In Vitro Growth-Inhibitory Synergistic Effect of Zinc Pyrithione in Combination with Gentamicin against Bacterial Skin Pathogens of Livestock
Abstract
:1. Introduction
2. Results
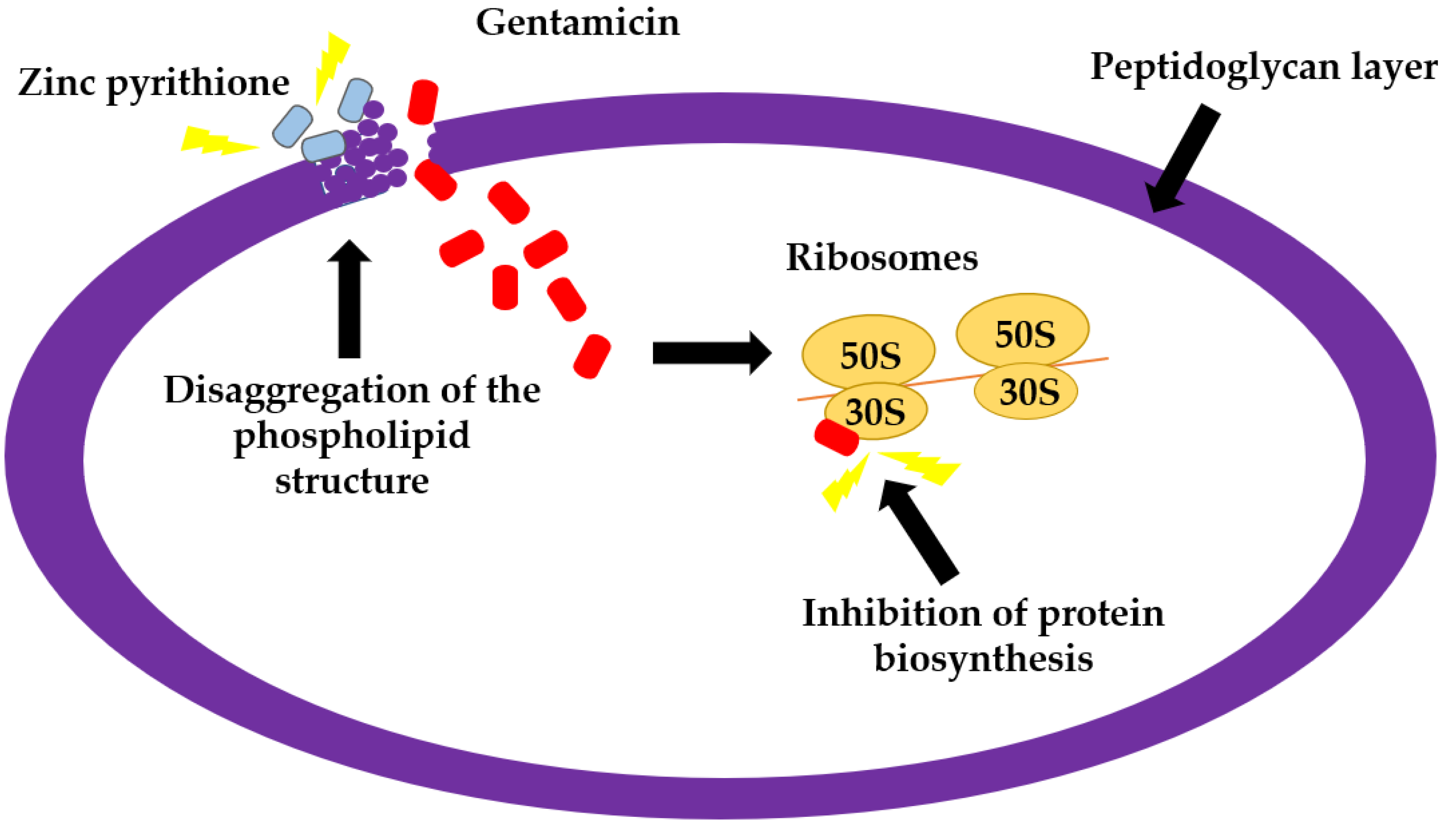
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains and Growth Media
4.3. Evaluation of Minimum Inhibitory Concentrations and Synergistic Combinatory Effect
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, M.C.; Joshi, C. Plants used in skin diseases of animals. Nat. Prod. Radiance 2004, 3, 293–299. [Google Scholar]
- Wassink, G.J.; George, T.R.; Kaler, J.; Green, L.E. Footrot and interdigital dermatitis in sheep: Farmer satisfaction with current management, their ideal management and sources used to adopt new strategies. Prev. Vet. Med. 2010, 96, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stulberg, D.; Pendrod, M.; Blatny, R.A. Caring for common skin condition: Common bacterial skin infections. Am. Fam. Physician 2002, 66, 119–125. [Google Scholar]
- Foster, A.P. Staphylococcal skin disease in livestock. Vet. Dermatol. 2012, 23, 342–351. [Google Scholar] [CrossRef]
- Apley, M.D.; Coetzee, J.F. Antimicrobial drug use in cattle. In Antimicrobial Therapy in Veterinary Medicine, 5th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; pp. 495–518. [Google Scholar]
- Clark, D. The Changing Nature of Farm Systems Research; The New Zealand Society of Animal Production: Hamilton, New Zealand, 2013. [Google Scholar]
- Rodvold, K.A.; McConeghy, K.W. Methicillin-resistant Staphylococcus aureus therapy: Past, present, and future. Clin. Infect. Dis. 2014, 58, 20–27. [Google Scholar] [CrossRef]
- Gomes, F.; Henriques, M. Control of bovine mastitis: Old and recent therapeutic approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and classification of mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef]
- Lozano, C.; Gharsa, H.; Ben Slama, K.; Zarazaga, M.; Torres, C. Staphylococcus aureus in animals and food: Methicillin resistance, prevalence and population structure. A review in the African continent. Microorganisms 2016, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Nöremark, M.; Frössling, J.; Sternber Lewerin, S. Application of routines that contribute to on-farm biosecurity as reported by Swedish livestock farmers. Transbound. Emerg. Dis. 2010, 57, 225–236. [Google Scholar] [CrossRef]
