon. Antibiotics, 11(5), 662. https://doi.org/10.3390/antibiotics11050662