- Al Sheikh, H.M.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-Sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 480. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Begashaw, B.; Mishra, B.; Tsegaw, A.; Shewamene, Z. Methanol leaves extract Hibiscus micranthus Linn exhibited antibacterial and wound healing activities. BMC Complementary Altern. Med. 2017, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Barak-Shinar, D.; Green, L.J. Scalp seborrheic dermatitis and dandruff therapy using a herbal and zinc pyrithione-based therapy of shampoo and scalp lotion. J. Clin. Aesthetic Dermatol. 2018, 11, 26–31. [Google Scholar]
- Deeksha, R.M.; Sharma, P.K. Advancement in shampoo (a dermal care product): Preparation methods, patents and commercial utility. Recent Pat. Inflamm. Allergy Drug Discov. 2014, 8, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kokoska, L.; Kloucek, P.; Leuner, O.; Novy, P. Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem. 2019, 26, 5501–5541. [Google Scholar] [CrossRef]
- Blanchard, C.; Brooks, L.; Ebsworth-Mojica, K.; Didione, L.; Wucher, B.; Dewhurst, S.; Krysan, D.; Dunman, P.M.; Wozniak, R.A.F.; Fey, P.D. Zinc pyrithione improves the antibacterial activity of silver sulfadiazine ointment. MSphere 2016, 1, e00194-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, J.R.; Bacon, R.A.; Shah, R.; Mizoguchi, H.; Tosti, A. Therapeutic efficacy of anti-dandruff shampoos: A randomized clinical trial comparing products based on potentiated zinc pyrithione and zinc pyrithione/climbazole. Int. J. Cosmet. Sci. 2013, 35, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Yechiel, E. Interactive vehicles in synergistic cosmeceuticals: Advances in nanoencapsulation, transportation, transfer, and targeting. In Delivery System Handbook for Personal Care and Cosmetic Products; William Andrew Publishing: Norwich, NY, USA, 2005; pp. 303–319. [Google Scholar]
- Boothe, D.M.; Auburn University, Auburn, AL, USA. Personal communication, 2015.
- Hu, Y.; Liu, A.; Vaudrey, J.; Vaiciunaite, B.; Moigboi, C.; McTavish, S.M.; Kearns, A.; Coates, A. Combinations of β-lactam or aminoglycoside antibiotics with plectasin are synergistic against methicillin-sensitive and methicillin-resistant Staphylococcus aureus. PLoS ONE 2015, 10, e0117664. [Google Scholar] [CrossRef] [Green Version]
- Ruppen, C.; Lupo, A.; Decosterd, L.; Sendi, P. Is penicillin plus gentamicin synergistic against clinical group B Streptococcus isolates?: An in vitro study. Front. Microbiol. 2016, 7, 1680. [Google Scholar] [CrossRef]
- Kalnins, N.J.; Haworth, M.; Croton, C.; Gibson, J.S.; Stewart, A.J.; Purcell, S.L. Treatment of moderate grad dog bite wounds using amoxicillin-clavuanic acid with and without enrofloxacin: A randomised non-inferiority trial. Aust. Vet. J. 2021, 99, 369–377. [Google Scholar] [CrossRef]
- Shin, B.; Park, W. Zoonotic diseases and phytochemical medicines for microbial infections in veterinary science: Current state ad future prospective. Front. Vet. Sci. 2018, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Maia, N.L.; Barros, M.; Oliveira, L.L.; Cardoso, S.A.; Santos, M.H.; Pieri, F.A.; Ramalho, T.C.; Cunha, E.F.F.; Moreira, M.A.S. Synergism of plant compound with traditional antimicrobials against Streptococcus spp. isolated from bovine mastitis. Front. Microbiol. 2018, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Sreepian, A.; Popruk, S.; Nutalai, D.; Phutthanu, C.; Sreepian, P.M. Antibacterial activities and synergistic interaction of citrus essential oils and limonene with gentamicin against clinically isolated methicillin-resistant Staphylococcus aureus. Sci. World J. 2022, 2022, 8418287. [Google Scholar] [CrossRef] [PubMed]
- Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI-2020.pdf (accessed on 20 January 2020).
- Paduszynska, M.A.; Greber, K.E.; Paduszynski, W.; Sawicki, W.; Kamysz, W. Activity of temporin A and short lipopeptides combined with gentamicin against biofilm formed by Staphylococcus aureus and Pseudomonas aeruginosa. Antibiotics 2020, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.R.; Baron, E.J.; Pfaller, M.A.; Tenover, F.C.; Yolken, R.H. Mannual of Clinical Microbiology, 7th ed.; American Society for Microbiology: Washington, DC, USA, 2007; pp. 1532–1533. [Google Scholar]
- Lin, L.; Huang, X.; Yang, H.; He, Y.; He, X.; Huang, J.; Li, S.; Wang, X.; Tang, S.; Liu, G.; et al. Molecular epidemiology, antimicrobial activity, and virulence gene clustering of Streptococcus agalactiae isolated from dairy cattle with mastitis in China. J. Dairy Sci. 2021, 104, 4893–4903. [Google Scholar] [CrossRef]
- Oh, S.I.; Kim, J.W.; Jung, J.Y.; Chae, M.; Lee, Y.R.; Kim, J.H.; So, B.J.; Kim, H.Y. Pathologic and molecular characterization of Streptococcus dysgalactiae subsp. equisimilis infection in neonatal piglets. J. Vet. Sci. 2018, 19, 313–317. [Google Scholar] [CrossRef] [Green Version]
- Moreno, M.G.; Trampuz, A.; di Luca, M. Synergistic antibiotic activity against planktonic and biofilm-embeded Streptococus agalactiae, Streptococcus pyogenes and Streptococcus oralis. J. Antimicrob. Chemother. 2017, 72, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.H.; Yu, C.M.; Yu, V.L.; Chow, J.W. Synergy assessed by checkerboard. A critical analysis. Diagn. Microbiol. Infect. Dis. 1993, 16, 343–349. [Google Scholar] [CrossRef]
- Albert, A.; Rees, C.W.; Tomlinson, A.J.H. The influence of chemical constitution on antibacterial activity. Part VIII. 2-mercaptopyridine N-oxide, and some general observations on metal-binding agents. Br. J. Exp. Pathol. 1956, 37, 500–511. [Google Scholar]
- Latchman, D.S. Transcription factors: An overview. Int. J. Exp. Pathol. 1993, 74, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Bairoch, A. The ENZYME database in 2000. Nucleic Acids Res. 2000, 28, 304–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Freisinger, E.; Sigel, R.K.O. From nucleotides to ribozymes-a comparison of their metal ion binding properties. Coord. Chem. Rev. 2007, 251, 1834–1851. [Google Scholar] [CrossRef] [Green Version]
- Dinning, A.J.; AL-Adham, I.S.; Austin, P.; Charlton, M.; Collier, P.J. Pyrithione biocide interactions with bacterial phospholipid head groups. J. Appl. Microbiol. 1998, 85, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almatrood, W.; Nakouti, I.; Hobbs, G. Microtiter plate with built-in oxygen sensors: A novel approach to investigate the dynamics of Pseudomonas aeruginosa growth suppression in the presence of divalent cations and antibiotics. Arch. Microbiol. 2022, 204, 297. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Joly, H.; Omri, A. Liposomes as a carrier for gentamicin delivery: Development and evaluation of the physicochemical properties. Int. J. Pharm. 2008, 359, 254–263. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Hayete, B.; Lawrence, C.A.; Collins, J.J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 2007, 130, 797–810. [Google Scholar] [CrossRef] [Green Version]
- Chandler, C.J.; Segel, I.H. Mechanism of the antibacterial action of pyrithione: Effects on membrane transport, ATP levels and protein synthesis. Antimicrob. Agents Chemother. 1978, 14, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, D.H.; Page, S.W. Antimicrobial stewardship in veterinary medicine. Microbiol. Spectr. 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- National Registration Authority for Agricultural and Veterinary Chemicals. Evaluation of the New Active Zinc Pyrithione in the Product International Intersmooth 360 Ecoloflex Antifouling; NRA: Canberra, Australia, 2001. [Google Scholar]
- Mangion, S.E.; Holmes, A.M.; Roberts, M.S. Targeted delivery of zinc pyrithione to skin epithelia. Int. J. Mol. Sci. 2021, 22, 9730. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on Zinc Pyrithione; SCCS: Luxembourg, 2013. [Google Scholar]
- Maglio, D.; Nightingale, C.H.; Nicolau, D.P. Extended interval aminoglycoside dosing: From concept to clinic. Int. J. Antimicrob. Agents 2002, 19, 341–348. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Rondevaldova, J.; Hummelova, J.; Tauchen, J.; Kokoska, L. In vitro antistaphylococcal synergistic effect of isoflavone metabolite demethyltexasin with amoxicillin and oxacillin. Microb. Drug Resist. 2018, 24, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. In Approved Standard M07, 11th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Cos, P.; Vlietinck, A.J.; Berghe, D.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Leber, A. synergism testing: Broth microdilution checkerboard and broth macrodilution methods. In Clinical Microbiology Procedures Handbook, 4th ed.; ASM Press: Washington, DC, USA, 2016; Volume 1–3, pp. 1–23. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
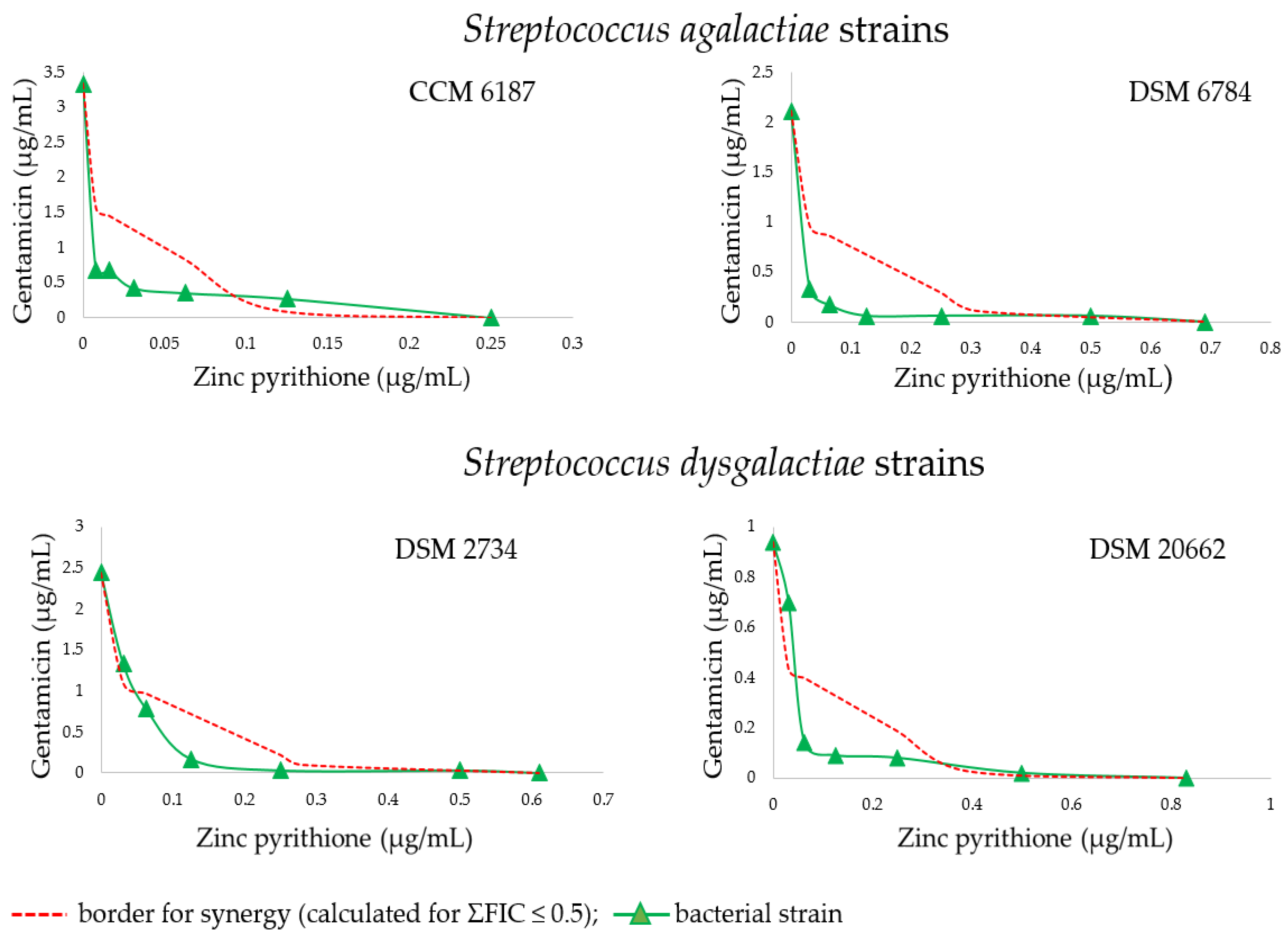
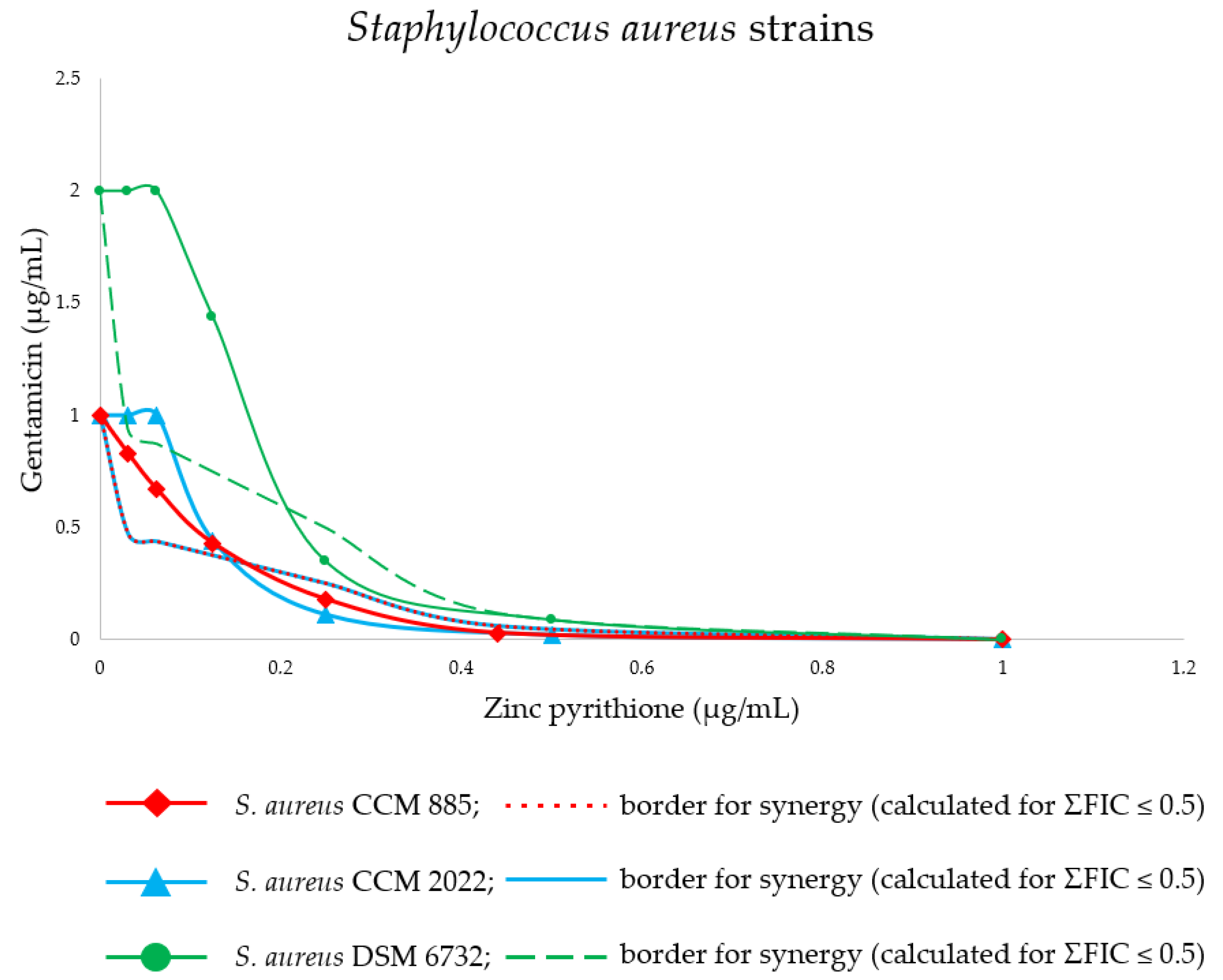

| Str.a. strain | Alone MICs (µg/mL) | GEN at concentration indicated in MIC column in combination with listed ZnP concentrations (µg/mL) | ||||||||||||||||
| GEN | ZnP | +ZnP 0.125 | +ZnP 0.063 | + ZnP 0.031 | +ZnP 0.016 | +ZnP 0.008 | ||||||||||||
| MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | |||||||||
| 6187 * | 3.33 | 0.25 | 0.27 | 0.58 b | 0.35 | 0.36cd | 0.42 | 0.25d | 0.67 | 0.26 | 0.67 | 0.23 | ||||||
| +ZnP 0.50 | +ZnP 0.25 | +ZnP 0.125 | +ZnP 0.063 | +ZnP 0.031 | ||||||||||||||
| GEN | ZnP | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | |||||||
| 6784 ** | 2.11 | 0.69 | 0.06 | 0.75 a | 0.06 | 0.39b | 0.06 | 0.26 c | 0.17 | 0.25 cd | 0.33 | 0.20d | ||||||
| Str.d. strain | Alone MICs (µg/mL) | GEN at concentration indicated in MIC column in combination with listed ZnP concentrations (µg/mL) | ||||||||||||||||
| GEN | ZnP | +ZnP 0.50 | +ZnP 0.25 | +ZnP 0.125 | +ZnP 0.063 | +ZnP 0.031 | ||||||||||||
| MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | |||||||||
| 2734 * | 2.44 | 0.61 | 0.03 | 0.83 a | 0.03 | 0.42b | 0.17 | 0.27c | 0.78 | 0.39cd | 1.33 | 0.59 c | ||||||
| 20662 * | 0.94 | 0.83 | 0.02 | 0.62 b | 0.08 | 0.39b | 0.09 | 0.24c | 0.14 | 0.22cd | 0.70 | 0.51 c | ||||||
| S.a. strain | Alone MICs (µg/mL) | GEN at concentration indicated in MIC column in combination with listed ZnP concentrations (µg/mL) | ||||||||||||||||
| GEN | ZnP | +ZnP 0.50 | +ZnP 0.25 | +ZnP 0.125 | +ZnP 0.063 | +ZnP 0.031 | ||||||||||||
| MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | MIC | ΣFIC | |||||||||
| SA1 * | 0.67 | 0.50 | 0.02 | 1.03 a | 0.03 | 0.55 a | 0.39 | 0.83 a | 1.12 | 1.80 a | 1.56 | 2.39 a | ||||||
| SA3 * | 1.00 | 0.50 | 0.02 | 1.02 a | 0.03 | 0.53 a | 0.5 | 0.75 a | 0.89 | 1.02 bc | 0.94 | 1.00 bc | ||||||
| 29213 ** | 1.00 | 0.50 | 0.02 | 1.02 a | 0.11 | 0.61 a | 0.63 | 0.88 a | 1.21 | 1.33 b | 1.33 | 1.39 b | ||||||
| 885 *** | 1.00 | 1.00 | 0.03 | 0.53 bc | 0.18 | 0.43b | 0.43 | 0.54 b | 0.67 | 0.73 c | 0.83 | 0.86 bc | ||||||
| 2022 *** | 1.00 | 1.00 | 0.02 | 0.52 c | 0.11 | 0.35bc | 0.44 | 0.57 b | 1.00 | 1.06 bc | 1.00 | 1.03 bc | ||||||
| 2773 *** | 2.00 | 0.50 | 0.02 | 1.01 a | 0.09 | 0.55 a | 0.54 | 0.52 b | 1.58 | 0.92 bc | 1.67 | 0.90 bc | ||||||
| 4516 *** | 1.00 | 0.50 | 0.02 | 1.02 a | 0.12 | 0.62 a | 0.64 | 0.89 a | 1.33 | 1.46 b | 1.61 | 1.67 b | ||||||
| 6732 **** | 2.00 | 1.00 | 0.09 | 0.55 bc | 0.35 | 0.42b | 1.44 | 0.85 b | 2.00 | 1.06 bc | 2.00 | 1.03 bc | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mala, L.; Lalouckova, K.; Skrivanova, E.; Houdkova, M.; Strakova, M.; Kokoska, L. In Vitro Growth-Inhibitory Synergistic Effect of Zinc Pyrithione in Combination with Gentamicin against Bacterial Skin Pathogens of Livestock. Antibiotics 2022, 11, 960. https://doi.org/10.3390/antibiotics11070960
Mala L, Lalouckova K, Skrivanova E, Houdkova M, Strakova M, Kokoska L. In Vitro Growth-Inhibitory Synergistic Effect of Zinc Pyrithione in Combination with Gentamicin against Bacterial Skin Pathogens of Livestock. Antibiotics. 2022; 11(7):960. https://doi.org/10.3390/antibiotics11070960
Chicago/Turabian StyleMala, Lucie, Klara Lalouckova, Eva Skrivanova, Marketa Houdkova, Marie Strakova, and Ladislav Kokoska. 2022. "In Vitro Growth-Inhibitory Synergistic Effect of Zinc Pyrithione in Combination with Gentamicin against Bacterial Skin Pathogens of Livestock" Antibiotics 11, no. 7: 960. https://doi.org/10.3390/antibiotics11070960
APA StyleMala, L., Lalouckova, K., Skrivanova, E., Houdkova, M., Strakova, M., & Kokoska, L. (2022). In Vitro Growth-Inhibitory Synergistic Effect of Zinc Pyrithione in Combination with Gentamicin against Bacterial Skin Pathogens of Livestock. Antibiotics, 11(7), 960. https://doi.org/10.3390/antibiotics11070960








